 Found 141 hits with Last Name = 'nielsen' and Initial = 'te'
Found 141 hits with Last Name = 'nielsen' and Initial = 'te' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50596272
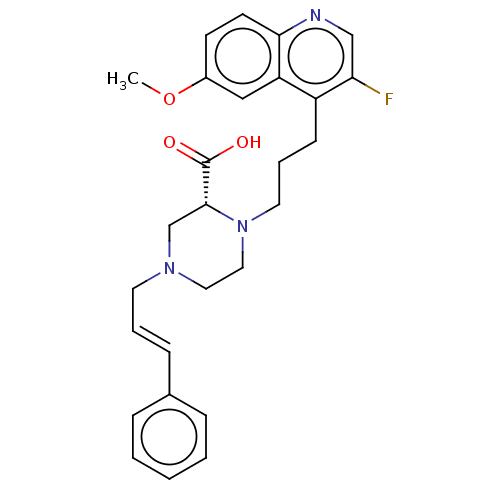
(CHEMBL5199813)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(C\C=C\c4ccccc4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50596278
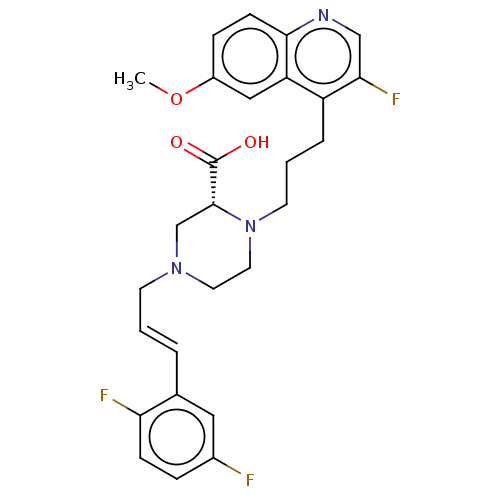
(CHEMBL5178214)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(C\C=C\c4cc(F)ccc4F)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50596263

(CHEMBL5207657)Show SMILES COc1ccc2ncc(F)c(\C=C/CN3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50393503
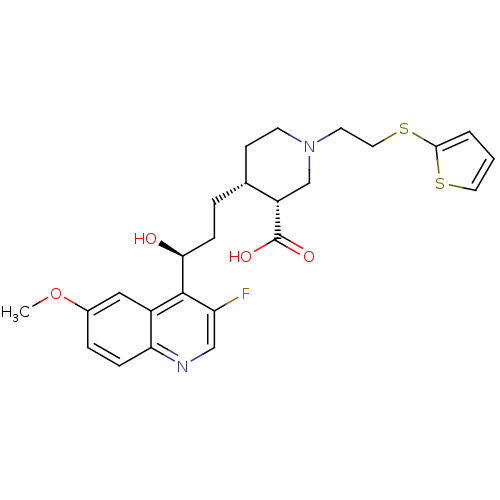
(CHEMBL2158050)Show SMILES COc1ccc2ncc(F)c([C@@H](O)CC[C@@H]3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| Show InChI InChI=1S/C25H29FN2O4S2/c1-32-17-5-6-21-18(13-17)24(20(26)14-27-21)22(29)7-4-16-8-9-28(15-19(16)25(30)31)10-12-34-23-3-2-11-33-23/h2-3,5-6,11,13-14,16,19,22,29H,4,7-10,12,15H2,1H3,(H,30,31)/t16-,19+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50596275
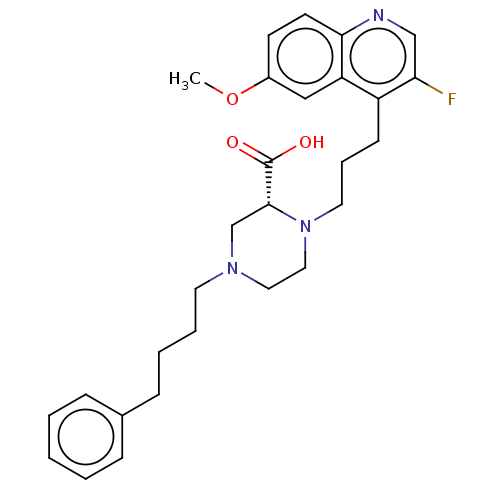
(CHEMBL5176348)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(CCCCc4ccccc4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50596261
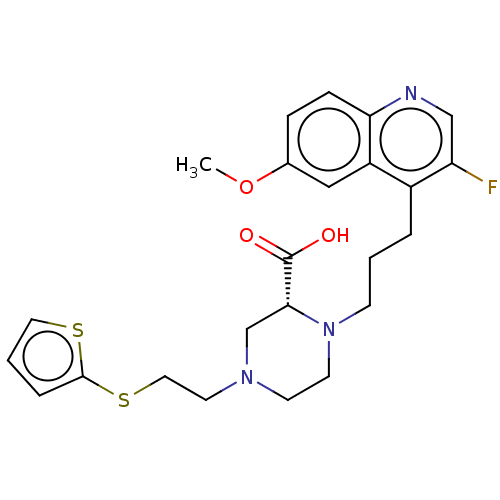
(CHEMBL5176134)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50596271
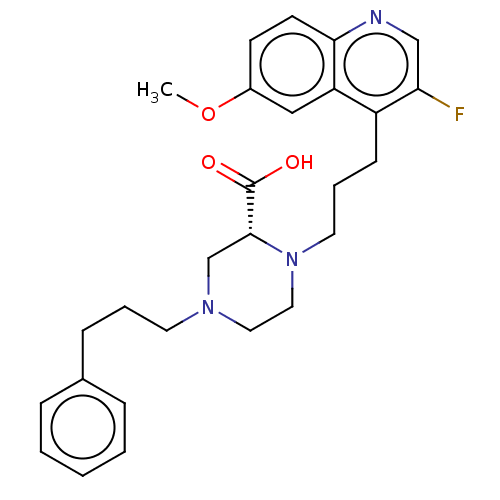
(CHEMBL5176046)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(CCCc4ccccc4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50596264

(CHEMBL5206355)Show SMILES COc1ccc2ncc(F)c(C#CCN3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50596274

(CHEMBL5204986)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(CCc4ccccc4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50596262
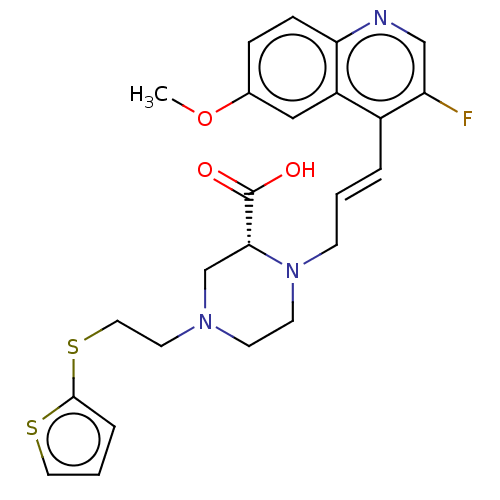
(CHEMBL5176331)Show SMILES COc1ccc2ncc(F)c(\C=C\CN3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50596262

(CHEMBL5176331)Show SMILES COc1ccc2ncc(F)c(\C=C\CN3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
HTH-type quorum-sensing regulator RhlR
(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50470115
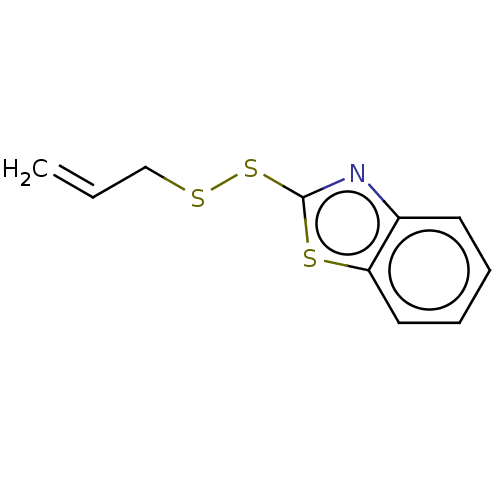
(CHEMBL4098007)Show InChI InChI=1S/C10H9NS3/c1-2-7-12-14-10-11-8-5-3-4-6-9(8)13-10/h2-6H,1,7H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University
Curated by ChEMBL
| Assay Description
Inhibition of quorum sensing regulator protein RhlR in Pseudomonas aeruginosa PAO1 harboring reporter plasmid rhlA-gfp assessed as reduction in rhlA ... |
J Med Chem 60: 215-227 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01025
BindingDB Entry DOI: 10.7270/Q2125WC6 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50596278

(CHEMBL5178214)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(C\C=C\c4cc(F)ccc4F)C[C@@H]3C(O)=O)c2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50596264

(CHEMBL5206355)Show SMILES COc1ccc2ncc(F)c(C#CCN3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50596272

(CHEMBL5199813)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(C\C=C\c4ccccc4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50596275

(CHEMBL5176348)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(CCCCc4ccccc4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50393503

(CHEMBL2158050)Show SMILES COc1ccc2ncc(F)c([C@@H](O)CC[C@@H]3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| Show InChI InChI=1S/C25H29FN2O4S2/c1-32-17-5-6-21-18(13-17)24(20(26)14-27-21)22(29)7-4-16-8-9-28(15-19(16)25(30)31)10-12-34-23-3-2-11-33-23/h2-3,5-6,11,13-14,16,19,22,29H,4,7-10,12,15H2,1H3,(H,30,31)/t16-,19+,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50596261

(CHEMBL5176134)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50596278

(CHEMBL5178214)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(C\C=C\c4cc(F)ccc4F)C[C@@H]3C(O)=O)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50596261

(CHEMBL5176134)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50393503

(CHEMBL2158050)Show SMILES COc1ccc2ncc(F)c([C@@H](O)CC[C@@H]3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| Show InChI InChI=1S/C25H29FN2O4S2/c1-32-17-5-6-21-18(13-17)24(20(26)14-27-21)22(29)7-4-16-8-9-28(15-19(16)25(30)31)10-12-34-23-3-2-11-33-23/h2-3,5-6,11,13-14,16,19,22,29H,4,7-10,12,15H2,1H3,(H,30,31)/t16-,19+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50596263

(CHEMBL5207657)Show SMILES COc1ccc2ncc(F)c(\C=C/CN3CCN(CCSc4cccs4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50596271

(CHEMBL5176046)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(CCCc4ccccc4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 4 subunit A/B
(Staphylococcus aureus) | BDBM50596274

(CHEMBL5204986)Show SMILES COc1ccc2ncc(F)c(CCCN3CCN(CCc4ccccc4)C[C@@H]3C(O)=O)c2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50596269

(CHEMBL5183842)Show SMILES COC(=O)[C@H]1CN(CCSc2cccs2)CCN1CCCc1c(F)cnc2ccc(OC)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
HTH-type quorum-sensing regulator RhlR
(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50240785

((E)-1-Allyldisulfanyl-3-(prop-2-ene-1-sulfinyl)-pr...)Show InChI InChI=1S/C9H14OS3/c1-3-6-11-12-7-5-9-13(10)8-4-2/h3-5,7H,1-2,6,8-9H2/b7-5+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University
Curated by ChEMBL
| Assay Description
Inhibition of quorum sensing regulator protein RhlR in Pseudomonas aeruginosa PAO1 harboring reporter plasmid rhlA-gfp assessed as reduction in rhlA ... |
J Med Chem 60: 215-227 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01025
BindingDB Entry DOI: 10.7270/Q2125WC6 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Staphylococcus aureus) | BDBM50596269

(CHEMBL5183842)Show SMILES COC(=O)[C@H]1CN(CCSc2cccs2)CCN1CCCc1c(F)cnc2ccc(OC)cc12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128499
BindingDB Entry DOI: 10.7270/Q2X06C35 |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50498101

(CHEMBL3403862)Show SMILES O=Cc1cccc(c1)-n1cc(CCC(=O)N[C@H]2CCOC2=O)nn1 |r| Show InChI InChI=1S/C16H16N4O4/c21-10-11-2-1-3-13(8-11)20-9-12(18-19-20)4-5-15(22)17-14-6-7-24-16(14)23/h1-3,8-10,14H,4-7H2,(H,17,22)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Antagonist activity at lasB in Pseudomonas aeruginosa harboring lasB-gfp gene assessed as inhibition of 3-oxo-C6-HSL-induced quorum sensing by green ... |
Bioorg Med Chem 23: 1638-50 (2015)
Article DOI: 10.1016/j.bmc.2015.01.038
BindingDB Entry DOI: 10.7270/Q2F192P6 |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50498105

(CHEMBL3403858)Show InChI InChI=1S/C14H22N4O3/c1-2-3-4-5-6-11-9-18(17-16-11)10-13(19)15-12-7-8-21-14(12)20/h9,12H,2-8,10H2,1H3,(H,15,19)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Antagonist activity at lasB in Pseudomonas aeruginosa harboring lasB-gfp gene assessed as inhibition of 3-oxo-C6-HSL-induced quorum sensing by green ... |
Bioorg Med Chem 23: 1638-50 (2015)
Article DOI: 10.1016/j.bmc.2015.01.038
BindingDB Entry DOI: 10.7270/Q2F192P6 |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50498098
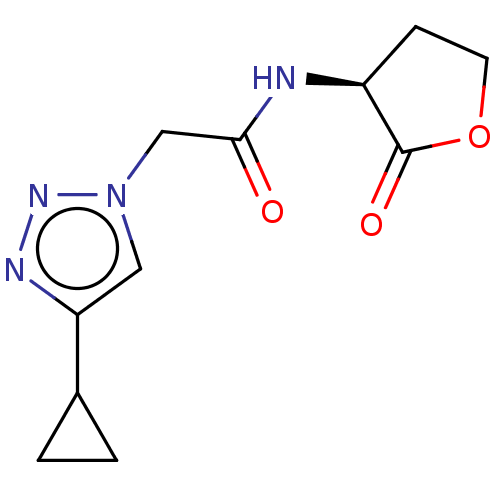
(CHEMBL3403859)Show InChI InChI=1S/C11H14N4O3/c16-10(12-8-3-4-18-11(8)17)6-15-5-9(13-14-15)7-1-2-7/h5,7-8H,1-4,6H2,(H,12,16)/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Antagonist activity at lasB in Pseudomonas aeruginosa harboring lasB-gfp gene assessed as inhibition of 3-oxo-C6-HSL-induced quorum sensing by green ... |
Bioorg Med Chem 23: 1638-50 (2015)
Article DOI: 10.1016/j.bmc.2015.01.038
BindingDB Entry DOI: 10.7270/Q2F192P6 |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50498104

(CHEMBL3403865)Show InChI InChI=1S/C12H12N4O3S/c17-11(13-9-1-3-19-12(9)18)6-16-5-10(14-15-16)8-2-4-20-7-8/h2,4-5,7,9H,1,3,6H2,(H,13,17)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Antagonist activity at lasB in Pseudomonas aeruginosa harboring lasB-gfp gene assessed as inhibition of 3-oxo-C6-HSL-induced quorum sensing by green ... |
Bioorg Med Chem 23: 1638-50 (2015)
Article DOI: 10.1016/j.bmc.2015.01.038
BindingDB Entry DOI: 10.7270/Q2F192P6 |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50498097
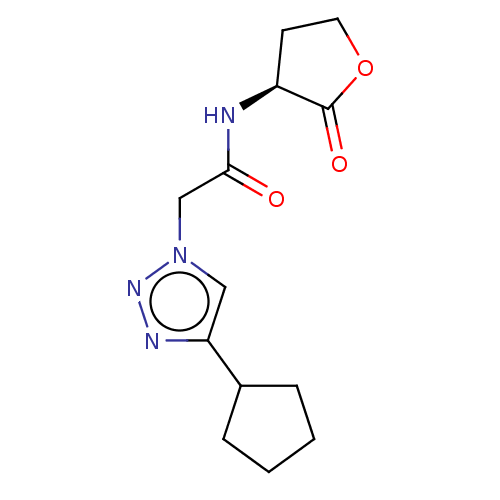
(CHEMBL3403860)Show InChI InChI=1S/C13H18N4O3/c18-12(14-10-5-6-20-13(10)19)8-17-7-11(15-16-17)9-3-1-2-4-9/h7,9-10H,1-6,8H2,(H,14,18)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Antagonist activity at lasB in Pseudomonas aeruginosa harboring lasB-gfp gene assessed as inhibition of 3-oxo-C6-HSL-induced quorum sensing by green ... |
Bioorg Med Chem 23: 1638-50 (2015)
Article DOI: 10.1016/j.bmc.2015.01.038
BindingDB Entry DOI: 10.7270/Q2F192P6 |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50498102

(CHEMBL3403856)Show InChI InChI=1S/C11H16N4O3/c1-2-3-8-6-15(14-13-8)7-10(16)12-9-4-5-18-11(9)17/h6,9H,2-5,7H2,1H3,(H,12,16)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Antagonist activity at lasB in Pseudomonas aeruginosa harboring lasB-gfp gene assessed as inhibition of 3-oxo-C6-HSL-induced quorum sensing by green ... |
Bioorg Med Chem 23: 1638-50 (2015)
Article DOI: 10.1016/j.bmc.2015.01.038
BindingDB Entry DOI: 10.7270/Q2F192P6 |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50498100

(CHEMBL3403864)Show SMILES O=C(Cn1cc(CSc2ccccc2)nn1)N[C@H]1CCOC1=O |r| Show InChI InChI=1S/C15H16N4O3S/c20-14(16-13-6-7-22-15(13)21)9-19-8-11(17-18-19)10-23-12-4-2-1-3-5-12/h1-5,8,13H,6-7,9-10H2,(H,16,20)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Antagonist activity at lasB in Pseudomonas aeruginosa harboring lasB-gfp gene assessed as inhibition of 3-oxo-C6-HSL-induced quorum sensing by green ... |
Bioorg Med Chem 23: 1638-50 (2015)
Article DOI: 10.1016/j.bmc.2015.01.038
BindingDB Entry DOI: 10.7270/Q2F192P6 |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50498103
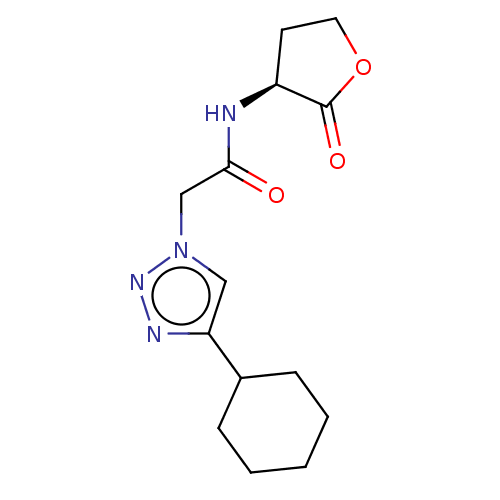
(CHEMBL3403861)Show InChI InChI=1S/C14H20N4O3/c19-13(15-11-6-7-21-14(11)20)9-18-8-12(16-17-18)10-4-2-1-3-5-10/h8,10-11H,1-7,9H2,(H,15,19)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Antagonist activity at lasB in Pseudomonas aeruginosa harboring lasB-gfp gene assessed as inhibition of 3-oxo-C6-HSL-induced quorum sensing by green ... |
Bioorg Med Chem 23: 1638-50 (2015)
Article DOI: 10.1016/j.bmc.2015.01.038
BindingDB Entry DOI: 10.7270/Q2F192P6 |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50498099
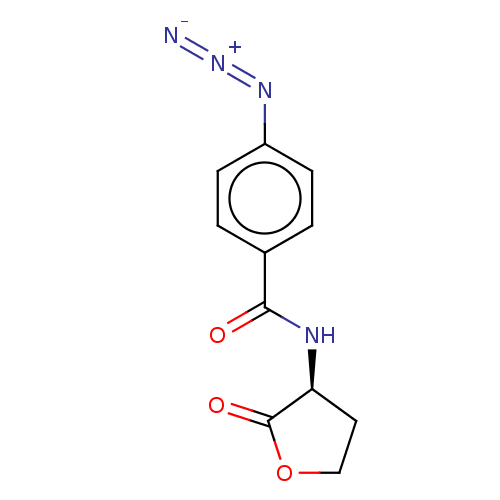
(CHEMBL3403868)Show SMILES [N-]=[N+]=Nc1ccc(cc1)C(=O)N[C@H]1CCOC1=O |r| Show InChI InChI=1S/C11H10N4O3/c12-15-14-8-3-1-7(2-4-8)10(16)13-9-5-6-18-11(9)17/h1-4,9H,5-6H2,(H,13,16)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark
Curated by ChEMBL
| Assay Description
Antagonist activity at lasB in Pseudomonas aeruginosa harboring lasB-gfp gene assessed as inhibition of 3-oxo-C6-HSL-induced quorum sensing by green ... |
Bioorg Med Chem 23: 1638-50 (2015)
Article DOI: 10.1016/j.bmc.2015.01.038
BindingDB Entry DOI: 10.7270/Q2F192P6 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50250214
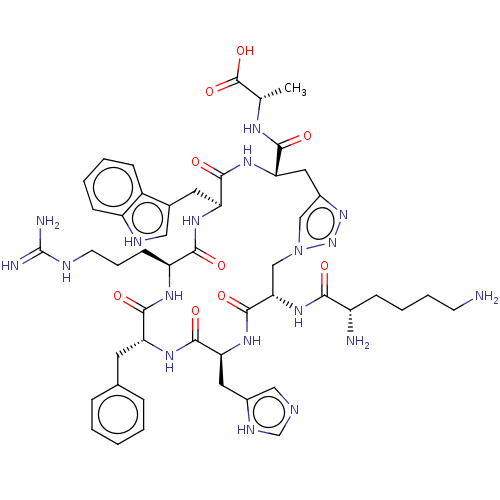
(CHEMBL4100598)Show SMILES C[C@H](NC(=O)[C@@H]1Cc2cn(C[C@H](NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N[C@H](Cc3ccccc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(=O)N1)nn2)C(O)=O |r| Show InChI InChI=1S/C49H66N18O9/c1-27(48(75)76)58-43(70)39-21-31-24-67(66-65-31)25-40(64-41(68)33(51)13-7-8-16-50)47(74)63-38(20-30-23-54-26-57-30)46(73)60-36(18-28-10-3-2-4-11-28)44(71)59-35(15-9-17-55-49(52)53)42(69)61-37(45(72)62-39)19-29-22-56-34-14-6-5-12-32(29)34/h2-6,10-12,14,22-24,26-27,33,35-40,56H,7-9,13,15-21,25,50-51H2,1H3,(H,54,57)(H,58,70)(H,59,71)(H,60,73)(H,61,69)(H,62,72)(H,63,74)(H,64,68)(H,75,76)(H4,52,53,55)/t27-,33-,35-,36+,37-,38-,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC4R expressed in HEK293 cells after 16 to 20 hrs by CRE-driven reporter assay |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50029747
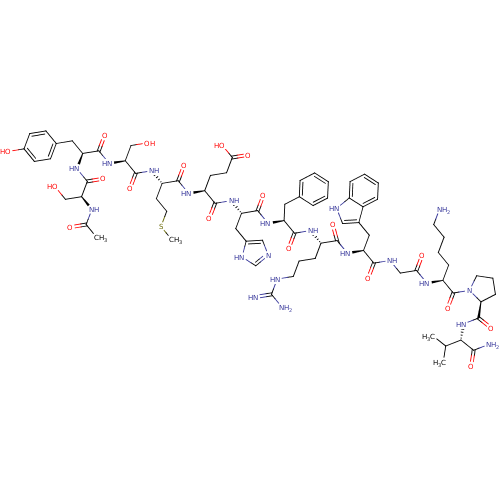
((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC4R expressed in HEK293 cells assessed as increase in cAMP accumulation after 30 mins by luminescent enzymatic ... |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50250212
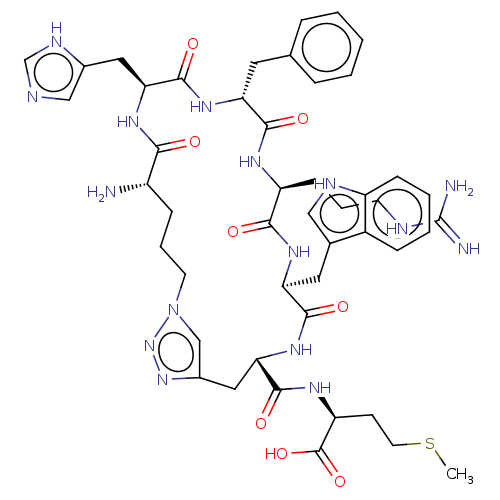
(CHEMBL4089119)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2cn(CCC[C@H](N)C(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N[C@H](Cc3ccccc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(=O)N1)nn2)C(O)=O |r| Show InChI InChI=1S/C47H62N16O8S/c1-72-18-15-35(46(70)71)56-44(68)39-22-30-25-63(62-61-30)17-8-12-32(48)40(64)57-38(21-29-24-51-26-54-29)45(69)58-36(19-27-9-3-2-4-10-27)42(66)55-34(14-7-16-52-47(49)50)41(65)59-37(43(67)60-39)20-28-23-53-33-13-6-5-11-31(28)33/h2-6,9-11,13,23-26,32,34-39,53H,7-8,12,14-22,48H2,1H3,(H,51,54)(H,55,66)(H,56,68)(H,57,64)(H,58,69)(H,59,65)(H,60,67)(H,70,71)(H4,49,50,52)/t32-,34-,35-,36+,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 185 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC4R expressed in HEK293 cells after 16 to 20 hrs by CRE-driven reporter assay |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50250213
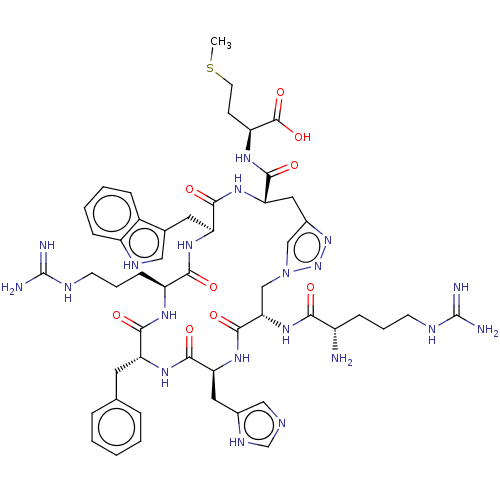
(CHEMBL4104927)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2cn(C[C@H](NC(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N[C@H](Cc3ccccc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(=O)N1)nn2)C(O)=O |r| Show InChI InChI=1S/C51H70N20O9S/c1-81-18-15-36(49(79)80)63-46(76)40-22-31-25-71(70-69-31)26-41(68-42(72)33(52)12-7-16-58-50(53)54)48(78)67-39(21-30-24-57-27-61-30)47(77)64-37(19-28-9-3-2-4-10-28)44(74)62-35(14-8-17-59-51(55)56)43(73)65-38(45(75)66-40)20-29-23-60-34-13-6-5-11-32(29)34/h2-6,9-11,13,23-25,27,33,35-41,60H,7-8,12,14-22,26,52H2,1H3,(H,57,61)(H,62,74)(H,63,76)(H,64,77)(H,65,73)(H,66,75)(H,67,78)(H,68,72)(H,79,80)(H4,53,54,58)(H4,55,56,59)/t33-,35-,36-,37+,38-,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC4R expressed in HEK293 cells after 16 to 20 hrs by CRE-driven reporter assay |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50250211
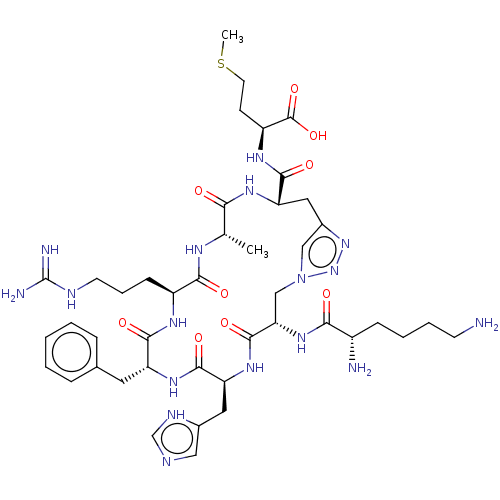
(CHEMBL4100221)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2cn(C[C@H](NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N[C@H](Cc3ccccc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N1)nn2)C(O)=O |r| Show InChI InChI=1S/C43H65N17O9S/c1-24-35(61)54-33(39(65)53-30(42(68)69)13-16-70-2)19-27-21-60(59-58-27)22-34(57-36(62)28(45)11-6-7-14-44)41(67)56-32(18-26-20-48-23-50-26)40(66)55-31(17-25-9-4-3-5-10-25)38(64)52-29(37(63)51-24)12-8-15-49-43(46)47/h3-5,9-10,20-21,23-24,28-34H,6-8,11-19,22,44-45H2,1-2H3,(H,48,50)(H,51,63)(H,52,64)(H,53,65)(H,54,61)(H,55,66)(H,56,67)(H,57,62)(H,68,69)(H4,46,47,49)/t24-,28-,29-,30-,31+,32-,33-,34-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC4R expressed in HEK293 cells assessed as increase in cAMP accumulation after 30 mins by luminescent enzymatic ... |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50029747

((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC5R expressed in HEK293 cells after 16 to 20 hrs by CRE-driven reporter assay |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50250205
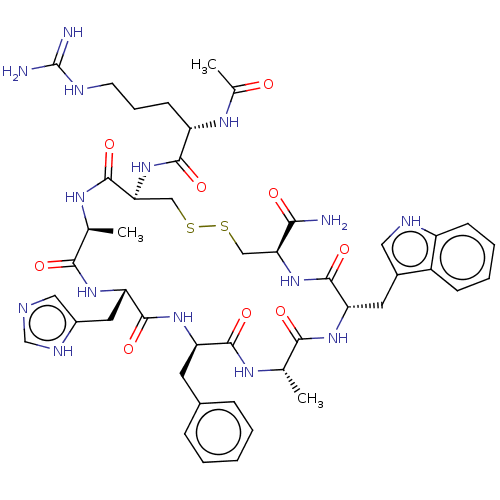
(CHEMBL4067491)Show SMILES C[C@@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](C)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O |r| Show InChI InChI=1S/C46H61N15O9S2/c1-24-39(64)57-34(17-28-19-52-31-13-8-7-12-30(28)31)43(68)60-36(38(47)63)21-71-72-22-37(61-41(66)32(56-26(3)62)14-9-15-51-46(48)49)45(70)55-25(2)40(65)58-35(18-29-20-50-23-53-29)44(69)59-33(42(67)54-24)16-27-10-5-4-6-11-27/h4-8,10-13,19-20,23-25,32-37,52H,9,14-18,21-22H2,1-3H3,(H2,47,63)(H,50,53)(H,54,67)(H,55,70)(H,56,62)(H,57,64)(H,58,65)(H,59,69)(H,60,68)(H,61,66)(H4,48,49,51)/t24-,25-,32-,33+,34-,35-,36-,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC5R expressed in HEK293 cells after 16 to 20 hrs by CRE-driven reporter assay |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50250206
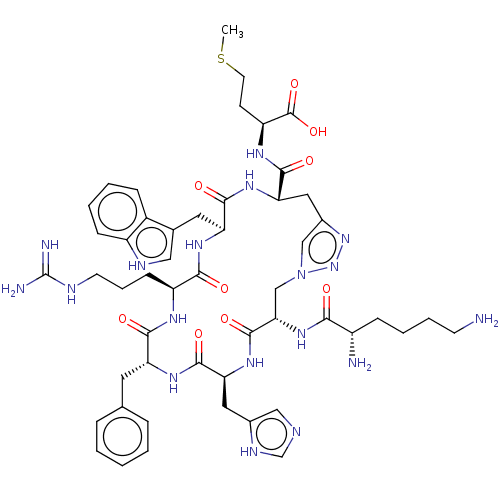
(CHEMBL4060816)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2cn(C[C@H](NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N[C@H](Cc3ccccc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(=O)N1)nn2)C(O)=O |r| Show InChI InChI=1S/C51H70N18O9S/c1-79-19-16-37(50(77)78)61-47(74)41-23-32-26-69(68-67-32)27-42(66-43(70)34(53)13-7-8-17-52)49(76)65-40(22-31-25-56-28-59-31)48(75)62-38(20-29-10-3-2-4-11-29)45(72)60-36(15-9-18-57-51(54)55)44(71)63-39(46(73)64-41)21-30-24-58-35-14-6-5-12-33(30)35/h2-6,10-12,14,24-26,28,34,36-42,58H,7-9,13,15-23,27,52-53H2,1H3,(H,56,59)(H,60,72)(H,61,74)(H,62,75)(H,63,71)(H,64,73)(H,65,76)(H,66,70)(H,77,78)(H4,54,55,57)/t34-,36-,37-,38+,39-,40-,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC4R expressed in HEK293 cells assessed as increase in cAMP accumulation after 30 mins by luminescent enzymatic ... |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50250207
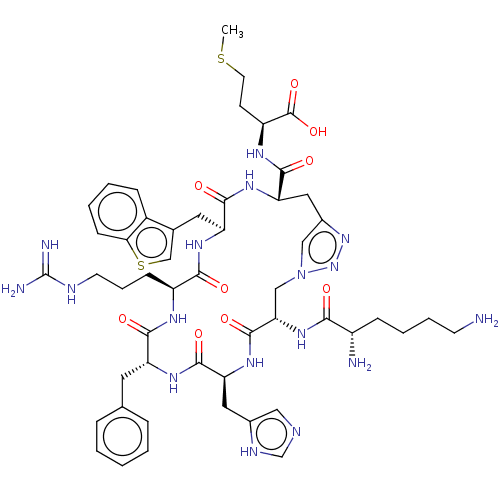
(CHEMBL4082585)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2cn(C[C@H](NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N[C@H](Cc3ccccc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3csc4ccccc34)C(=O)N1)nn2)C(O)=O |r| Show InChI InChI=1S/C51H69N17O9S2/c1-78-19-16-36(50(76)77)60-47(73)40-23-32-25-68(67-66-32)26-41(65-43(69)34(53)13-7-8-17-52)49(75)64-39(22-31-24-56-28-58-31)48(74)61-37(20-29-10-3-2-4-11-29)45(71)59-35(14-9-18-57-51(54)55)44(70)62-38(46(72)63-40)21-30-27-79-42-15-6-5-12-33(30)42/h2-6,10-12,15,24-25,27-28,34-41H,7-9,13-14,16-23,26,52-53H2,1H3,(H,56,58)(H,59,71)(H,60,73)(H,61,74)(H,62,70)(H,63,72)(H,64,75)(H,65,69)(H,76,77)(H4,54,55,57)/t34-,35-,36-,37+,38-,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 64 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC4R expressed in HEK293 cells assessed as increase in cAMP accumulation after 30 mins by luminescent enzymatic ... |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50250208

(CHEMBL4062443)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2cn(C[C@H](NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N[C@@H](Cc3ccccc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(=O)N1)nn2)C(O)=O |r| Show InChI InChI=1S/C51H70N18O9S/c1-79-19-16-37(50(77)78)61-47(74)41-23-32-26-69(68-67-32)27-42(66-43(70)34(53)13-7-8-17-52)49(76)65-40(22-31-25-56-28-59-31)48(75)62-38(20-29-10-3-2-4-11-29)45(72)60-36(15-9-18-57-51(54)55)44(71)63-39(46(73)64-41)21-30-24-58-35-14-6-5-12-33(30)35/h2-6,10-12,14,24-26,28,34,36-42,58H,7-9,13,15-23,27,52-53H2,1H3,(H,56,59)(H,60,72)(H,61,74)(H,62,75)(H,63,71)(H,64,73)(H,65,76)(H,66,70)(H,77,78)(H4,54,55,57)/t34-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 792 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC4R expressed in HEK293 cells assessed as increase in cAMP accumulation after 30 mins by luminescent enzymatic ... |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50250209
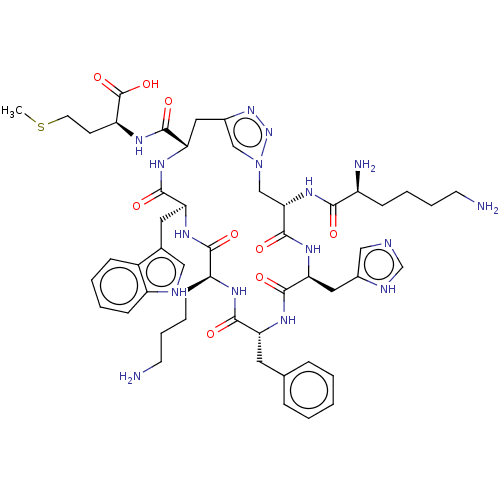
(CHEMBL4090425)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2cn(C[C@H](NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](Cc3cnc[nH]3)C(=O)N[C@H](Cc3ccccc3)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(=O)N1)nn2)C(O)=O |r| Show InChI InChI=1S/C51H70N16O9S/c1-77-20-17-38(51(75)76)59-48(72)42-24-33-27-67(66-65-33)28-43(64-44(68)35(54)14-7-9-18-52)50(74)63-41(23-32-26-55-29-57-32)49(73)60-39(21-30-11-3-2-4-12-30)46(70)58-37(16-8-10-19-53)45(69)61-40(47(71)62-42)22-31-25-56-36-15-6-5-13-34(31)36/h2-6,11-13,15,25-27,29,35,37-43,56H,7-10,14,16-24,28,52-54H2,1H3,(H,55,57)(H,58,70)(H,59,72)(H,60,73)(H,61,69)(H,62,71)(H,63,74)(H,64,68)(H,75,76)/t35-,37-,38-,39+,40-,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 124 | n/a | n/a | n/a | n/a |
CECB, Department of Chemistry, University of Copenhagen , Universitetsparken 5, 2100 Copenhagen, Denmark.
Curated by ChEMBL
| Assay Description
Agonist activity at EYFP-fused human MC4R expressed in HEK293 cells assessed as increase in cAMP accumulation after 30 mins by luminescent enzymatic ... |
J Med Chem 60: 8716-8730 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00353
BindingDB Entry DOI: 10.7270/Q2VQ3535 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data