 Found 117 hits with Last Name = 'naito' and Initial = 'm'
Found 117 hits with Last Name = 'naito' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50390978

(VALSPODAR)Show SMILES C\C=C\C[C@@H](C)C(=O)[C@@H]1N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C(=O)[C@@H](NC1=O)C(C)C)C(C)C |r| Show InChI InChI=1S/C63H111N11O12/c1-26-27-28-41(16)53(76)52-57(80)67-49(38(10)11)61(84)68(19)33-48(75)69(20)44(29-34(2)3)56(79)66-50(39(12)13)62(85)70(21)45(30-35(4)5)55(78)64-42(17)54(77)65-43(18)58(81)71(22)46(31-36(6)7)59(82)72(23)47(32-37(8)9)60(83)73(24)51(40(14)15)63(86)74(52)25/h26-27,34-47,49-52H,28-33H2,1-25H3,(H,64,78)(H,65,77)(H,66,79)(H,67,80)/b27-26+/t41-,42+,43-,44+,45+,46+,47+,49+,50+,51+,52+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Takarazuka Research Institute
Curated by ChEMBL
| Assay Description
TP_TRANSPORTER: inhibition of ATPase activity (Verapamil) in P-gp-MRP-16-Protein A complex |
Br J Pharmacol 122: 241-8 (1997)
Article DOI: 10.1038/sj.bjp.0701377
BindingDB Entry DOI: 10.7270/Q28055FW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50171495
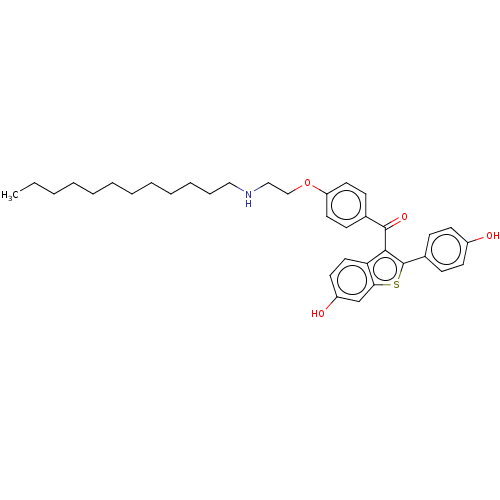
(CHEMBL3805318)Show SMILES CCCCCCCCCCCCNCCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C35H43NO4S/c1-2-3-4-5-6-7-8-9-10-11-22-36-23-24-40-30-19-14-26(15-20-30)34(39)33-31-21-18-29(38)25-32(31)41-35(33)27-12-16-28(37)17-13-27/h12-21,25,36-38H,2-11,22-24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha (unknown origin) transfected in 17beta-estradiol induced-HEK293 cells assessed as inhibition of estradiol-mediated pro... |
Bioorg Med Chem 24: 2914-2919 (2016)
Article DOI: 10.1016/j.bmc.2016.04.068
BindingDB Entry DOI: 10.7270/Q21V5GWW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay |
Bioorg Med Chem 24: 2914-2919 (2016)
Article DOI: 10.1016/j.bmc.2016.04.068
BindingDB Entry DOI: 10.7270/Q21V5GWW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50171494
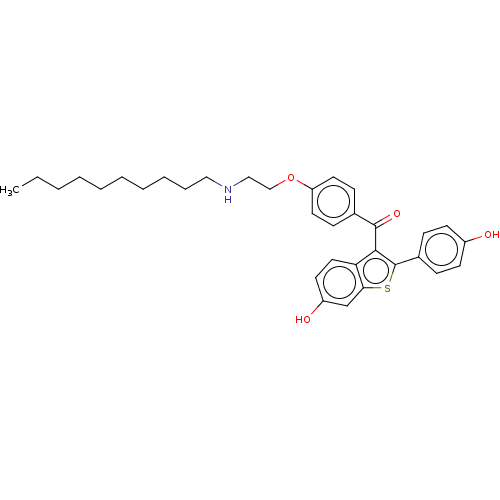
(CHEMBL3806167)Show SMILES CCCCCCCCCCNCCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C33H39NO4S/c1-2-3-4-5-6-7-8-9-20-34-21-22-38-28-17-12-24(13-18-28)32(37)31-29-19-16-27(36)23-30(29)39-33(31)25-10-14-26(35)15-11-25/h10-19,23,34-36H,2-9,20-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha (unknown origin) transfected in 17beta-estradiol induced-HEK293 cells assessed as inhibition of estradiol-mediated pro... |
Bioorg Med Chem 24: 2914-2919 (2016)
Article DOI: 10.1016/j.bmc.2016.04.068
BindingDB Entry DOI: 10.7270/Q21V5GWW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method |
Bioorg Med Chem 23: 4132-8 (2015)
Article DOI: 10.1016/j.bmc.2015.06.067
BindingDB Entry DOI: 10.7270/Q2V989T0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50459869
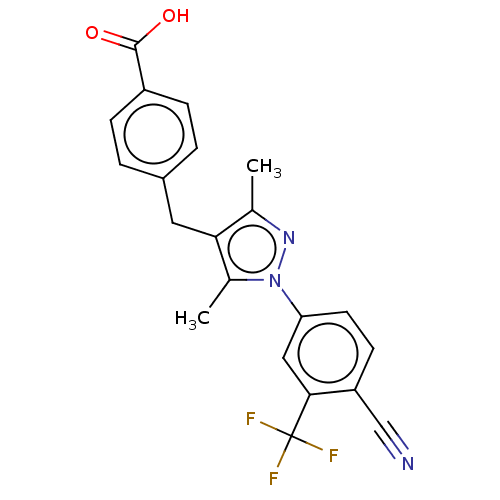
(CHEMBL4227715)Show SMILES Cc1nn(c(C)c1Cc1ccc(cc1)C(O)=O)-c1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C21H16F3N3O2/c1-12-18(9-14-3-5-15(6-4-14)20(28)29)13(2)27(26-12)17-8-7-16(11-25)19(10-17)21(22,23)24/h3-8,10H,9H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to androgen receptor (unknown origin) |
J Med Chem 61: 543-575 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00168
BindingDB Entry DOI: 10.7270/Q2VT1VR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50459874
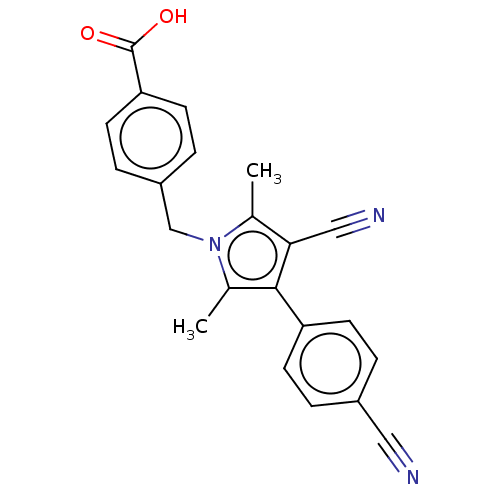
(CHEMBL4226259)Show SMILES Cc1c(C#N)c(c(C)n1Cc1ccc(cc1)C(O)=O)-c1ccc(cc1)C#N Show InChI InChI=1S/C22H17N3O2/c1-14-20(12-24)21(18-7-3-16(11-23)4-8-18)15(2)25(14)13-17-5-9-19(10-6-17)22(26)27/h3-10H,13H2,1-2H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to androgen receptor (unknown origin) |
J Med Chem 61: 543-575 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00168
BindingDB Entry DOI: 10.7270/Q2VT1VR7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50171499
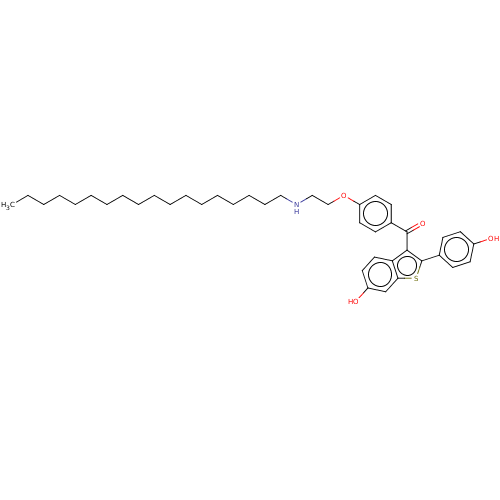
(CHEMBL3805385)Show SMILES CCCCCCCCCCCCCCCCCCNCCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C41H55NO4S/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-28-42-29-30-46-36-25-20-32(21-26-36)40(45)39-37-27-24-35(44)31-38(37)47-41(39)33-18-22-34(43)23-19-33/h18-27,31,42-44H,2-17,28-30H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay |
Bioorg Med Chem 24: 2914-2919 (2016)
Article DOI: 10.1016/j.bmc.2016.04.068
BindingDB Entry DOI: 10.7270/Q21V5GWW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50092526
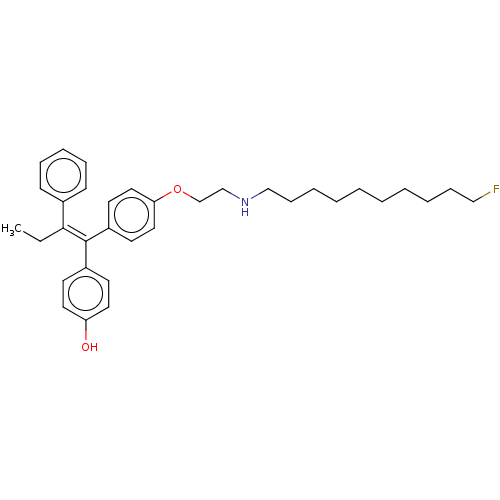
(CHEMBL3586195)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCNCCCCCCCCCCF)cc1)c1ccccc1 Show InChI InChI=1S/C34H44FNO2/c1-2-33(28-14-10-9-11-15-28)34(29-16-20-31(37)21-17-29)30-18-22-32(23-19-30)38-27-26-36-25-13-8-6-4-3-5-7-12-24-35/h9-11,14-23,36-37H,2-8,12-13,24-27H2,1H3/b34-33- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha (unknown origin) by fluorescence polarization-based competition binding assay |
Bioorg Med Chem 23: 3091-6 (2015)
Article DOI: 10.1016/j.bmc.2015.05.002
BindingDB Entry DOI: 10.7270/Q28P6277 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50092484
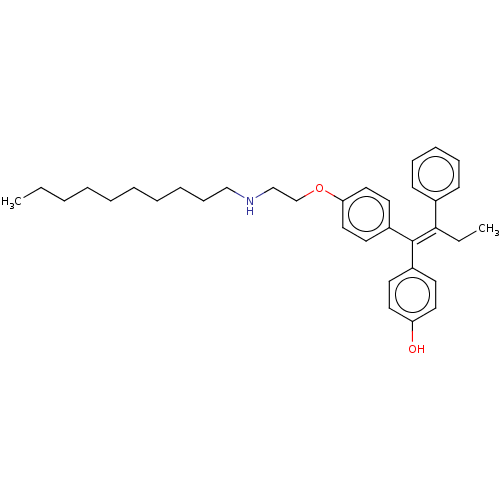
(CHEMBL3586191)Show SMILES CCCCCCCCCCNCCOc1ccc(cc1)C(=C(\CC)c1ccccc1)\c1ccc(O)cc1 Show InChI InChI=1S/C34H45NO2/c1-3-5-6-7-8-9-10-14-25-35-26-27-37-32-23-19-30(20-24-32)34(29-17-21-31(36)22-18-29)33(4-2)28-15-12-11-13-16-28/h11-13,15-24,35-36H,3-10,14,25-27H2,1-2H3/b34-33- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha (unknown origin) by fluorescence polarization-based competition binding assay |
Bioorg Med Chem 23: 3091-6 (2015)
Article DOI: 10.1016/j.bmc.2015.05.002
BindingDB Entry DOI: 10.7270/Q28P6277 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50092523
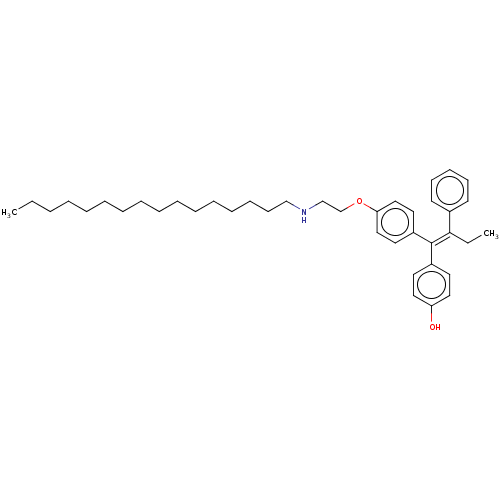
(CHEMBL3586193)Show SMILES CCCCCCCCCCCCCCCCNCCOc1ccc(cc1)C(=C(\CC)c1ccccc1)\c1ccc(O)cc1 Show InChI InChI=1S/C40H57NO2/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-20-31-41-32-33-43-38-29-25-36(26-30-38)40(35-23-27-37(42)28-24-35)39(4-2)34-21-18-17-19-22-34/h17-19,21-30,41-42H,3-16,20,31-33H2,1-2H3/b40-39- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha (unknown origin) by fluorescence polarization-based competition binding assay |
Bioorg Med Chem 23: 3091-6 (2015)
Article DOI: 10.1016/j.bmc.2015.05.002
BindingDB Entry DOI: 10.7270/Q28P6277 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50279272
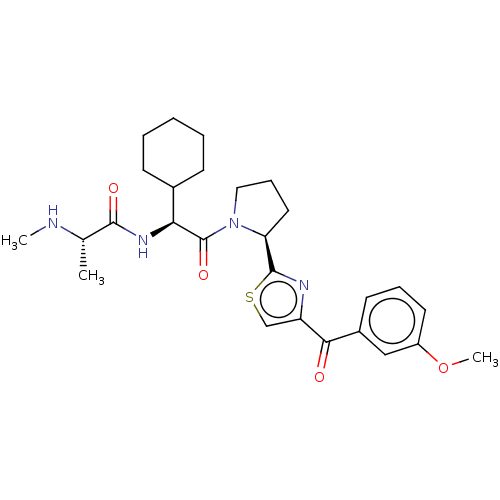
(CHEMBL4164385)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1c1nc(cs1)C(=O)c1cccc(OC)c1 |r| Show InChI InChI=1S/C27H36N4O4S/c1-17(28-2)25(33)30-23(18-9-5-4-6-10-18)27(34)31-14-8-13-22(31)26-29-21(16-36-26)24(32)19-11-7-12-20(15-19)35-3/h7,11-12,15-18,22-23,28H,4-6,8-10,13-14H2,1-3H3,(H,30,33)/t17-,22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells |
ACS Med Chem Lett 8: 1042-1047 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00247
BindingDB Entry DOI: 10.7270/Q2XG9TNP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50437046

(CHEMBL2403356)Show InChI InChI=1S/C21H28O2/c1-5-11-21(12-6-2,17-7-9-19(22)15(3)13-17)18-8-10-20(23)16(4)14-18/h7-10,13-14,22-23H,5-6,11-12H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Antagonist activity at ERalpha (unknown origin) expressed in human MCF7 cells assessed as inhibition of E2-induced response by dual luciferase report... |
Bioorg Med Chem 26: 1638-1642 (2018)
Article DOI: 10.1016/j.bmc.2018.02.010
BindingDB Entry DOI: 10.7270/Q24170Q8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608
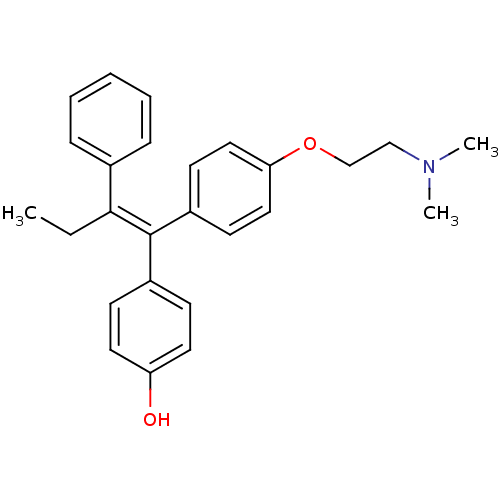
(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha (unknown origin) by fluorescence polarization-based competition binding assay |
Bioorg Med Chem 23: 3091-6 (2015)
Article DOI: 10.1016/j.bmc.2015.05.002
BindingDB Entry DOI: 10.7270/Q28P6277 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50279272

(CHEMBL4164385)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1c1nc(cs1)C(=O)c1cccc(OC)c1 |r| Show InChI InChI=1S/C27H36N4O4S/c1-17(28-2)25(33)30-23(18-9-5-4-6-10-18)27(34)31-14-8-13-22(31)26-29-21(16-36-26)24(32)19-11-7-12-20(15-19)35-3/h7,11-12,15-18,22-23,28H,4-6,8-10,13-14H2,1-3H3,(H,30,33)/t17-,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against SO561945 (HIV 1 mutant RT) viral viral infection of MT-4 cells |
ACS Med Chem Lett 8: 1042-1047 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00247
BindingDB Entry DOI: 10.7270/Q2XG9TNP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50171495

(CHEMBL3805318)Show SMILES CCCCCCCCCCCCNCCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C35H43NO4S/c1-2-3-4-5-6-7-8-9-10-11-22-36-23-24-40-30-19-14-26(15-20-30)34(39)33-31-21-18-29(38)25-32(31)41-35(33)27-12-16-28(37)17-13-27/h12-21,25,36-38H,2-11,22-24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay |
Bioorg Med Chem 24: 2914-2919 (2016)
Article DOI: 10.1016/j.bmc.2016.04.068
BindingDB Entry DOI: 10.7270/Q21V5GWW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50437046

(CHEMBL2403356)Show InChI InChI=1S/C21H28O2/c1-5-11-21(12-6-2,17-7-9-19(22)15(3)13-17)18-8-10-20(23)16(4)14-18/h7-10,13-14,22-23H,5-6,11-12H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nagasaki University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone ES2 from full length recombinant human untagged ERalpha expressed in Sf insect cells by fluorescence polarization assay |
Bioorg Med Chem 26: 1638-1642 (2018)
Article DOI: 10.1016/j.bmc.2018.02.010
BindingDB Entry DOI: 10.7270/Q24170Q8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50171498
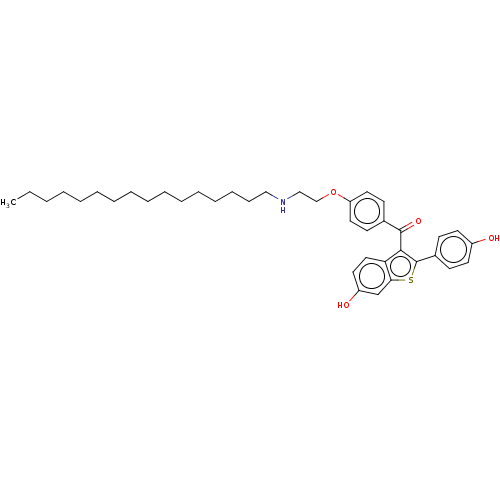
(CHEMBL3805025)Show SMILES CCCCCCCCCCCCCCCCNCCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C39H51NO4S/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-26-40-27-28-44-34-23-18-30(19-24-34)38(43)37-35-25-22-33(42)29-36(35)45-39(37)31-16-20-32(41)21-17-31/h16-25,29,40-42H,2-15,26-28H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay |
Bioorg Med Chem 24: 2914-2919 (2016)
Article DOI: 10.1016/j.bmc.2016.04.068
BindingDB Entry DOI: 10.7270/Q21V5GWW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50092522
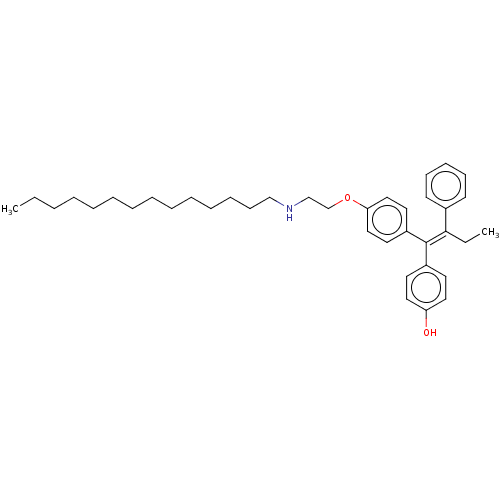
(CHEMBL3586192)Show SMILES CCCCCCCCCCCCCCNCCOc1ccc(cc1)C(=C(\CC)c1ccccc1)\c1ccc(O)cc1 Show InChI InChI=1S/C38H53NO2/c1-3-5-6-7-8-9-10-11-12-13-14-18-29-39-30-31-41-36-27-23-34(24-28-36)38(33-21-25-35(40)26-22-33)37(4-2)32-19-16-15-17-20-32/h15-17,19-28,39-40H,3-14,18,29-31H2,1-2H3/b38-37- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha (unknown origin) by fluorescence polarization-based competition binding assay |
Bioorg Med Chem 23: 3091-6 (2015)
Article DOI: 10.1016/j.bmc.2015.05.002
BindingDB Entry DOI: 10.7270/Q28P6277 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50104834
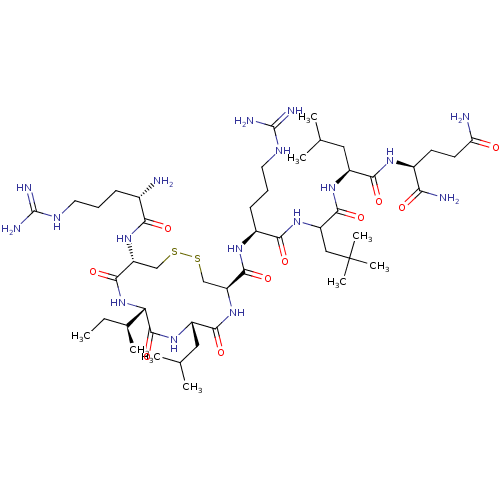
(CHEMBL3597499)Show SMILES [H][C@]1(NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N)[C@@H](C)CC |r| Show InChI InChI=1S/C48H89N17O10S2/c1-10-26(6)36-45(75)61-31(20-25(4)5)41(71)64-33(22-76-77-23-34(44(74)65-36)63-38(68)27(49)13-11-17-56-46(52)53)43(73)59-29(14-12-18-57-47(54)55)39(69)62-32(21-48(7,8)9)42(72)60-30(19-24(2)3)40(70)58-28(37(51)67)15-16-35(50)66/h24-34,36H,10-23,49H2,1-9H3,(H2,50,66)(H2,51,67)(H,58,70)(H,59,73)(H,60,72)(H,61,75)(H,62,69)(H,63,68)(H,64,71)(H,65,74)(H4,52,53,56)(H4,54,55,57)/t26-,27-,28-,29-,30-,31-,32?,33-,34+,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method |
Bioorg Med Chem 23: 4132-8 (2015)
Article DOI: 10.1016/j.bmc.2015.06.067
BindingDB Entry DOI: 10.7270/Q2V989T0 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50092501
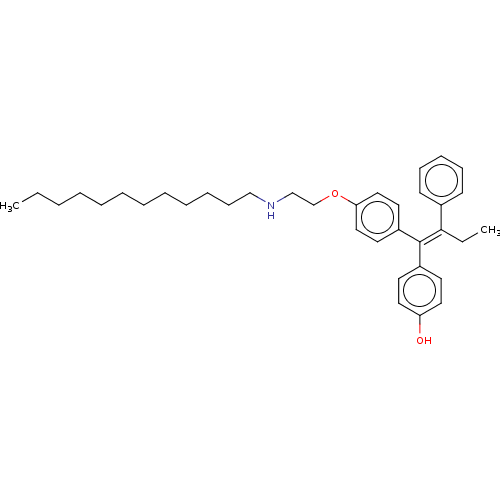
(CHEMBL3098274)Show SMILES CCCCCCCCCCCCNCCOc1ccc(cc1)C(=C(\CC)c1ccccc1)\c1ccc(O)cc1 Show InChI InChI=1S/C36H49NO2/c1-3-5-6-7-8-9-10-11-12-16-27-37-28-29-39-34-25-21-32(22-26-34)36(31-19-23-33(38)24-20-31)35(4-2)30-17-14-13-15-18-30/h13-15,17-26,37-38H,3-12,16,27-29H2,1-2H3/b36-35- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha (unknown origin) by fluorescence polarization-based competition binding assay |
Bioorg Med Chem 23: 3091-6 (2015)
Article DOI: 10.1016/j.bmc.2015.05.002
BindingDB Entry DOI: 10.7270/Q28P6277 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50550011
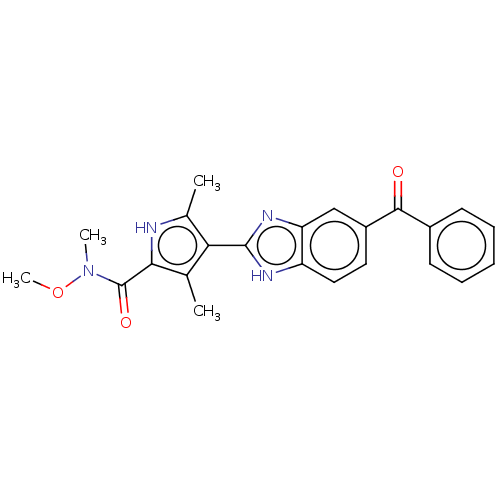
(CHEMBL4747168)Show SMILES Cl.CON(C)C(=O)c1[nH]c(C)c(c1C)-c1nc2cc(ccc2[nH]1)C(=O)c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HPGDS |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50459873
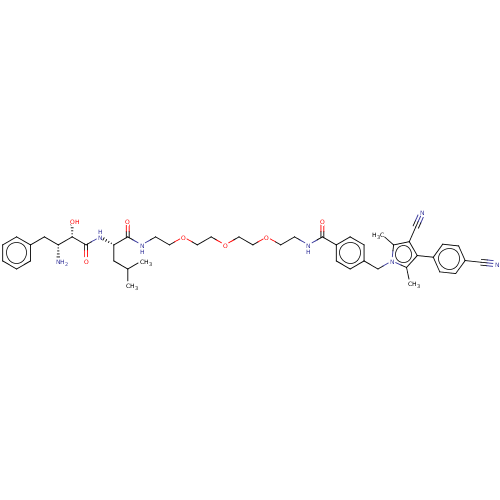
(CHEMBL4225186)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(=O)NCCOCCOCCOCCNC(=O)c1ccc(Cn2c(C)c(C#N)c(c2C)-c2ccc(cc2)C#N)cc1 |r| Show InChI InChI=1S/C46H57N7O7/c1-31(2)26-41(52-46(57)43(54)40(49)27-34-8-6-5-7-9-34)45(56)51-19-21-59-23-25-60-24-22-58-20-18-50-44(55)38-16-12-36(13-17-38)30-53-32(3)39(29-48)42(33(53)4)37-14-10-35(28-47)11-15-37/h5-17,31,40-41,43,54H,18-27,30,49H2,1-4H3,(H,50,55)(H,51,56)(H,52,57)/t40-,41+,43+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-H-3] mibolerone from wild-type androgen receptor (unknown origin) expressed in human Freestyle293F cells measured af... |
J Med Chem 61: 543-575 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00168
BindingDB Entry DOI: 10.7270/Q2VT1VR7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50171497
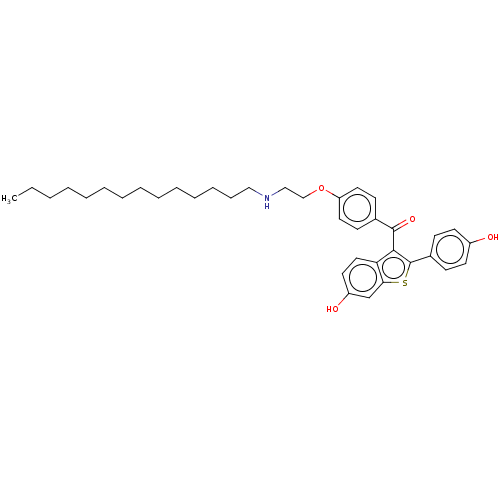
(CHEMBL3804907)Show SMILES CCCCCCCCCCCCCCNCCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C37H47NO4S/c1-2-3-4-5-6-7-8-9-10-11-12-13-24-38-25-26-42-32-21-16-28(17-22-32)36(41)35-33-23-20-31(40)27-34(33)43-37(35)29-14-18-30(39)19-15-29/h14-23,27,38-40H,2-13,24-26H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay |
Bioorg Med Chem 24: 2914-2919 (2016)
Article DOI: 10.1016/j.bmc.2016.04.068
BindingDB Entry DOI: 10.7270/Q21V5GWW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50279265
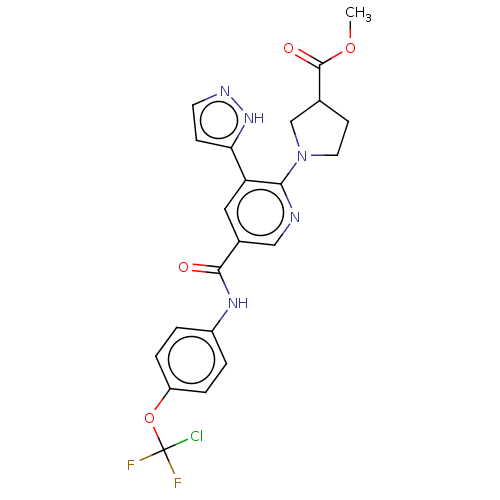
(CHEMBL4162041)Show SMILES COC(=O)C1CCN(C1)c1ncc(cc1-c1ccn[nH]1)C(=O)Nc1ccc(OC(F)(F)Cl)cc1 Show InChI InChI=1S/C22H20ClF2N5O4/c1-33-21(32)13-7-9-30(12-13)19-17(18-6-8-27-29-18)10-14(11-26-19)20(31)28-15-2-4-16(5-3-15)34-22(23,24)25/h2-6,8,10-11,13H,7,9,12H2,1H3,(H,27,29)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged full length ABL1 allosteric site expressed in baculovirus expression system by TR-FRET assay |
ACS Med Chem Lett 8: 1042-1047 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00247
BindingDB Entry DOI: 10.7270/Q2XG9TNP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50104835
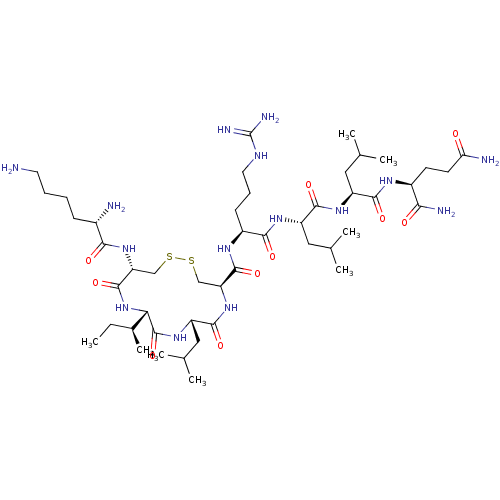
(CHEMBL3597498)Show SMILES [H][C@]1(NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCCN)[C@@H](C)CC |r| Show InChI InChI=1S/C47H87N15O10S2/c1-9-27(8)37-46(72)59-33(21-26(6)7)43(69)61-34(22-73-74-23-35(45(71)62-37)60-39(65)28(49)13-10-11-17-48)44(70)56-30(14-12-18-54-47(52)53)40(66)57-32(20-25(4)5)42(68)58-31(19-24(2)3)41(67)55-29(38(51)64)15-16-36(50)63/h24-35,37H,9-23,48-49H2,1-8H3,(H2,50,63)(H2,51,64)(H,55,67)(H,56,70)(H,57,66)(H,58,68)(H,59,72)(H,60,65)(H,61,69)(H,62,71)(H4,52,53,54)/t27-,28-,29-,30-,31-,32-,33-,34-,35+,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method |
Bioorg Med Chem 23: 4132-8 (2015)
Article DOI: 10.1016/j.bmc.2015.06.067
BindingDB Entry DOI: 10.7270/Q2V989T0 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50171494

(CHEMBL3806167)Show SMILES CCCCCCCCCCNCCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C33H39NO4S/c1-2-3-4-5-6-7-8-9-20-34-21-22-38-28-17-12-24(13-18-28)32(37)31-29-19-16-27(36)23-30(29)39-33(31)25-10-14-26(35)15-11-25/h10-19,23,34-36H,2-9,20-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay |
Bioorg Med Chem 24: 2914-2919 (2016)
Article DOI: 10.1016/j.bmc.2016.04.068
BindingDB Entry DOI: 10.7270/Q21V5GWW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50171493
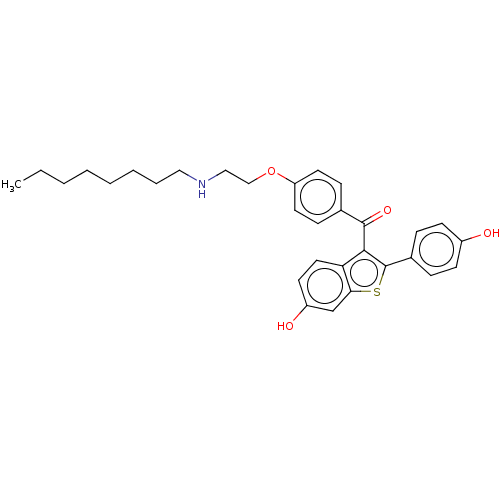
(CHEMBL3805170)Show SMILES CCCCCCCCNCCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C31H35NO4S/c1-2-3-4-5-6-7-18-32-19-20-36-26-15-10-22(11-16-26)30(35)29-27-17-14-25(34)21-28(27)37-31(29)23-8-12-24(33)13-9-23/h8-17,21,32-34H,2-7,18-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay |
Bioorg Med Chem 24: 2914-2919 (2016)
Article DOI: 10.1016/j.bmc.2016.04.068
BindingDB Entry DOI: 10.7270/Q21V5GWW |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50092483
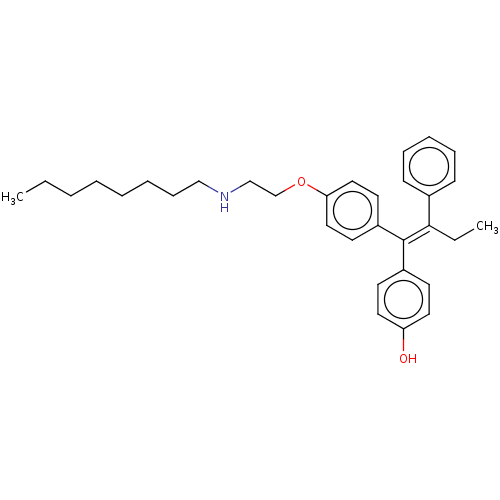
(CHEMBL3586190)Show SMILES CCCCCCCCNCCOc1ccc(cc1)C(=C(\CC)c1ccccc1)\c1ccc(O)cc1 Show InChI InChI=1S/C32H41NO2/c1-3-5-6-7-8-12-23-33-24-25-35-30-21-17-28(18-22-30)32(27-15-19-29(34)20-16-27)31(4-2)26-13-10-9-11-14-26/h9-11,13-22,33-34H,3-8,12,23-25H2,1-2H3/b32-31- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha (unknown origin) by fluorescence polarization-based competition binding assay |
Bioorg Med Chem 23: 3091-6 (2015)
Article DOI: 10.1016/j.bmc.2015.05.002
BindingDB Entry DOI: 10.7270/Q28P6277 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50459875
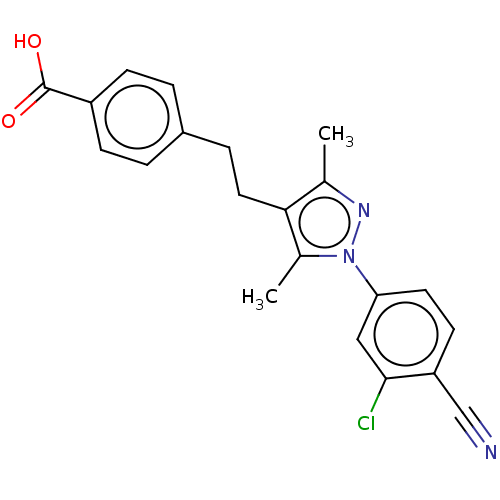
(CHEMBL4226844)Show SMILES Cc1nn(c(C)c1CCc1ccc(cc1)C(O)=O)-c1ccc(C#N)c(Cl)c1 Show InChI InChI=1S/C21H18ClN3O2/c1-13-19(10-5-15-3-6-16(7-4-15)21(26)27)14(2)25(24-13)18-9-8-17(12-23)20(22)11-18/h3-4,6-9,11H,5,10H2,1-2H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to androgen receptor (unknown origin) |
J Med Chem 61: 543-575 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00168
BindingDB Entry DOI: 10.7270/Q2VT1VR7 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50459868
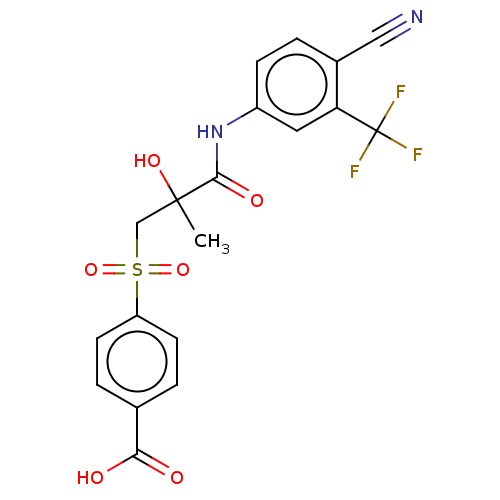
(CHEMBL4225339)Show SMILES CC(O)(CS(=O)(=O)c1ccc(cc1)C(O)=O)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C19H15F3N2O6S/c1-18(28,10-31(29,30)14-6-3-11(4-7-14)16(25)26)17(27)24-13-5-2-12(9-23)15(8-13)19(20,21)22/h2-8,28H,10H2,1H3,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to androgen receptor (unknown origin) |
J Med Chem 61: 543-575 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00168
BindingDB Entry DOI: 10.7270/Q2VT1VR7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50171492
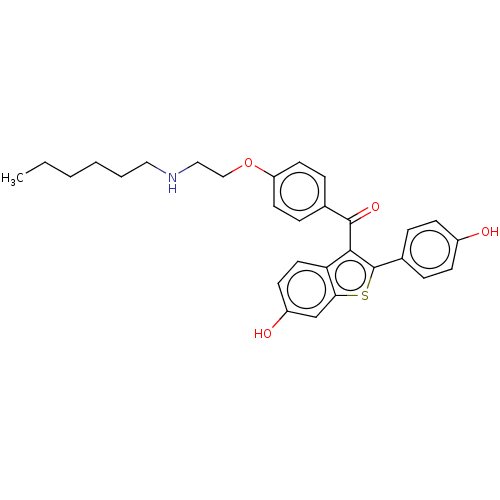
(CHEMBL3805921)Show SMILES CCCCCCNCCOc1ccc(cc1)C(=O)c1c(sc2cc(O)ccc12)-c1ccc(O)cc1 Show InChI InChI=1S/C29H31NO4S/c1-2-3-4-5-16-30-17-18-34-24-13-8-20(9-14-24)28(33)27-25-15-12-23(32)19-26(25)35-29(27)21-6-10-22(31)11-7-21/h6-15,19,30-32H,2-5,16-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of Fluormone ES2 from human recombinant full length untagged-ERalpha by fluorescence polarization competition binding assay |
Bioorg Med Chem 24: 2914-2919 (2016)
Article DOI: 10.1016/j.bmc.2016.04.068
BindingDB Entry DOI: 10.7270/Q21V5GWW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50279272

(CHEMBL4164385)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1c1nc(cs1)C(=O)c1cccc(OC)c1 |r| Show InChI InChI=1S/C27H36N4O4S/c1-17(28-2)25(33)30-23(18-9-5-4-6-10-18)27(34)31-14-8-13-22(31)26-29-21(16-36-26)24(32)19-11-7-12-20(15-19)35-3/h7,11-12,15-18,22-23,28H,4-6,8-10,13-14H2,1-3H3,(H,30,33)/t17-,22-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged XIAP (Asn252 to Thr356 residues) expressed in Escherichia coli by TR-FRET assay |
ACS Med Chem Lett 8: 1042-1047 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00247
BindingDB Entry DOI: 10.7270/Q2XG9TNP |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463722
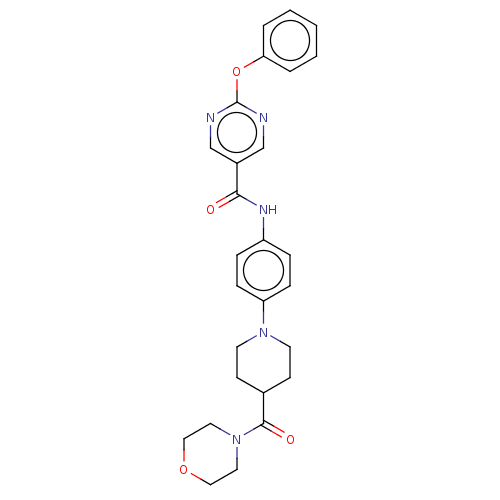
(CHEMBL4238186)Show SMILES O=C(Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCOCC1)c1cnc(Oc2ccccc2)nc1 Show InChI InChI=1S/C27H29N5O4/c33-25(21-18-28-27(29-19-21)36-24-4-2-1-3-5-24)30-22-6-8-23(9-7-22)31-12-10-20(11-13-31)26(34)32-14-16-35-17-15-32/h1-9,18-20H,10-17H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HPGDS expressed in Escherichia coli BL21 (DE3) measured after 3 mins by spectrophotometric analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50591607

(CHEMBL5193611)Show SMILES Cn1cccc1C(=O)N1CCN(CC1)C(=O)Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCN(CC1)c1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00284a
BindingDB Entry DOI: 10.7270/Q2V4106T |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463722

(CHEMBL4238186)Show SMILES O=C(Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCOCC1)c1cnc(Oc2ccccc2)nc1 Show InChI InChI=1S/C27H29N5O4/c33-25(21-18-28-27(29-19-21)36-24-4-2-1-3-5-24)30-22-6-8-23(9-7-22)31-12-10-20(11-13-31)26(34)32-14-16-35-17-15-32/h1-9,18-20H,10-17H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HPGDS using [14C]-PGH2 as substrate preincubated for 1 min in presence of MgCl2 followed by substrate addition and me... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00605
BindingDB Entry DOI: 10.7270/Q2348Q13 |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 2
(Homo sapiens (Human)) | BDBM50279273
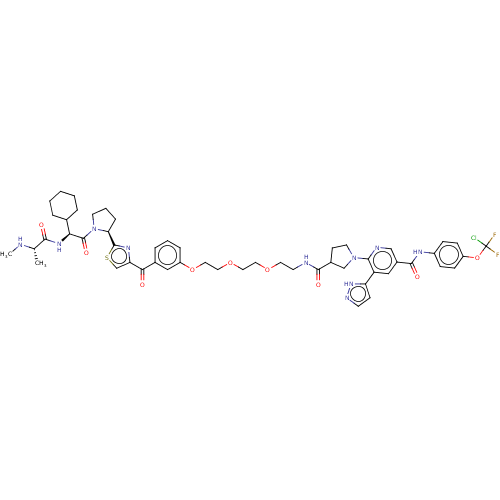
(CHEMBL4160980)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1c1nc(cs1)C(=O)c1cccc(OCCOCCOCCNC(=O)C2CCN(C2)c2ncc(cc2-c2ccn[nH]2)C(=O)Nc2ccc(OC(F)(F)Cl)cc2)c1 |r| Show InChI InChI=1S/C53H63ClF2N10O9S/c1-33(57-2)48(68)63-45(34-8-4-3-5-9-34)52(71)66-21-7-12-44(66)51-62-43(32-76-51)46(67)35-10-6-11-40(28-35)74-27-26-73-25-24-72-23-20-58-49(69)36-18-22-65(31-36)47-41(42-17-19-60-64-42)29-37(30-59-47)50(70)61-38-13-15-39(16-14-38)75-53(54,55)56/h6,10-11,13-17,19,28-30,32-34,36,44-45,57H,3-5,7-9,12,18,20-27,31H2,1-2H3,(H,58,69)(H,60,64)(H,61,70)(H,63,68)/t33-,36?,44-,45-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells |
ACS Med Chem Lett 8: 1042-1047 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00247
BindingDB Entry DOI: 10.7270/Q2XG9TNP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50104800

(CHEMBL3597509)Show SMILES [H][C@]1(NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)NCC(=O)NCC(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N)[C@@H](C)CC |r| Show InChI InChI=1S/C96H182N48O20S2/c1-10-50(6)70-86(164)140-62(41-49(4)5)82(160)143-64(46-165-166-47-65(85(163)144-70)142-72(150)51(97)20-11-31-118-87(100)101)84(162)137-59(28-19-39-126-95(116)117)80(158)141-63(42-96(7,8)9)83(161)139-61(40-48(2)3)81(159)138-60(29-30-66(98)145)73(151)129-44-68(147)127-43-67(146)128-45-69(148)130-53(22-13-33-120-89(104)105)74(152)132-55(24-15-35-122-91(108)109)76(154)134-57(26-17-37-124-93(112)113)78(156)136-58(27-18-38-125-94(114)115)79(157)135-56(25-16-36-123-92(110)111)77(155)133-54(23-14-34-121-90(106)107)75(153)131-52(71(99)149)21-12-32-119-88(102)103/h48-65,70H,10-47,97H2,1-9H3,(H2,98,145)(H2,99,149)(H,127,147)(H,128,146)(H,129,151)(H,130,148)(H,131,153)(H,132,152)(H,133,155)(H,134,154)(H,135,157)(H,136,156)(H,137,162)(H,138,159)(H,139,161)(H,140,164)(H,141,158)(H,142,150)(H,143,160)(H,144,163)(H4,100,101,118)(H4,102,103,119)(H4,104,105,120)(H4,106,107,121)(H4,108,109,122)(H4,110,111,123)(H4,112,113,124)(H4,114,115,125)(H4,116,117,126)/t50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,63?,64-,65+,70-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method |
Bioorg Med Chem 23: 4132-8 (2015)
Article DOI: 10.1016/j.bmc.2015.06.067
BindingDB Entry DOI: 10.7270/Q2V989T0 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50279274
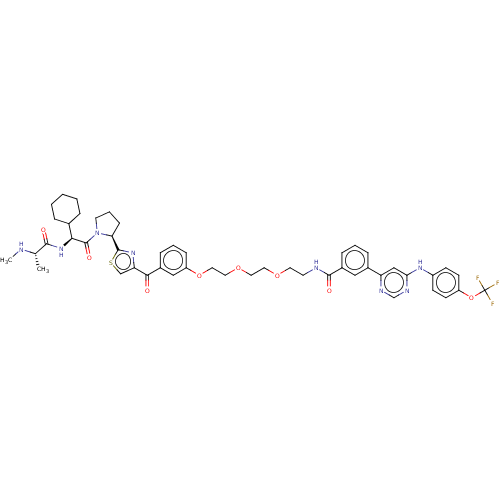
(CHEMBL4172268)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1c1nc(cs1)C(=O)c1cccc(OCCOCCOCCNC(=O)c2cccc(c2)-c2cc(Nc3ccc(OC(F)(F)F)cc3)ncn2)c1 |r| Show InChI InChI=1S/C50H57F3N8O8S/c1-32(54-2)46(63)60-44(33-9-4-3-5-10-33)49(65)61-21-8-15-42(61)48-59-41(30-70-48)45(62)35-12-7-14-39(28-35)68-26-25-67-24-23-66-22-20-55-47(64)36-13-6-11-34(27-36)40-29-43(57-31-56-40)58-37-16-18-38(19-17-37)69-50(51,52)53/h6-7,11-14,16-19,27-33,42,44,54H,3-5,8-10,15,20-26H2,1-2H3,(H,55,64)(H,60,63)(H,56,57,58)/t32-,42-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Concentration required to protect the cell against HIV-1 strain IIIB viral cytopathogenicity by 50% in MT-4 cells |
ACS Med Chem Lett 8: 1042-1047 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00247
BindingDB Entry DOI: 10.7270/Q2XG9TNP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50104804
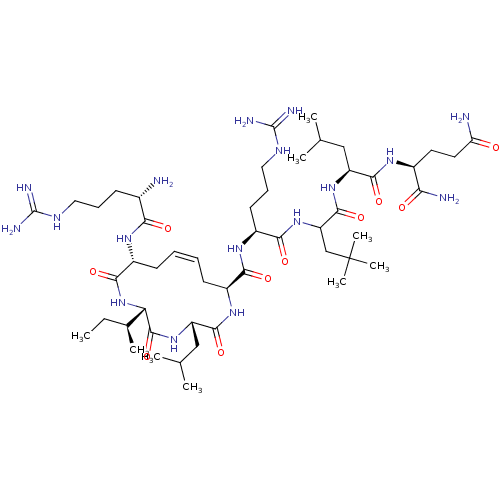
(CHEMBL3597505)Show SMILES [H][C@]1(NC(=O)[C@@H](C\C=C/C[C@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC(CC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N)[C@@H](C)CC |r,c:7| Show InChI InChI=1S/C50H91N17O10/c1-10-28(6)38-47(77)65-35(24-27(4)5)45(75)63-31(16-11-12-17-32(43(73)67-38)61-40(70)29(51)15-13-21-58-48(54)55)41(71)62-33(18-14-22-59-49(56)57)42(72)66-36(25-50(7,8)9)46(76)64-34(23-26(2)3)44(74)60-30(39(53)69)19-20-37(52)68/h11-12,26-36,38H,10,13-25,51H2,1-9H3,(H2,52,68)(H2,53,69)(H,60,74)(H,61,70)(H,62,71)(H,63,75)(H,64,76)(H,65,77)(H,66,72)(H,67,73)(H4,54,55,58)(H4,56,57,59)/b12-11-/t28-,29-,30-,31-,32+,33-,34-,35-,36?,38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor alpha (unknown origin)-SRC1 coactivator interaction incubated for 1 hr by receptor cofactor assay system based method |
Bioorg Med Chem 23: 4132-8 (2015)
Article DOI: 10.1016/j.bmc.2015.06.067
BindingDB Entry DOI: 10.7270/Q2V989T0 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50587270
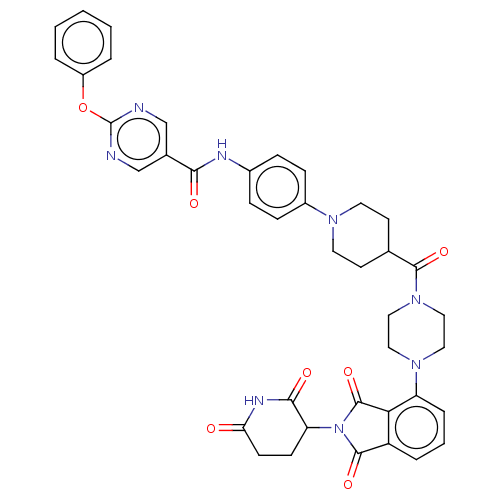
(CHEMBL5076100)Show SMILES O=C(Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCN(CC1)c1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)c1cnc(Oc2ccccc2)nc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00284a
BindingDB Entry DOI: 10.7270/Q2V4106T |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase XIAP
(Homo sapiens (Human)) | BDBM50279273

(CHEMBL4160980)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1c1nc(cs1)C(=O)c1cccc(OCCOCCOCCNC(=O)C2CCN(C2)c2ncc(cc2-c2ccn[nH]2)C(=O)Nc2ccc(OC(F)(F)Cl)cc2)c1 |r| Show InChI InChI=1S/C53H63ClF2N10O9S/c1-33(57-2)48(68)63-45(34-8-4-3-5-9-34)52(71)66-21-7-12-44(66)51-62-43(32-76-51)46(67)35-10-6-11-40(28-35)74-27-26-73-25-24-72-23-20-58-49(69)36-18-22-65(31-36)47-41(42-17-19-60-64-42)29-37(30-59-47)50(70)61-38-13-15-39(16-14-38)75-53(54,55)56/h6,10-11,13-17,19,28-30,32-34,36,44-45,57H,3-5,7-9,12,18,20-27,31H2,1-2H3,(H,58,69)(H,60,64)(H,61,70)(H,63,68)/t33-,36?,44-,45-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged XIAP (Asn252 to Thr356 residues) expressed in Escherichia coli by TR-FRET assay |
ACS Med Chem Lett 8: 1042-1047 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00247
BindingDB Entry DOI: 10.7270/Q2XG9TNP |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50591608
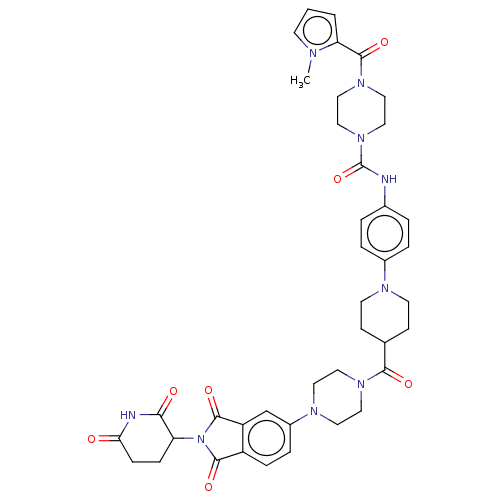
(CHEMBL5207946)Show SMILES Cn1cccc1C(=O)N1CCN(CC1)C(=O)Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCN(CC1)c1ccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00284a
BindingDB Entry DOI: 10.7270/Q2V4106T |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM136516
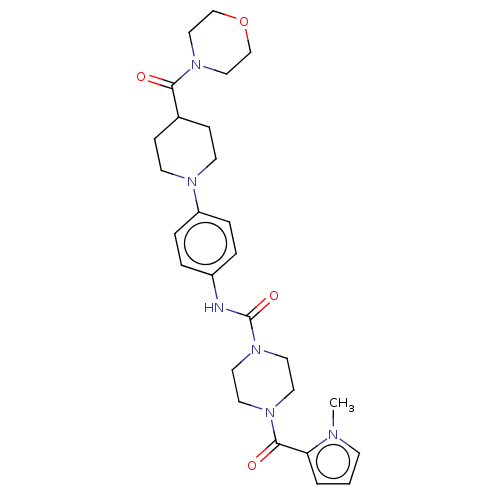
(US8865714, 3)Show SMILES Cn1cccc1C(=O)N1CCN(CC1)C(=O)Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCOCC1 Show InChI InChI=1S/C27H36N6O4/c1-29-10-2-3-24(29)26(35)31-13-15-33(16-14-31)27(36)28-22-4-6-23(7-5-22)30-11-8-21(9-12-30)25(34)32-17-19-37-20-18-32/h2-7,10,21H,8-9,11-20H2,1H3,(H,28,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00284a
BindingDB Entry DOI: 10.7270/Q2V4106T |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50587271

(CHEMBL5075403)Show SMILES CN1C(=O)CCC(N2C(=O)c3cccc(N4CCN(CC4)C(=O)C4CCN(CC4)c4ccc(NC(=O)c5cnc(Oc6ccccc6)nc5)cc4)c3C2=O)C1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of H-PGDS (unknown origin) using fluorescent probe by competition binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01206
BindingDB Entry DOI: 10.7270/Q2T157KT |
More data for this
Ligand-Target Pair | |
Baculoviral IAP repeat-containing protein 3
(Homo sapiens (Human)) | BDBM50279273

(CHEMBL4160980)Show SMILES CN[C@@H](C)C(=O)N[C@@H](C1CCCCC1)C(=O)N1CCC[C@H]1c1nc(cs1)C(=O)c1cccc(OCCOCCOCCNC(=O)C2CCN(C2)c2ncc(cc2-c2ccn[nH]2)C(=O)Nc2ccc(OC(F)(F)Cl)cc2)c1 |r| Show InChI InChI=1S/C53H63ClF2N10O9S/c1-33(57-2)48(68)63-45(34-8-4-3-5-9-34)52(71)66-21-7-12-44(66)51-62-43(32-76-51)46(67)35-10-6-11-40(28-35)74-27-26-73-25-24-72-23-20-58-49(69)36-18-22-65(31-36)47-41(42-17-19-60-64-42)29-37(30-59-47)50(70)61-38-13-15-39(16-14-38)75-53(54,55)56/h6,10-11,13-17,19,28-30,32-34,36,44-45,57H,3-5,7-9,12,18,20-27,31H2,1-2H3,(H,58,69)(H,60,64)(H,61,70)(H,63,68)/t33-,36?,44-,45-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged cIAP2 (Gln238 to Ser349 residues) expressed in Escherichia coli by TR-FRET assay |
ACS Med Chem Lett 8: 1042-1047 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00247
BindingDB Entry DOI: 10.7270/Q2XG9TNP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50092482
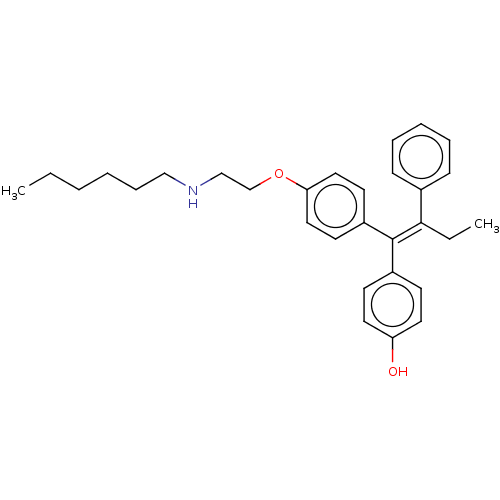
(CHEMBL3098273)Show SMILES CCCCCCNCCOc1ccc(cc1)C(=C(\CC)c1ccccc1)\c1ccc(O)cc1 Show InChI InChI=1S/C30H37NO2/c1-3-5-6-10-21-31-22-23-33-28-19-15-26(16-20-28)30(25-13-17-27(32)18-14-25)29(4-2)24-11-8-7-9-12-24/h7-9,11-20,31-32H,3-6,10,21-23H2,1-2H3/b30-29- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Inhibition of ERalpha (unknown origin) by fluorescence polarization-based competition binding assay |
Bioorg Med Chem 23: 3091-6 (2015)
Article DOI: 10.1016/j.bmc.2015.05.002
BindingDB Entry DOI: 10.7270/Q28P6277 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50587270

(CHEMBL5076100)Show SMILES O=C(Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCN(CC1)c1cccc2C(=O)N(C3CCC(=O)NC3=O)C(=O)c12)c1cnc(Oc2ccccc2)nc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of H-PGDS (unknown origin) using fluorescent probe by competition binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01206
BindingDB Entry DOI: 10.7270/Q2T157KT |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50463722

(CHEMBL4238186)Show SMILES O=C(Nc1ccc(cc1)N1CCC(CC1)C(=O)N1CCOCC1)c1cnc(Oc2ccccc2)nc1 Show InChI InChI=1S/C27H29N5O4/c33-25(21-18-28-27(29-19-21)36-24-4-2-1-3-5-24)30-22-6-8-23(9-7-22)31-12-10-20(11-13-31)26(34)32-14-16-35-17-15-32/h1-9,18-20H,10-17H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of H-PGDS (unknown origin) using fluorescent probe by competition binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01206
BindingDB Entry DOI: 10.7270/Q2T157KT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50459866
(CHEMBL4226899)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(=O)NCCOCCOCCOCCNC(=O)c1ccc(Cc2c(C)nn(c2C)-c2ccc(C#N)c(c2)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C45H56F3N7O7/c1-29(2)24-40(53-44(59)41(56)39(50)26-32-8-6-5-7-9-32)43(58)52-17-19-61-21-23-62-22-20-60-18-16-51-42(57)34-12-10-33(11-13-34)25-37-30(3)54-55(31(37)4)36-15-14-35(28-49)38(27-36)45(46,47)48/h5-15,27,29,39-41,56H,16-26,50H2,1-4H3,(H,51,57)(H,52,58)(H,53,59)/t39-,40+,41+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Health Sciences
Curated by ChEMBL
| Assay Description
Displacement of [17-alpha-methyl-H-3] mibolerone from wild-type androgen receptor (unknown origin) expressed in human Freestyle293F cells measured af... |
J Med Chem 61: 543-575 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00168
BindingDB Entry DOI: 10.7270/Q2VT1VR7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data