

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275051 (US9878996, Compound 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275052 (US9878996, Compound 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275054 (US9878996, Compound 27) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275056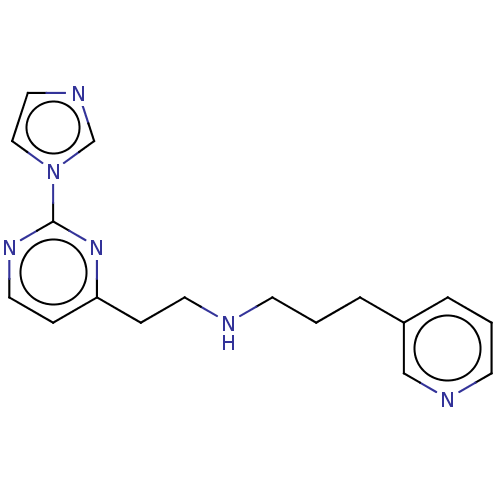 (US9878996, Compound 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275053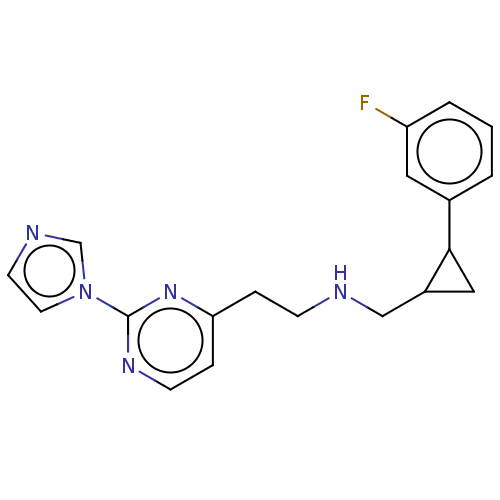 (US9878996, Compound 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM275052 (US9878996, Compound 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM275056 (US9878996, Compound 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM275055 (US9878996, Compound 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275050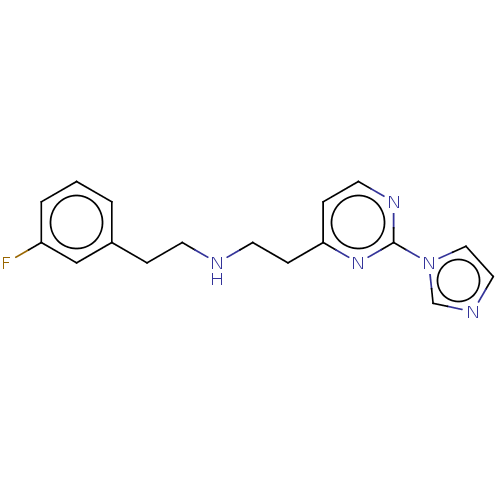 (US9878996, Compound 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275055 (US9878996, Compound 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM275051 (US9878996, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM275054 (US9878996, Compound 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 358 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM275053 (US9878996, Compound 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 359 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275046 (US9878996, Compound 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275058 (US9878996, Compound 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 667 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM275050 (US9878996, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 758 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM275051 (US9878996, Compound 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM275050 (US9878996, Compound 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM275056 (US9878996, Compound 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM275053 (US9878996, Compound 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM275052 (US9878996, Compound 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275049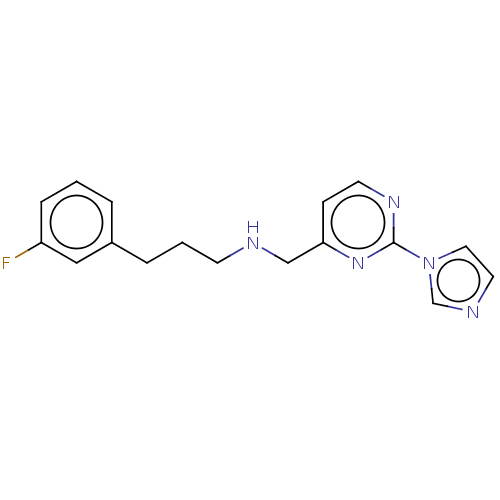 (US9878996, Compound 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM275054 (US9878996, Compound 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM275055 (US9878996, Compound 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM275050 (US9878996, Compound 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM275053 (US9878996, Compound 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM275058 (US9878996, Compound 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50070068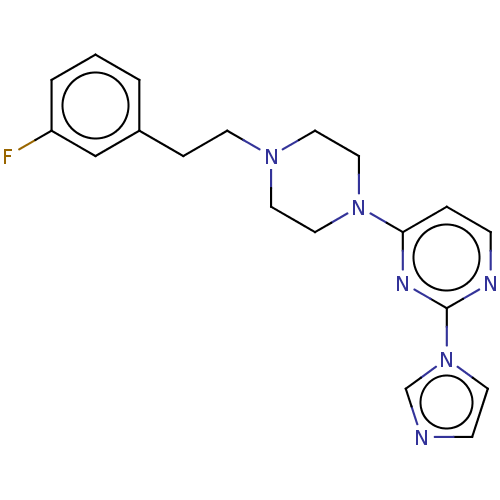 (CHEMBL3407805 | US9878996, Compound 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM275051 (US9878996, Compound 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275059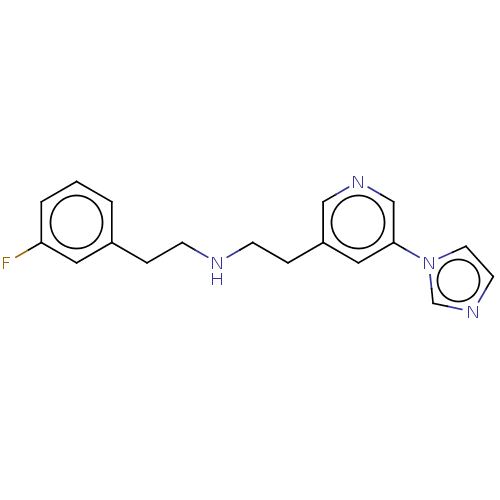 (US9878996, Compound 48) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM275046 (US9878996, Compound 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM275052 (US9878996, Compound 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275048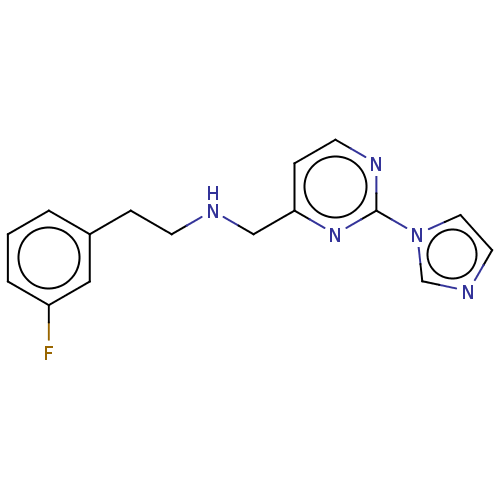 (US9878996, Compound 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM275055 (US9878996, Compound 28) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM275049 (US9878996, Compound 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM275056 (US9878996, Compound 29) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM275054 (US9878996, Compound 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM275046 (US9878996, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM275057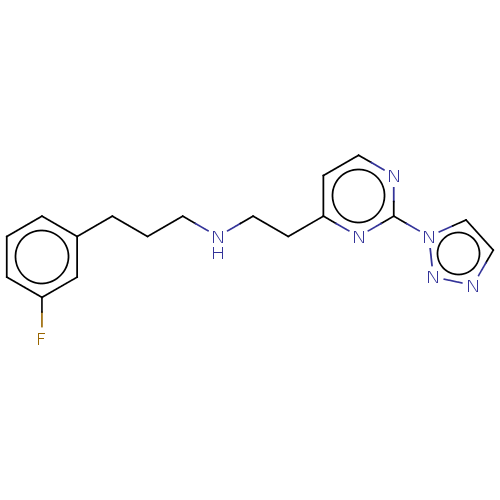 (US9878996, Compound 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM275049 (US9878996, Compound 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM275058 (US9878996, Compound 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM275058 (US9878996, Compound 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. | US Patent US9878996 (2018) BindingDB Entry DOI: 10.7270/Q2PN97PN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50511579 (CHEBI:61033 | Fondaparinux) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of coagulation factor 10a (unknown origin) incubated for 2 mins in presence of AT3 by chromogenic substrate based fluorescence assay | J Med Chem 63: 4227-4255 (2020) Article DOI: 10.1021/acs.jmedchem.0c00156 BindingDB Entry DOI: 10.7270/Q2SN0D99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50511576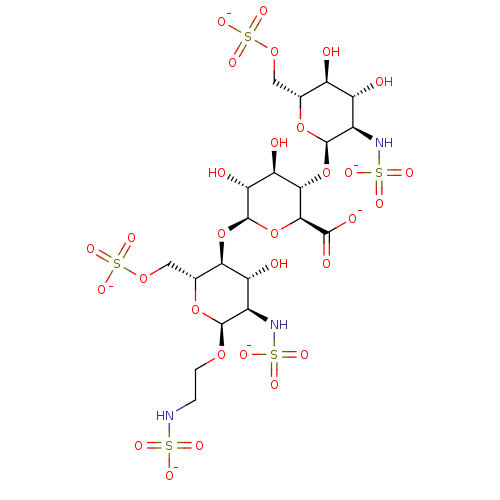 (CHEMBL4537045) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 393 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of heparanase (unknown origin) using biotin-heparan sulfate-Eu cryptate as substrate preincubated for 10 mins followed by substrate additi... | J Med Chem 63: 4227-4255 (2020) Article DOI: 10.1021/acs.jmedchem.0c00156 BindingDB Entry DOI: 10.7270/Q2SN0D99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50511576 (CHEMBL4537045) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of coagulation factor 2a (unknown origin) incubated for 2 mins in presence of AT3 by chromogenic substrate based fluorescence assay | J Med Chem 63: 4227-4255 (2020) Article DOI: 10.1021/acs.jmedchem.0c00156 BindingDB Entry DOI: 10.7270/Q2SN0D99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50511576 (CHEMBL4537045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of coagulation factor 10a (unknown origin) incubated for 2 mins in presence of AT3 by chromogenic substrate based fluorescence assay | J Med Chem 63: 4227-4255 (2020) Article DOI: 10.1021/acs.jmedchem.0c00156 BindingDB Entry DOI: 10.7270/Q2SN0D99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50511571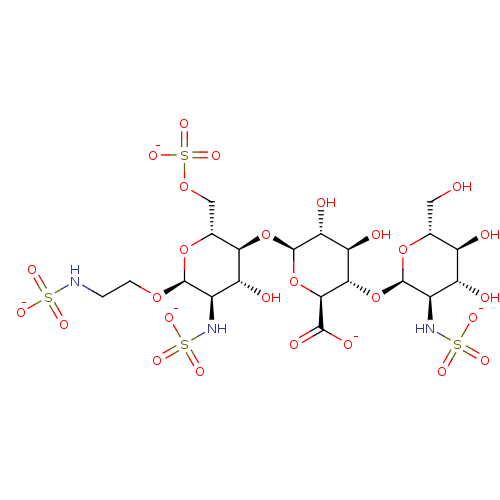 (CHEMBL4462283) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of heparanase (unknown origin) using biotin-heparan sulfate-Eu cryptate as substrate preincubated for 10 mins followed by substrate additi... | J Med Chem 63: 4227-4255 (2020) Article DOI: 10.1021/acs.jmedchem.0c00156 BindingDB Entry DOI: 10.7270/Q2SN0D99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50511572 (CHEMBL4514370) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of heparanase (unknown origin) using biotin-heparan sulfate-Eu cryptate as substrate preincubated for 10 mins followed by substrate additi... | J Med Chem 63: 4227-4255 (2020) Article DOI: 10.1021/acs.jmedchem.0c00156 BindingDB Entry DOI: 10.7270/Q2SN0D99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50511582 (CHEMBL4469617) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of heparanase (unknown origin) using biotin-heparan sulfate-Eu cryptate as substrate preincubated for 10 mins followed by substrate additi... | J Med Chem 63: 4227-4255 (2020) Article DOI: 10.1021/acs.jmedchem.0c00156 BindingDB Entry DOI: 10.7270/Q2SN0D99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heparanase (Homo sapiens (Human)) | BDBM50511583 (CHEMBL4447606) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Inhibition of heparanase (unknown origin) using biotin-heparan sulfate-Eu cryptate as substrate preincubated for 10 mins followed by substrate additi... | J Med Chem 63: 4227-4255 (2020) Article DOI: 10.1021/acs.jmedchem.0c00156 BindingDB Entry DOI: 10.7270/Q2SN0D99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |