

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24616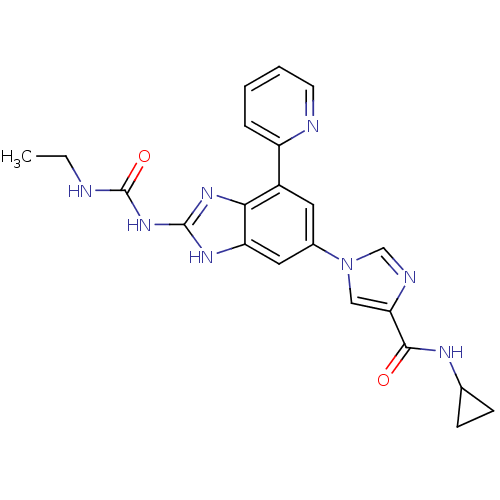 (1-(6-(4-(Cyclopropylcarbamoyl)-1H-imidazol-1-yl)-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24617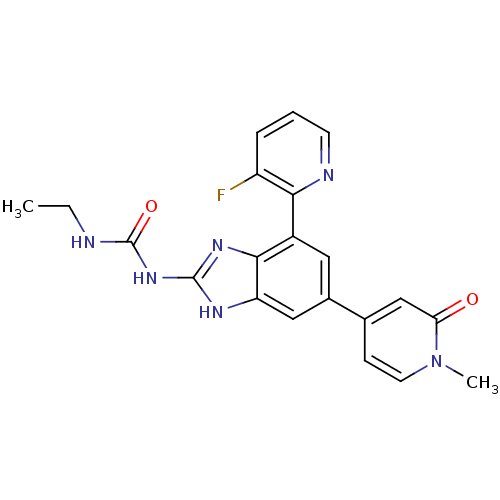 (3-ethyl-1-[7-(3-fluoropyridin-2-yl)-5-(1-methyl-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50393079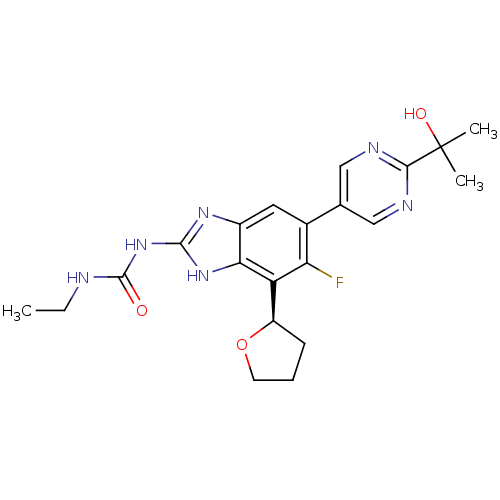 (CHEMBL2152855 | US9040542, 23) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VERTEX PHARMACEUTICALS INCORPORATED US Patent | Assay Description The conversion of ATP to ADP by S. aureus TopoIV enzyme is coupled to the conversion of NADH to NAD+, and the progress of the reaction is measured by... | US Patent US9040542 (2015) BindingDB Entry DOI: 10.7270/Q2F18XHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24615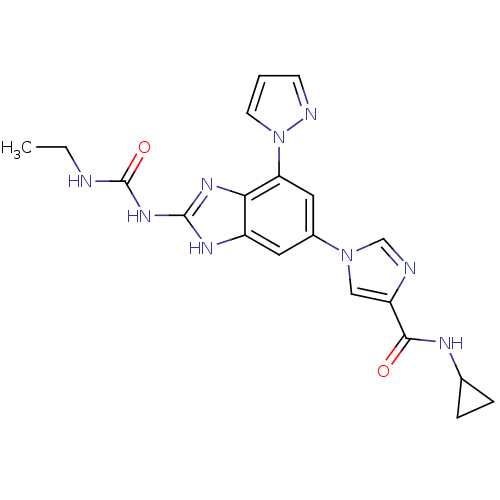 (Benzimidazole urea analogue, 19 | N-cyclopropyl-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24609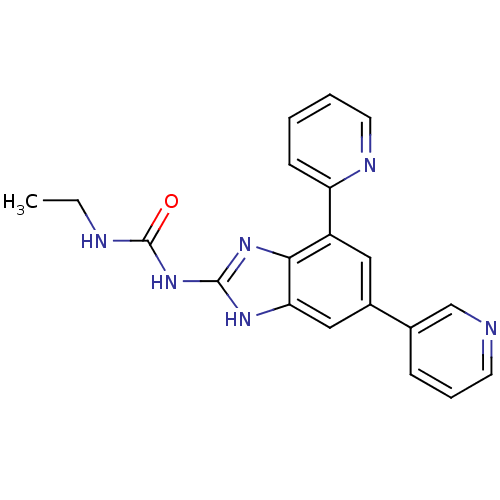 (3-ethyl-1-[7-(pyridin-2-yl)-5-(pyridin-3-yl)-1H-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24611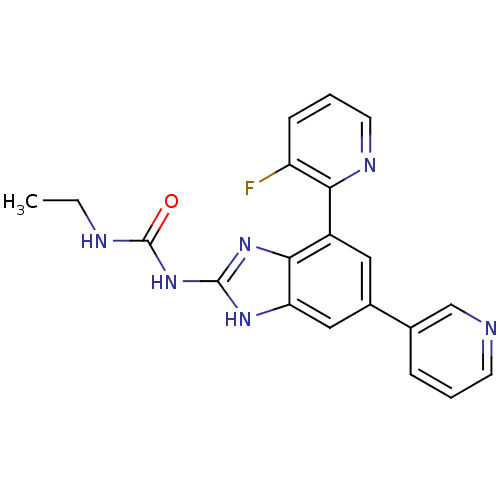 (3-ethyl-1-[7-(3-fluoropyridin-2-yl)-5-(pyridin-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50393079 (CHEMBL2152855 | US9040542, 23) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA topoisomerase 4 | ACS Med Chem Lett 6: 822-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00196 BindingDB Entry DOI: 10.7270/Q2J67JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24606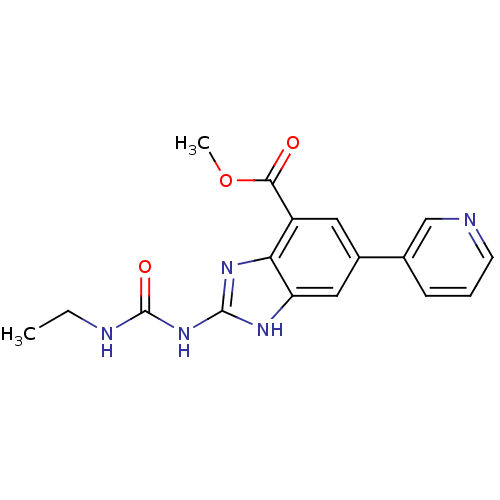 (Benzimidazole urea analogue, 10 | methyl 2-[(ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24608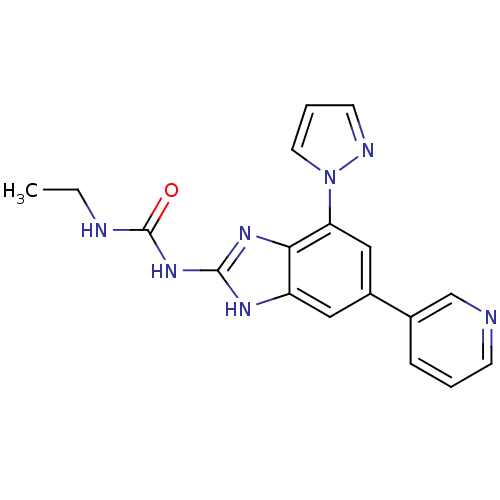 (3-ethyl-1-[7-(1H-pyrazol-1-yl)-5-(pyridin-3-yl)-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24614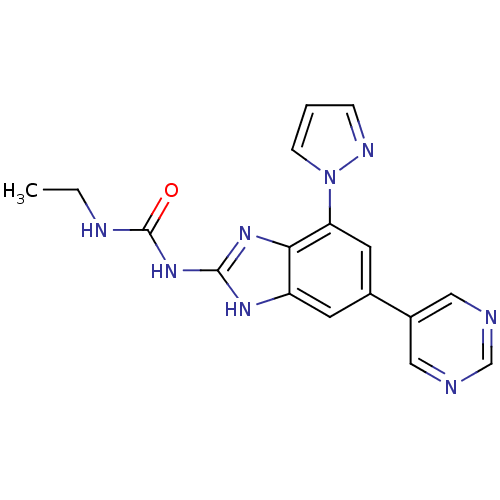 (3-ethyl-1-[7-(1H-pyrazol-1-yl)-5-(pyrimidin-5-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50112815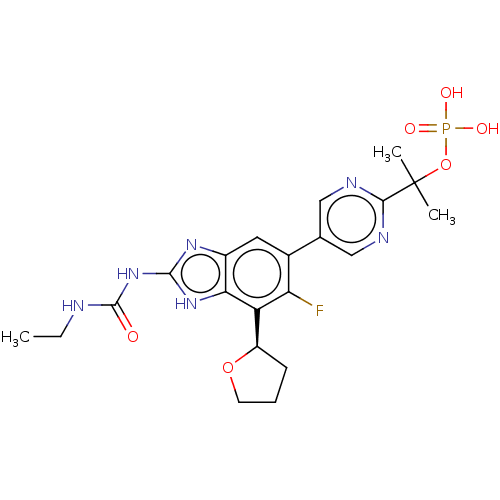 (CHEMBL2221212) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA topoisomerase 4 | ACS Med Chem Lett 6: 822-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00196 BindingDB Entry DOI: 10.7270/Q2J67JPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24601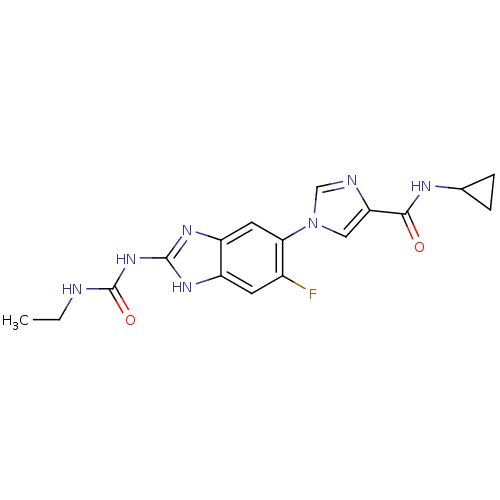 (Benzimidazole urea analogue, 5 | N-cyclopropyl-1-{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM282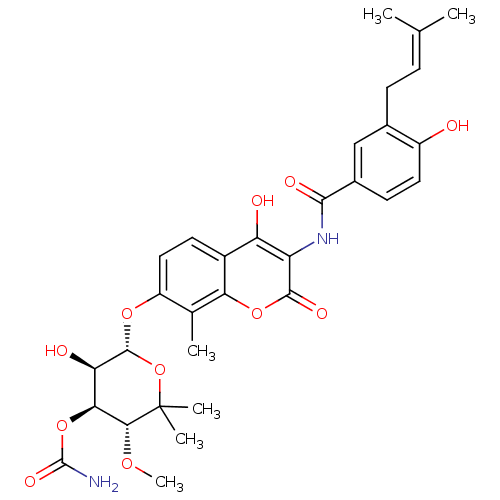 ((3R,4S,5R,6R)-5-hydroxy-6-[(2-hydroxy-3-{[4-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24607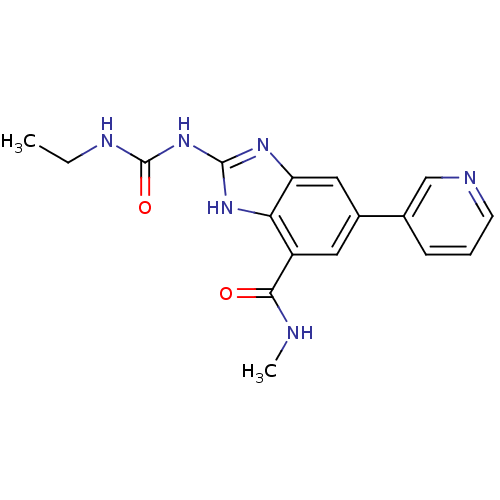 (2-[(ethylcarbamoyl)amino]-N-methyl-5-(pyridin-3-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24612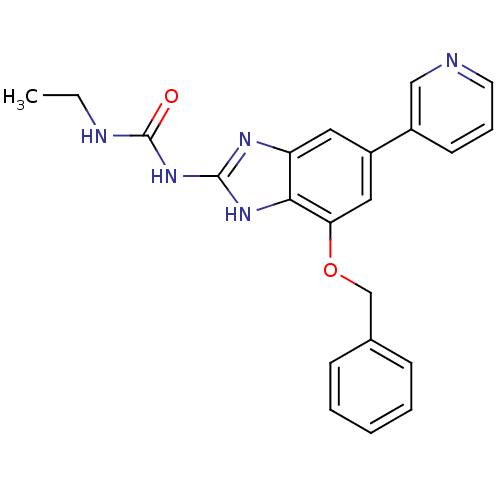 (1-[7-(benzyloxy)-5-(pyridin-3-yl)-1H-1,3-benzodiaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24602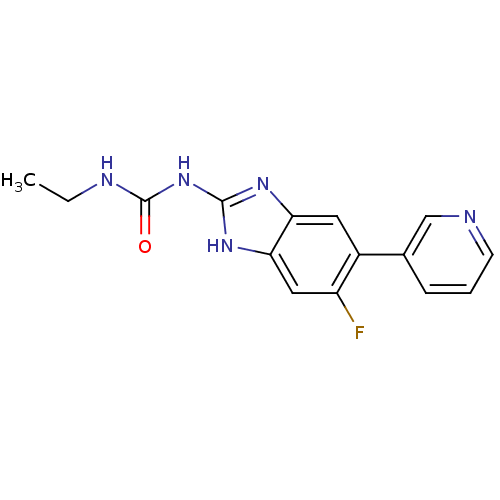 (3-ethyl-1-[6-fluoro-5-(pyridin-3-yl)-1H-1,3-benzod...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24613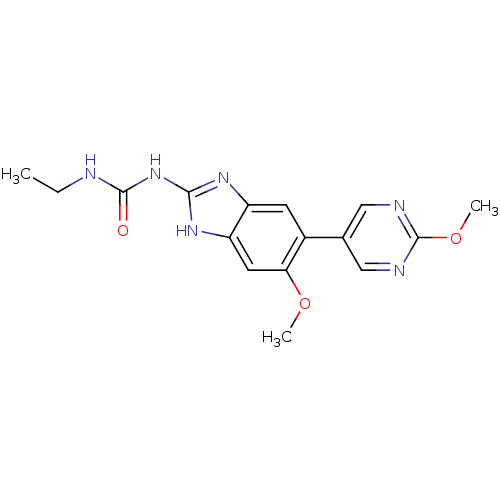 (3-ethyl-1-[6-methoxy-5-(2-methoxypyrimidin-5-yl)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24600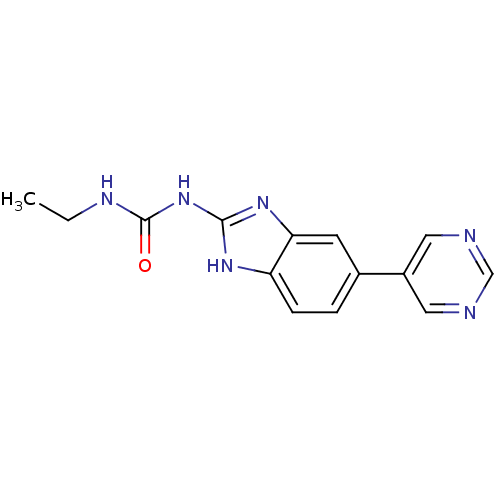 (3-ethyl-1-[5-(pyrimidin-5-yl)-1H-1,3-benzodiazol-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24610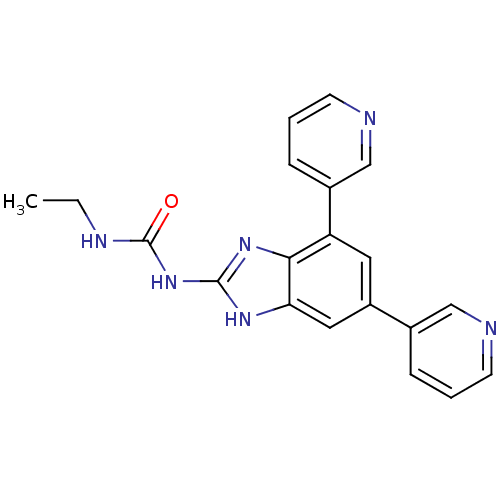 (1-[5,7-bis(pyridin-3-yl)-1H-1,3-benzodiazol-2-yl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24603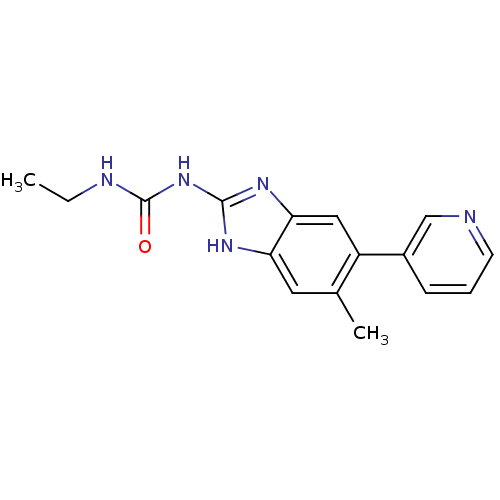 (3-ethyl-1-[6-methyl-5-(pyridin-3-yl)-1H-1,3-benzod...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24598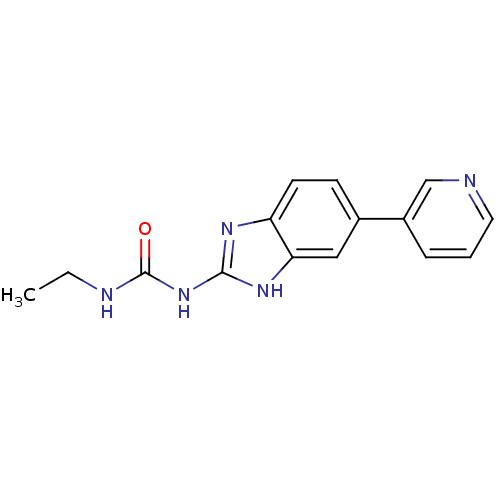 (3-ethyl-1-[5-(pyridin-3-yl)-1H-1,3-benzodiazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24605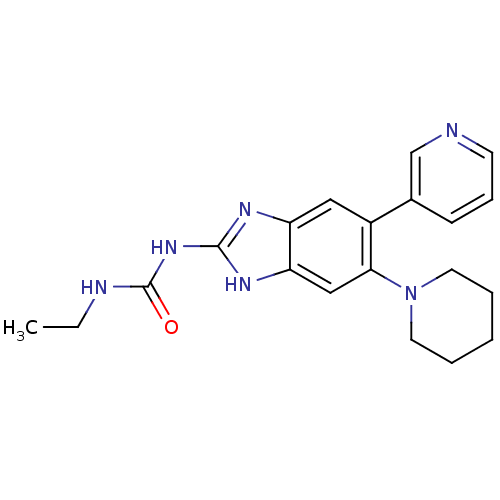 (3-ethyl-1-[6-(piperidin-1-yl)-5-(pyridin-3-yl)-1H-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24604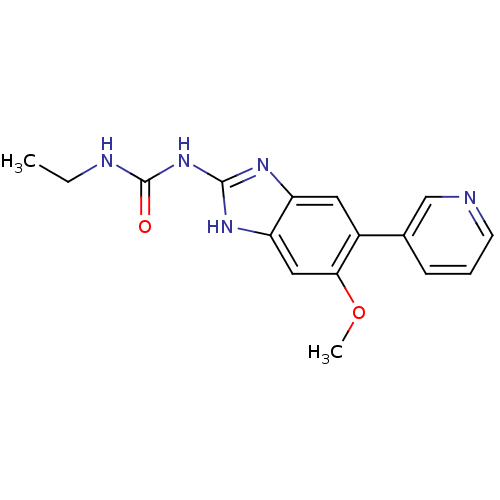 (3-ethyl-1-[6-methoxy-5-(pyridin-3-yl)-1H-1,3-benzo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24599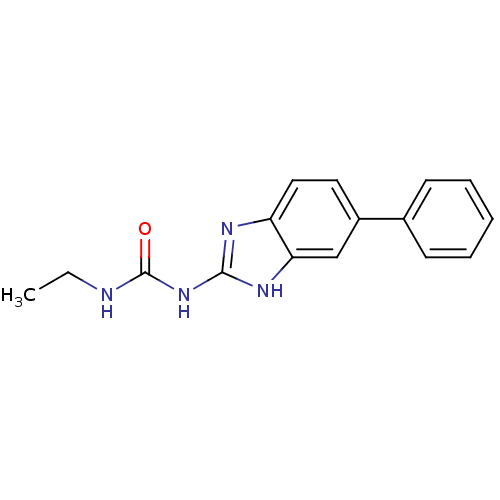 (3-ethyl-1-(5-phenyl-1H-1,3-benzodiazol-2-yl)urea |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Escherichia coli (strain K12)) | BDBM24597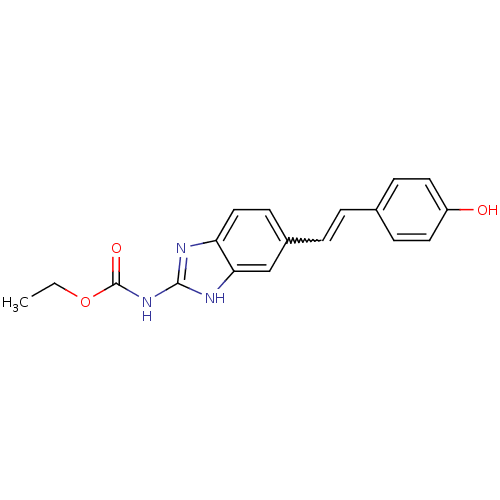 (Benzimidazole carbamate analogue, 1 | ethyl N-{5-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex | Assay Description Enzymatic hydrolysis of ATP to ADP was coupled to the conversion of NADH to NAD+. The decrease in NADH absorbance was monitored at 340 nm for 20 min ... | J Med Chem 51: 5243-63 (2008) Article DOI: 10.1021/jm800318d BindingDB Entry DOI: 10.7270/Q2J67F7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50494811 (CHEMBL3094350) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Holdings Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA topoisomerase 4 subunit ParC2/ParE2 after 60 mins by malachite green staining assay | Bioorg Med Chem Lett 23: 6598-603 (2013) Article DOI: 10.1016/j.bmcl.2013.10.058 BindingDB Entry DOI: 10.7270/Q2HD7ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50494806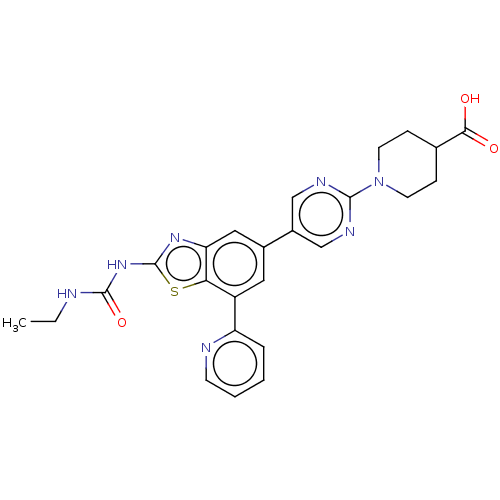 (CHEMBL3094349) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Holdings Ltd Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA topoisomerase 4 subunit ParC2/ParE2 after 60 mins by malachite green staining assay | Bioorg Med Chem Lett 23: 6598-603 (2013) Article DOI: 10.1016/j.bmcl.2013.10.058 BindingDB Entry DOI: 10.7270/Q2HD7ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50572198 (CHEMBL4873479) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase 4 assessed as reduction in decatenation using supercoiled pNO1 plasmid DNA as substrate incubated f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00345 BindingDB Entry DOI: 10.7270/Q28D012J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50572196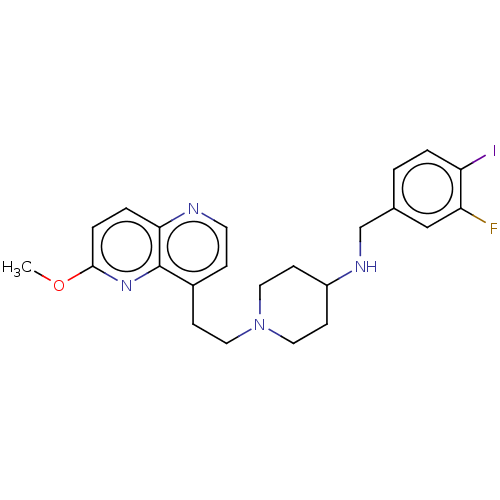 (CHEMBL4879265) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase 4 assessed as reduction in decatenation using supercoiled pNO1 plasmid DNA as substrate incubated f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00345 BindingDB Entry DOI: 10.7270/Q28D012J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50440321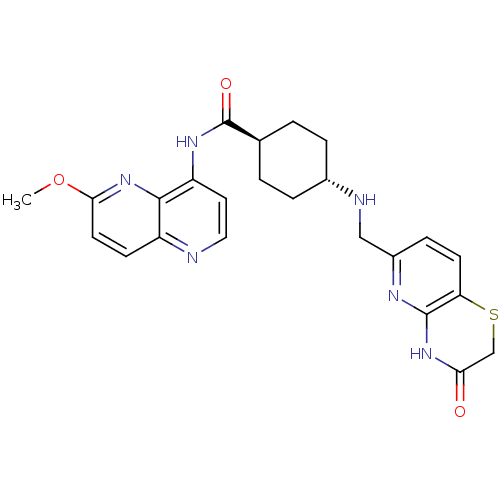 (CHEMBL2424893) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus ATCC 29213 topoisomerase-4 subunit 2GrlA/2GrlB assessed as pBR322 relaxation after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50561963 (CHEMBL4787821) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase-4 assessed as reduction in decatenation of kinetoplast DNA agarose gel electrophoresis method | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00428 BindingDB Entry DOI: 10.7270/Q2Z89H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50572197 (CHEMBL4879088) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase 4 assessed as reduction in decatenation using supercoiled pNO1 plasmid DNA as substrate incubated f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00345 BindingDB Entry DOI: 10.7270/Q28D012J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50582949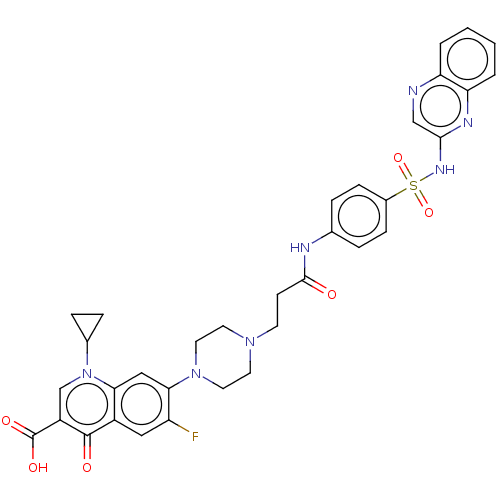 (CHEMBL5090088) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase 4 assessed as reduction in decatenation using kDNA as substrate incubated for 30 mins by fluorimetr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114021 BindingDB Entry DOI: 10.7270/Q2DZ0D6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50572195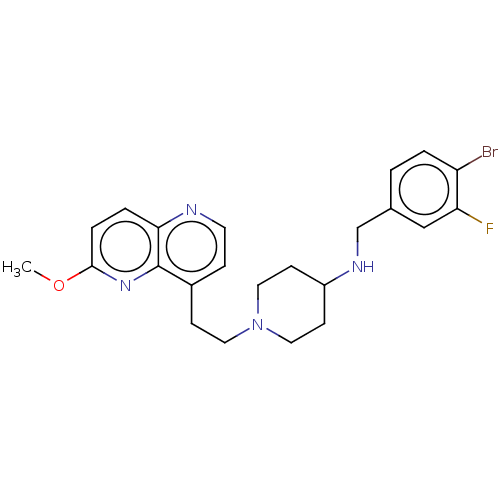 (CHEMBL4868908) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 241 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase 4 assessed as reduction in decatenation using supercoiled pNO1 plasmid DNA as substrate incubated f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00345 BindingDB Entry DOI: 10.7270/Q28D012J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50547867 (CHEMBL4793192) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus DNA topoisomerase 4 using supercoiled pNO1 plasmid DNA as substrate incubated for 30 mins by fluorescence assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00416 BindingDB Entry DOI: 10.7270/Q2JW8JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50547859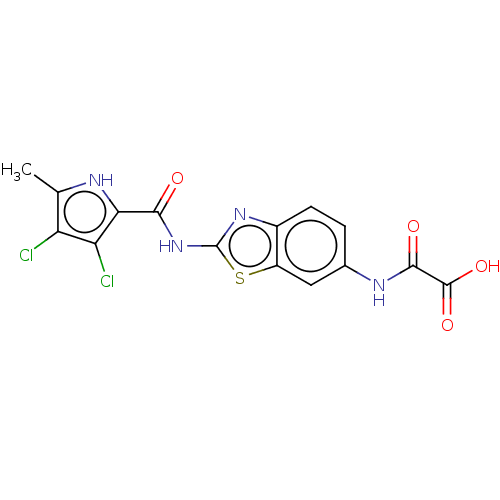 (CHEMBL4752708) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus DNA topoisomerase 4 using supercoiled pNO1 plasmid DNA as substrate incubated for 30 mins by fluorescence assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00416 BindingDB Entry DOI: 10.7270/Q2JW8JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50393503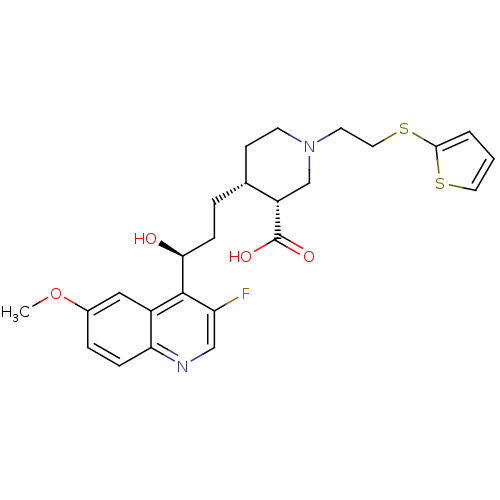 (CHEMBL2158050) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus RN4220 DNA topoisomerase 4 expressed in Escherichia coli BL21(DE3) cells using supercoiled pBR322 DNA as substrat... | J Med Chem 63: 5664-5674 (2020) Article DOI: 10.1021/acs.jmedchem.9b01738 BindingDB Entry DOI: 10.7270/Q2HD8064 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50561965 (CHEMBL4787145) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase-4 assessed as reduction in decatenation of kinetoplast DNA agarose gel electrophoresis method | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00428 BindingDB Entry DOI: 10.7270/Q2Z89H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50577588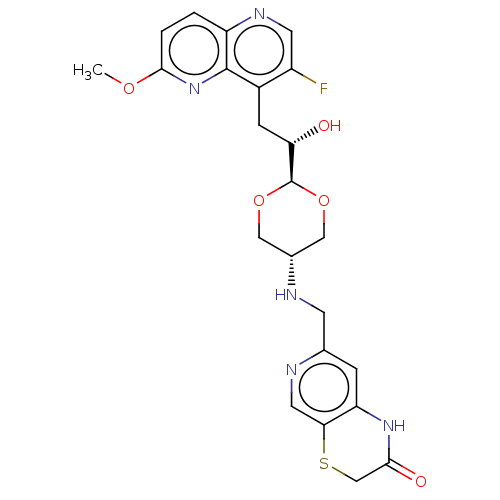 (CHEMBL4875924) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase IV decatenation activity using kDNA as substrate by ethidium bromide/bromophenol blue staining base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01250 BindingDB Entry DOI: 10.7270/Q2CN77R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50577587 (CHEMBL4869929) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase IV decatenation activity using kDNA as substrate by ethidium bromide/bromophenol blue staining base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01250 BindingDB Entry DOI: 10.7270/Q2CN77R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50582944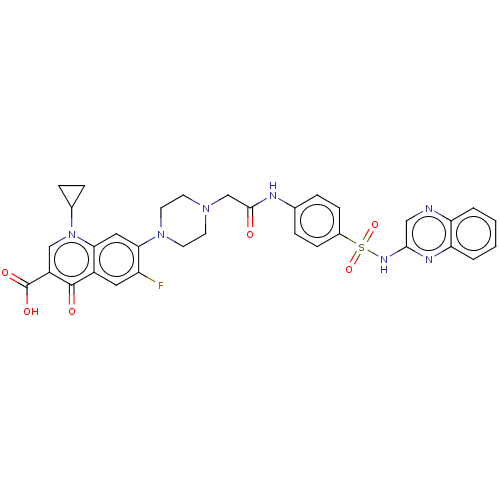 (CHEMBL5086095) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase 4 assessed as reduction in decatenation using kDNA as substrate incubated for 30 mins by fluorimetr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114021 BindingDB Entry DOI: 10.7270/Q2DZ0D6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50577579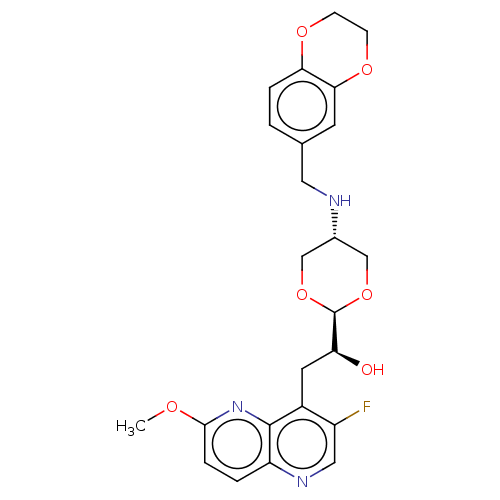 (CHEMBL4867964) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase IV decatenation activity using kDNA as substrate by ethidium bromide/bromophenol blue staining base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01250 BindingDB Entry DOI: 10.7270/Q2CN77R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50582952 (CHEMBL5087299) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase 4 assessed as reduction in decatenation using kDNA as substrate incubated for 30 mins by fluorimetr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114021 BindingDB Entry DOI: 10.7270/Q2DZ0D6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50597278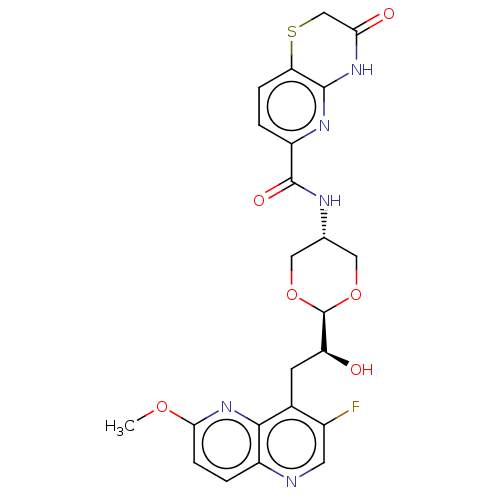 (CHEMBL5196889) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00111 BindingDB Entry DOI: 10.7270/Q2TT4W0M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50577585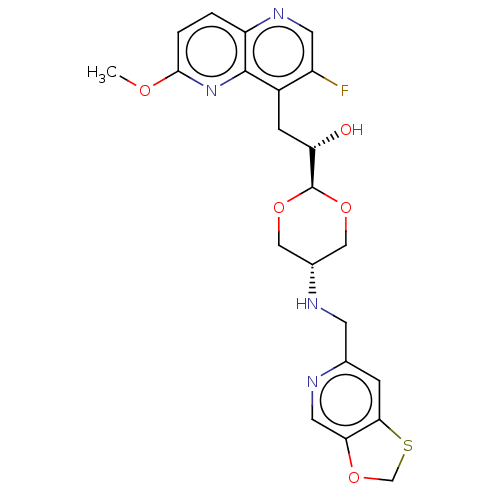 (CHEMBL4856026) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase IV decatenation activity using kDNA as substrate by ethidium bromide/bromophenol blue staining base... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01250 BindingDB Entry DOI: 10.7270/Q2CN77R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50582950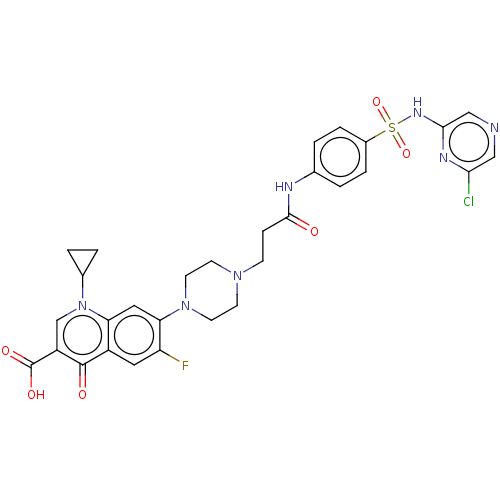 (CHEMBL5075535) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Staphylococcus aureus topoisomerase 4 assessed as reduction in decatenation using kDNA as substrate incubated for 30 mins by fluorimetr... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114021 BindingDB Entry DOI: 10.7270/Q2DZ0D6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50440332 (CHEMBL2424885) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus ATCC 29213 topoisomerase-4 subunit 2GrlA/2GrlB assessed as pBR322 relaxation after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50440323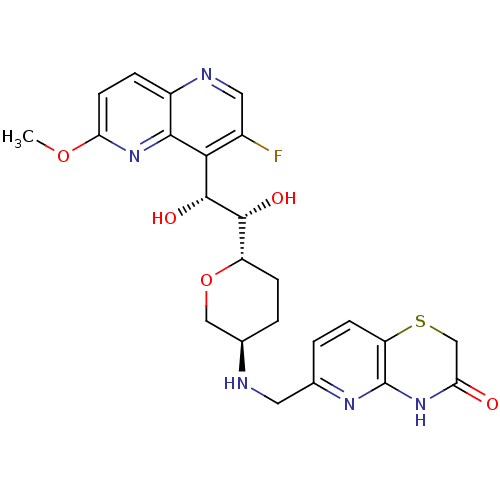 (CHEMBL2424833) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus ATCC 29213 topoisomerase-4 subunit 2GrlA/2GrlB assessed as pBR322 relaxation after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50440327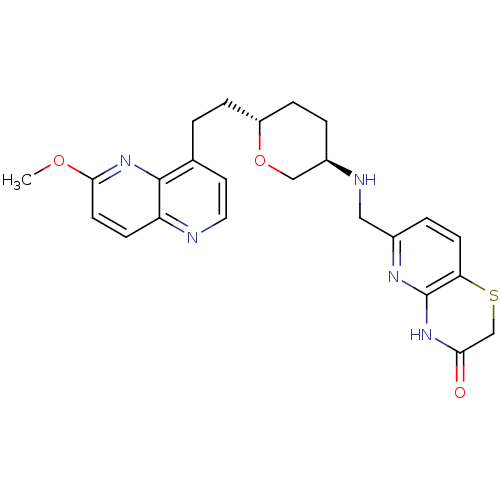 (CHEMBL2424883) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus ATCC 29213 topoisomerase-4 subunit 2GrlA/2GrlB assessed as pBR322 relaxation after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A/B (Staphylococcus aureus) | BDBM50440325 (CHEMBL2424890) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of wild type Staphylococcus aureus ATCC 29213 topoisomerase-4 subunit 2GrlA/2GrlB assessed as pBR322 relaxation after 1 hr | J Med Chem 56: 7396-415 (2013) Article DOI: 10.1021/jm400963y BindingDB Entry DOI: 10.7270/Q2HT2QRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 202 total ) | Next | Last >> |