 Found 379 hits with Last Name = 'namba' and Initial = 'k'
Found 379 hits with Last Name = 'namba' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Antagonist activity at dopamine D1 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Antagonist activity at dopamine D5 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50004822
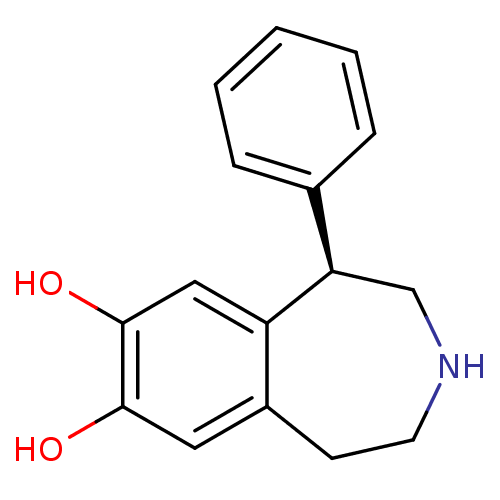
((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D5 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50487259
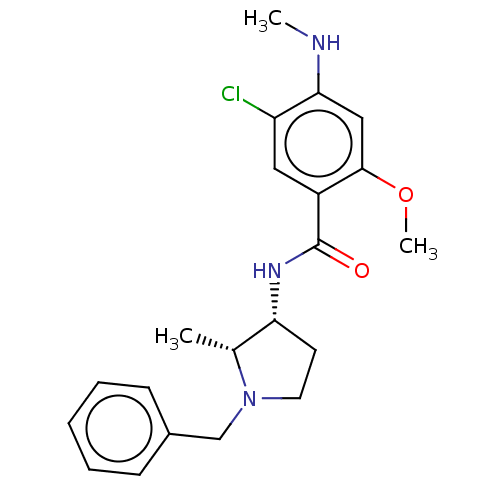
(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D4 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00365
BindingDB Entry DOI: 10.7270/Q2GX4GN3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WAY100635 from human 5-HT1A receptor expresssed in stable CHO cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50487259

(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-Methylspiperone from human dopamine D4 receptor expressed in stable HEK cells incubated for 90 mins by microbeta counting meth... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50487259

(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004822

((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...)Show InChI InChI=1S/C16H17NO2/c18-15-8-12-6-7-17-10-14(13(12)9-16(15)19)11-4-2-1-3-5-11/h1-5,8-9,14,17-19H,6-7,10H2/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D1 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50087033

((1R,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...)Show SMILES CO[C@]1(C)c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45 Show InChI InChI=1S/C20H21NO/c1-20(22-3)14-6-4-5-13-11-16-18-12(9-10-21(16)2)7-8-15(20)19(18)17(13)14/h4-8,16H,9-11H2,1-3H3/t16-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7 receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50570808
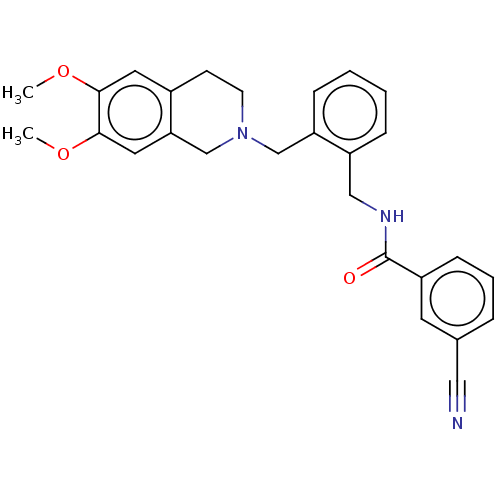
(CHEMBL4846574)Show SMILES COc1cc2CCN(Cc3ccccc3CNC(=O)c3cccc(c3)C#N)Cc2cc1OC | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50487259

(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-Methylspiperone from dopamine D3 receptor (unknown origin) incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50010301
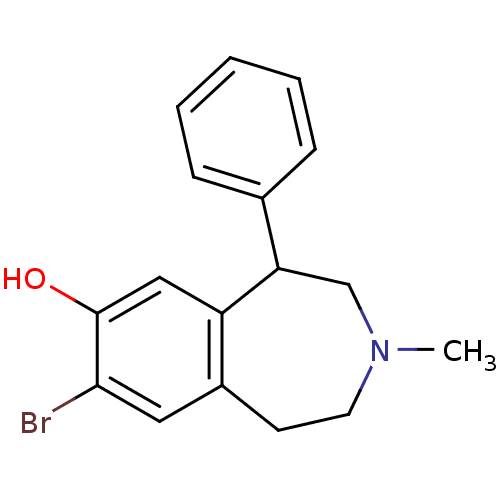
(8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C17H18BrNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]SCH23390 from dopamine D5 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to human D2 receptor |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to human D1 receptor |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50570806

(CHEMBL4876260)Show SMILES Oc1cc2CCN(Cc3ccccc3CNC(=O)c3cccc(Br)c3)Cc2cc1O | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50010301

(8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C17H18BrNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to human D5 receptor |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50487259

(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-Ketanserin from 5-HT2A receptor (unknown origin) incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A receptor |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50570809
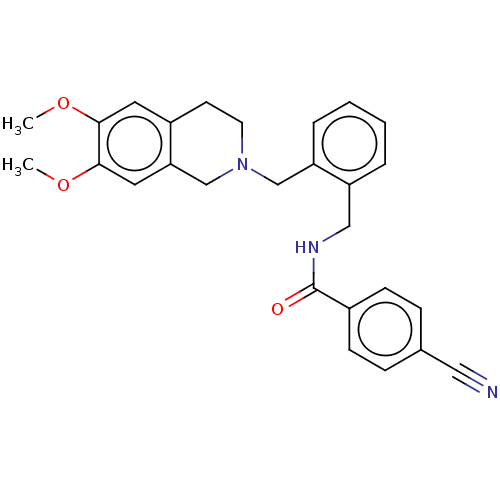
(CHEMBL4878587)Show SMILES COc1cc2CCN(Cc3ccccc3CNC(=O)c3ccc(cc3)C#N)Cc2cc1OC | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098549

((6aR,aR)-3-Methyl-2-(6-methyl-5,6,6a,7-tetrahydro-...)Show SMILES CN1CCc2cccc-3c2[C@H]1Cc1cccc(-c2c(C)cccc2C#N)c-31 |wD:10.11,(21.5,-8.8,;21.5,-7.27,;22.84,-6.51,;22.87,-4.98,;21.53,-4.21,;21.56,-2.65,;20.23,-1.86,;18.86,-2.62,;18.86,-4.18,;20.19,-4.98,;20.19,-6.51,;18.86,-7.27,;17.55,-6.51,;16.21,-7.27,;14.91,-6.51,;14.91,-4.98,;16.21,-4.18,;16.18,-2.49,;14.78,-1.86,;13.54,-2.72,;14.66,-.33,;15.93,.56,;17.3,-.11,;17.42,-1.63,;18.76,-.84,;20.07,-.07,;17.55,-4.95,)| Show InChI InChI=1S/C25H22N2/c1-16-6-3-9-19(15-26)23(16)20-10-5-8-18-14-22-25-17(12-13-27(22)2)7-4-11-21(25)24(18)20/h3-11,22H,12-14H2,1-2H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7 receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50599264
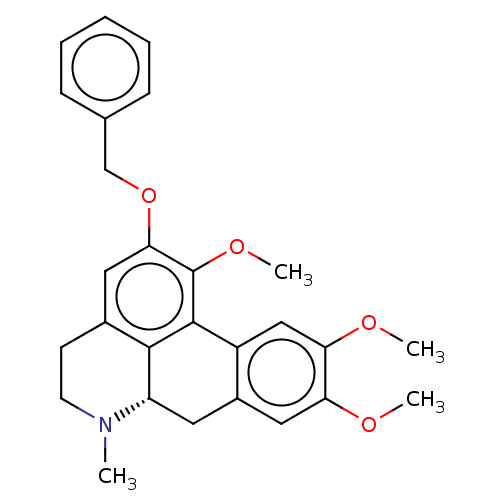
(CHEMBL5204813)Show SMILES [H][C@@]12Cc3cc(OC)c(OC)cc3-c3c(OC)c(OCc4ccccc4)cc(CCN1C)c23 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00365
BindingDB Entry DOI: 10.7270/Q2GX4GN3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6 receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50010301

(8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...)Show InChI InChI=1S/C17H18BrNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D5 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in HEKT cells incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50544133
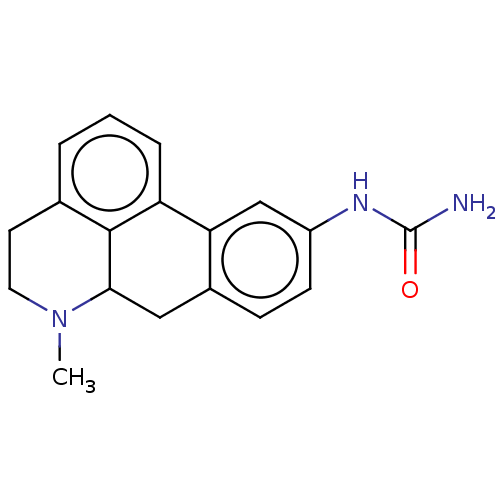
(CHEMBL4636148)Show InChI InChI=1S/C18H19N3O/c1-21-8-7-11-3-2-4-14-15-10-13(20-18(19)22)6-5-12(15)9-16(21)17(11)14/h2-6,10,16H,7-9H2,1H3,(H3,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7A receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-Methylspiperone from human dopamine D2 receptor expressed in stable fibroblast cells incubated for 90 mins by microbeta counti... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens) | BDBM50467926
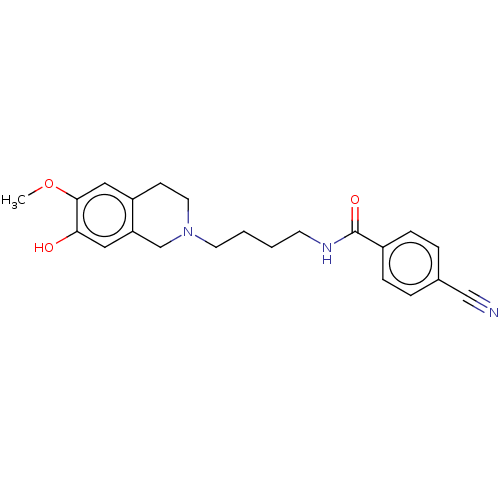
(CHEMBL4290759)Show InChI InChI=1S/C22H25N3O3/c1-28-21-13-18-8-11-25(15-19(18)12-20(21)26)10-3-2-9-24-22(27)17-6-4-16(14-23)5-7-17/h4-7,12-13,26H,2-3,8-11,15H2,1H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-methylspiperone from dopamine D3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50544139
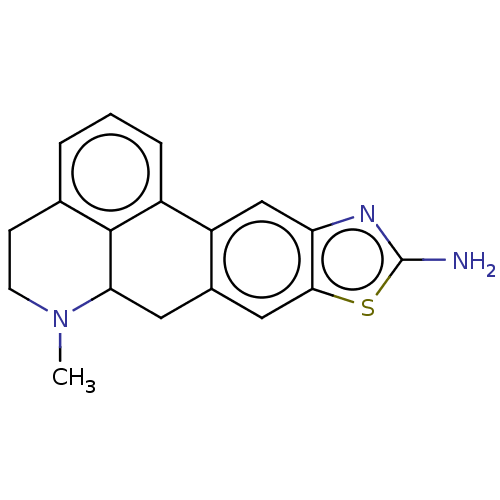
(CHEMBL4633786)Show InChI InChI=1S/C18H17N3S/c1-21-6-5-10-3-2-4-12-13-9-14-16(22-18(19)20-14)8-11(13)7-15(21)17(10)12/h2-4,8-9,15H,5-7H2,1H3,(H2,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7A receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50487259

(CHEBI:64219 | [3H]NEMONAPRIDE)Show SMILES CNc1cc(OC)c(cc1Cl)C(=O)N[C@@H]1CCN(Cc2ccccc2)[C@@H]1C |r| Show InChI InChI=1S/C21H26ClN3O2/c1-14-18(9-10-25(14)13-15-7-5-4-6-8-15)24-21(26)16-11-17(22)19(23-2)12-20(16)27-3/h4-8,11-12,14,18,23H,9-10,13H2,1-3H3,(H,24,26)/t14-,18-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DTG from sigma2 receptor(unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50544140
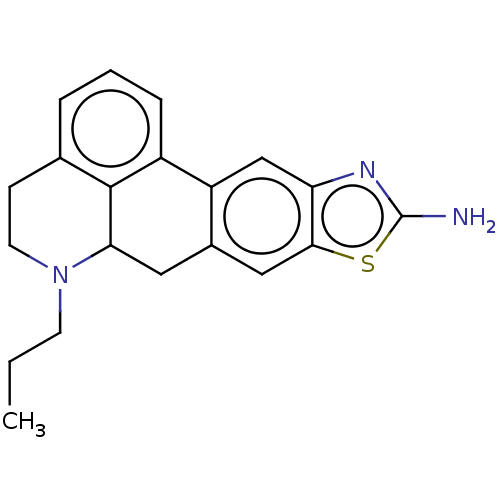
(CHEMBL4633042)Show InChI InChI=1S/C20H21N3S/c1-2-7-23-8-6-12-4-3-5-14-15-11-16-18(24-20(21)22-16)10-13(15)9-17(23)19(12)14/h3-5,10-11,17H,2,6-9H2,1H3,(H2,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WAY100635 from human 5-HT1A receptor expresssed in stable CHO cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50544137
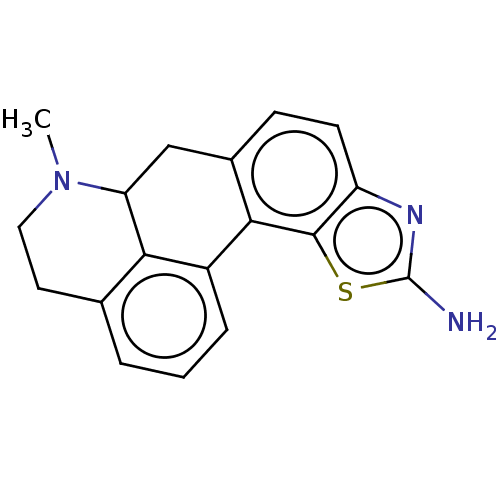
(CHEMBL4641502)Show InChI InChI=1S/C18H17N3S/c1-21-8-7-10-3-2-4-12-15(10)14(21)9-11-5-6-13-17(16(11)12)22-18(19)20-13/h2-6,14H,7-9H2,1H3,(H2,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7A receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50089979
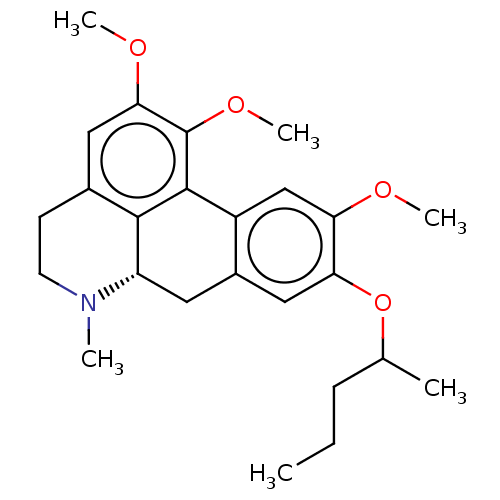
(CHEMBL3581250)Show SMILES [H][C@@]12Cc3cc(OC(C)CCC)c(OC)cc3-c3c(OC)c(OC)cc(CCN1C)c23 |r| Show InChI InChI=1S/C25H33NO4/c1-7-8-15(2)30-21-13-17-11-19-23-16(9-10-26(19)3)12-22(28-5)25(29-6)24(23)18(17)14-20(21)27-4/h12-15,19H,7-11H2,1-6H3/t15?,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00365
BindingDB Entry DOI: 10.7270/Q2GX4GN3 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50137618
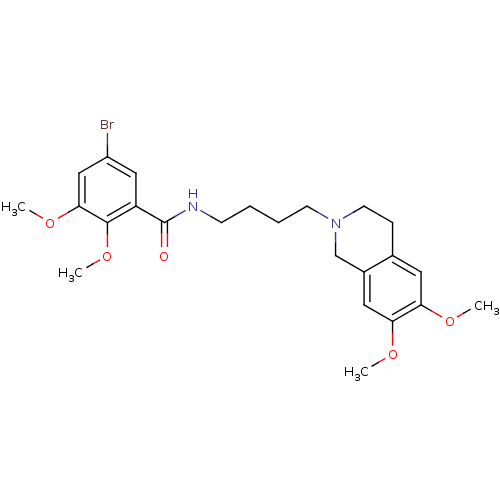
(5-Bromo-N-[4-(6,7-dimethoxy-3,4-dihydro-1H-isoquin...)Show SMILES COc1cc2CCN(CCCCNC(=O)c3cc(Br)cc(OC)c3OC)Cc2cc1OC Show InChI InChI=1S/C24H31BrN2O5/c1-29-20-11-16-7-10-27(15-17(16)12-21(20)30-2)9-6-5-8-26-24(28)19-13-18(25)14-22(31-3)23(19)32-4/h11-14H,5-10,15H2,1-4H3,(H,26,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DTG from sigma2 receptor(unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128047
BindingDB Entry DOI: 10.7270/Q2CJ8J8S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7A receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50544077

(CHEMBL4643069)Show InChI InChI=1S/C18H20ClNO2/c1-20-8-7-12-9-18(22-2)17(21)10-14(12)15(11-20)13-5-3-4-6-16(13)19/h3-6,9-10,15,21H,7-8,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50599254
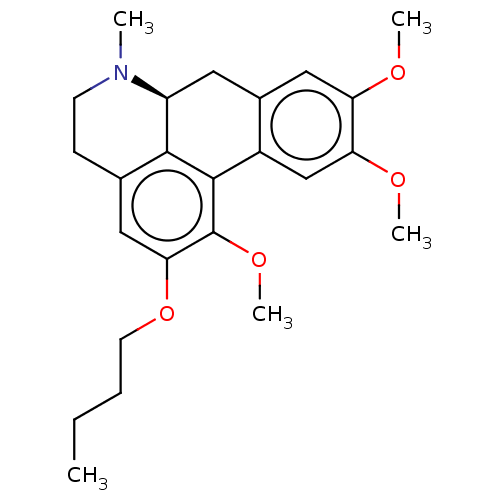
(CHEMBL5174366)Show SMILES [H][C@@]12Cc3cc(OC)c(OC)cc3-c3c(OC)c(OCCCC)cc(CCN1C)c23 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00365
BindingDB Entry DOI: 10.7270/Q2GX4GN3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
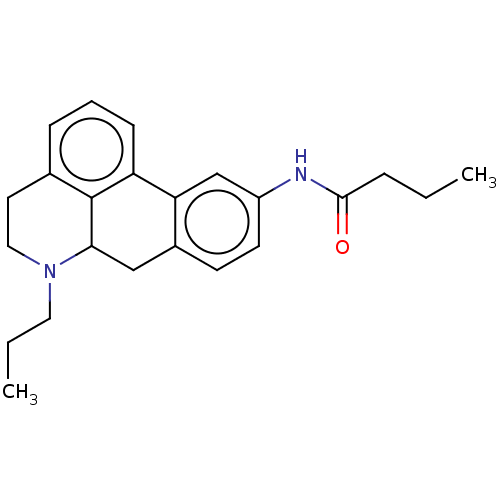
(Homo sapiens (Human)) | BDBM50544130
(CHEMBL4635676)Show InChI InChI=1S/C23H28N2O/c1-3-6-22(26)24-18-10-9-17-14-21-23-16(11-13-25(21)12-4-2)7-5-8-19(23)20(17)15-18/h5,7-10,15,21H,3-4,6,11-14H2,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WAY100635 from human 5-HT1A receptor expresssed in stable CHO cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from human D5 receptor expressed in CH4Cl cells incubated for 60 mins by radio ligand binding assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127053
BindingDB Entry DOI: 10.7270/Q2H135KF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50090078
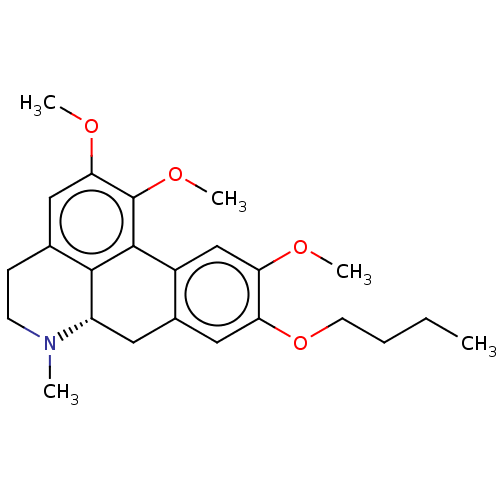
(CHEMBL3581245)Show SMILES [H][C@@]12Cc3cc(OCCCC)c(OC)cc3-c3c(OC)c(OC)cc(CCN1C)c23 |r| Show InChI InChI=1S/C24H31NO4/c1-6-7-10-29-20-13-16-11-18-22-15(8-9-25(18)2)12-21(27-4)24(28-5)23(22)17(16)14-19(20)26-3/h12-14,18H,6-11H2,1-5H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7 receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50544079
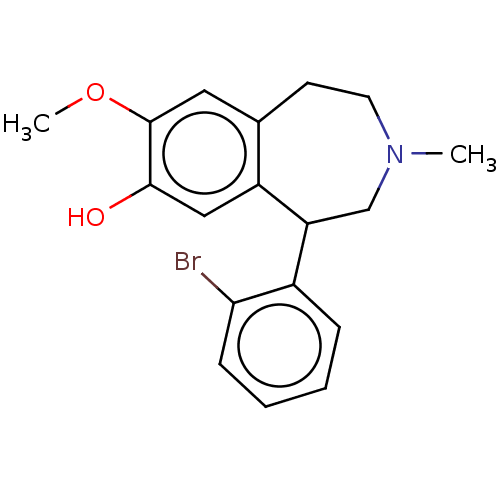
(CHEMBL4643426)Show InChI InChI=1S/C18H20BrNO2/c1-20-8-7-12-9-18(22-2)17(21)10-14(12)15(11-20)13-5-3-4-6-16(13)19/h3-6,9-10,15,21H,7-8,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127305
BindingDB Entry DOI: 10.7270/Q2377D85 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50599252

(CHEMBL5180016)Show SMILES [H][C@@]12Cc3cc(OC)c(OC)cc3-c3c(OC)c(OCC=C)cc(CCN1C)c23 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00365
BindingDB Entry DOI: 10.7270/Q2GX4GN3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50087033

((1R,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...)Show SMILES CO[C@]1(C)c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45 Show InChI InChI=1S/C20H21NO/c1-20(22-3)14-6-4-5-13-11-16-18-12(9-10-21(16)2)7-8-15(20)19(18)17(13)14/h4-8,16H,9-11H2,1-3H3/t16-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WAY100635 from human 5-HT1A receptor expresssed in stable CHO cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50544135
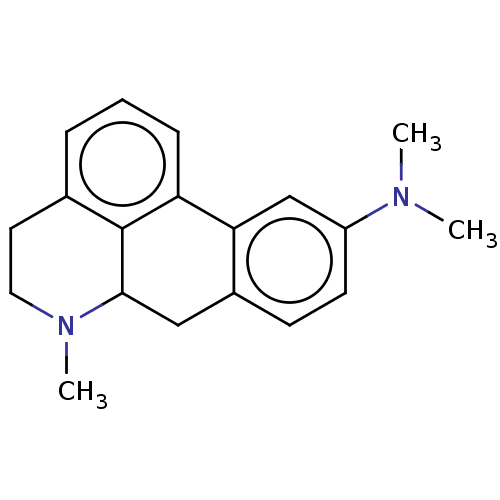
(CHEMBL4636329)Show InChI InChI=1S/C19H22N2/c1-20(2)15-8-7-14-11-18-19-13(9-10-21(18)3)5-4-6-16(19)17(14)12-15/h4-8,12,18H,9-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT7A receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115578
BindingDB Entry DOI: 10.7270/Q22B92KJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50060417
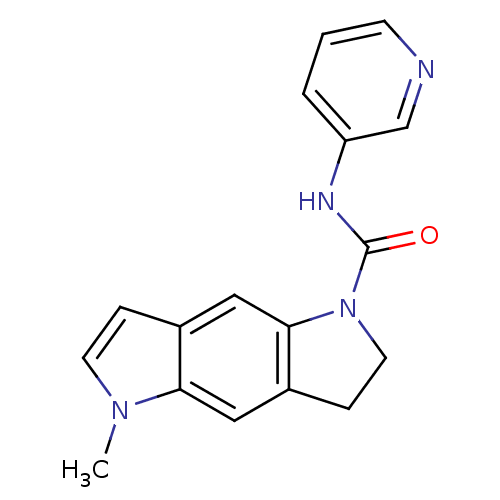
(5-Methyl-3,5-dihydro-2H-pyrrolo[2,3-f]indole-1-car...)Show InChI InChI=1S/C17H16N4O/c1-20-7-4-12-10-16-13(9-15(12)20)5-8-21(16)17(22)19-14-3-2-6-18-11-14/h2-4,6-7,9-11H,5,8H2,1H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00365
BindingDB Entry DOI: 10.7270/Q2GX4GN3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data