

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004490 (CCK7 analogue | CHEMBL412238 | N-(1-Carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004483 (CCK7 analogue | CHEMBL269355) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004477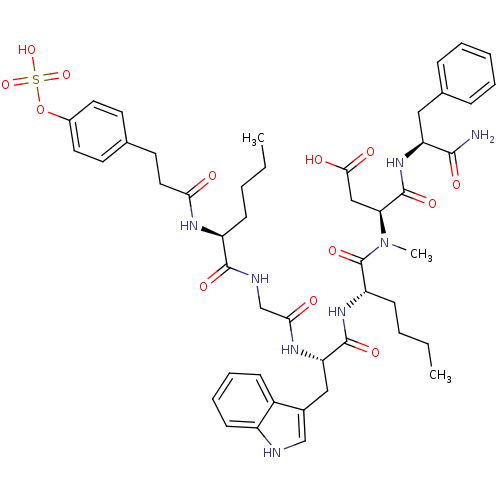 (CCK7 analogue | CHEMBL269016 | N-(1-Carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50004477 (CCK7 analogue | CHEMBL269016 | N-(1-Carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004474 (CCK7 analogue | CHEMBL316949 | N-(1-Carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004480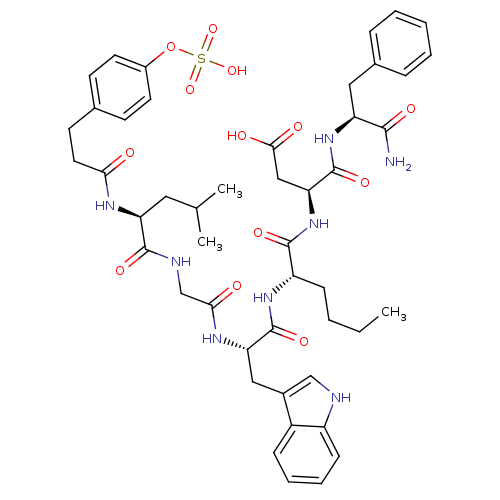 (CCK7 analogue | CHEMBL316944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004480 (CCK7 analogue | CHEMBL316944) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004474 (CCK7 analogue | CHEMBL316949 | N-(1-Carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004474 (CCK7 analogue | CHEMBL316949 | N-(1-Carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50421964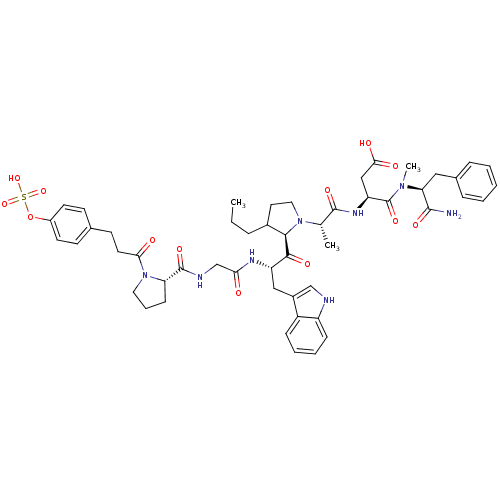 (CHEMBL2310858) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50016425 ((S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxyca...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]propionyl-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50004482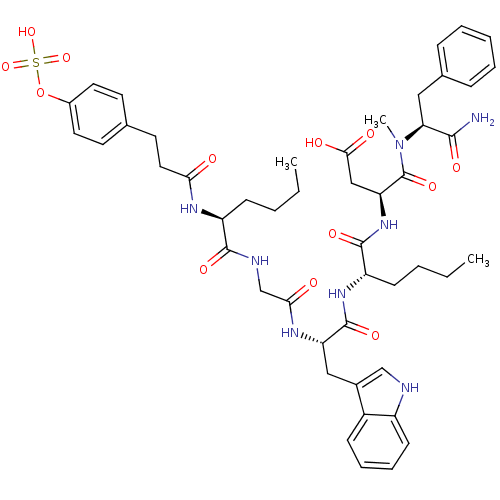 (CCK7 analogue | CHEMBL263742 | N-(1-Carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004482 (CCK7 analogue | CHEMBL263742 | N-(1-Carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004485 (CCK7 analogue | CHEMBL98631) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004475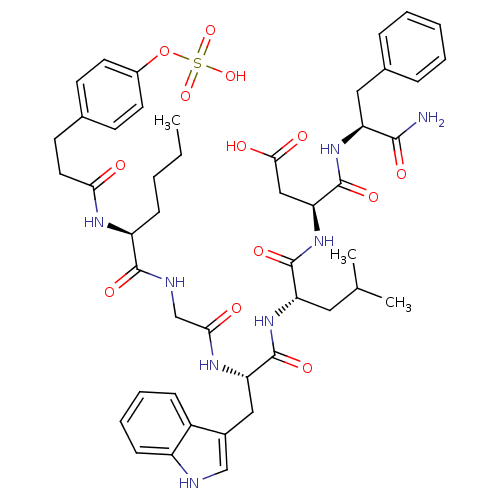 (CCK7 analogue | CHEMBL439591 | N-(1-Carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50421963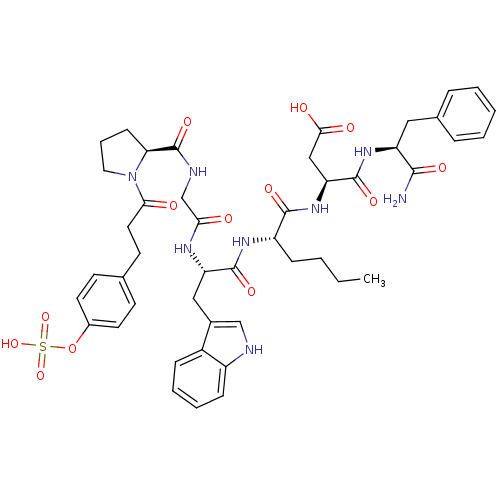 (CHEMBL2310856) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50421963 (CHEMBL2310856) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004489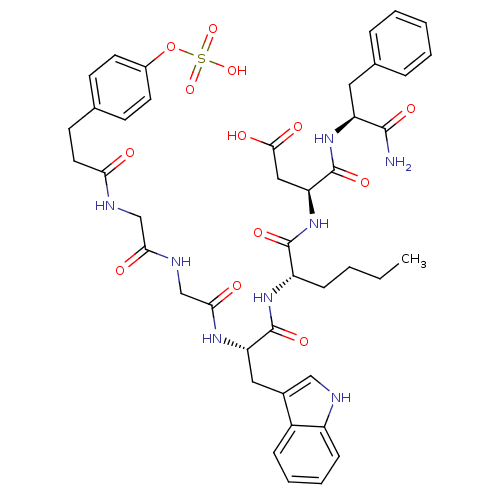 (CCK7 analogue | CHEMBL318010) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040523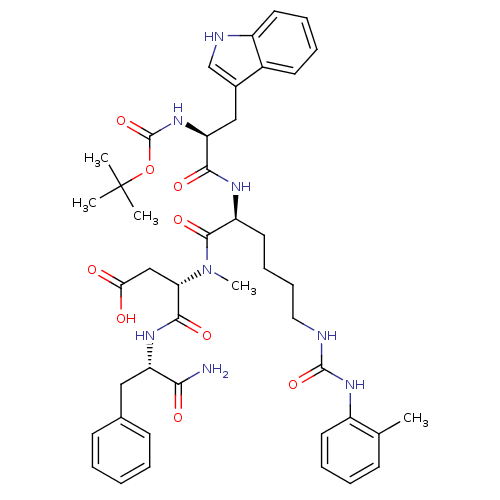 ((S)-3-{[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50007925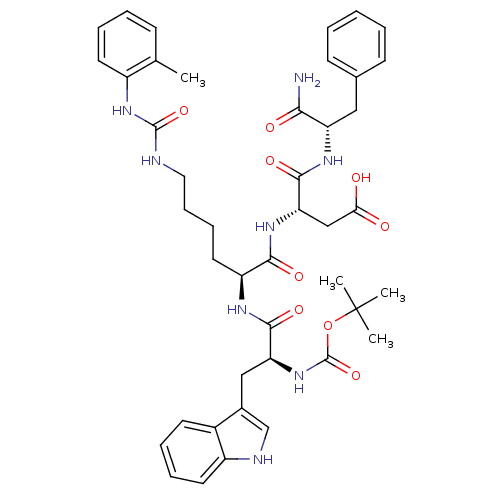 ((S)-3-[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50004483 (CCK7 analogue | CHEMBL269355) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040523 ((S)-3-{[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004475 (CCK7 analogue | CHEMBL439591 | N-(1-Carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004475 (CCK7 analogue | CHEMBL439591 | N-(1-Carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040520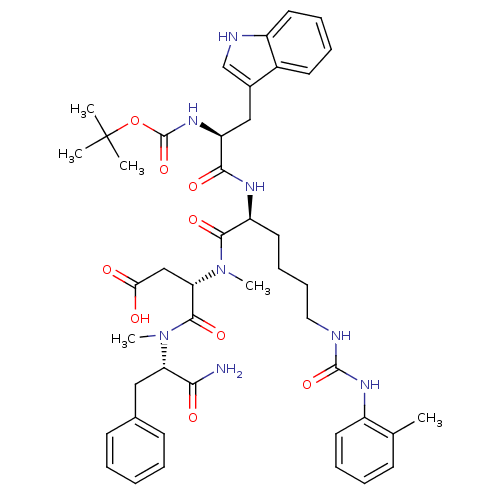 ((S)-3-{[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50004481 (CCK7 analogue | CHEMBL99499) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004489 (CCK7 analogue | CHEMBL318010) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50421964 (CHEMBL2310858) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004478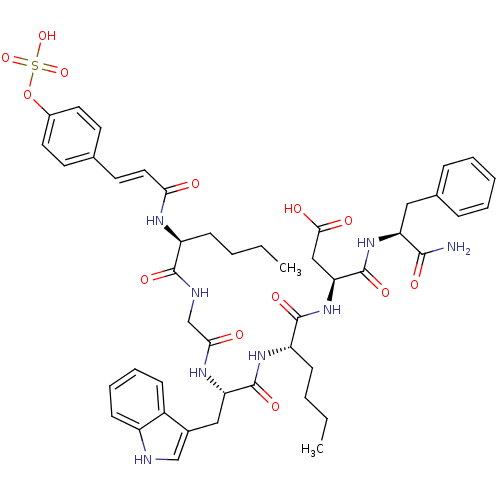 (CCK7 analogue | CHEMBL98332) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004491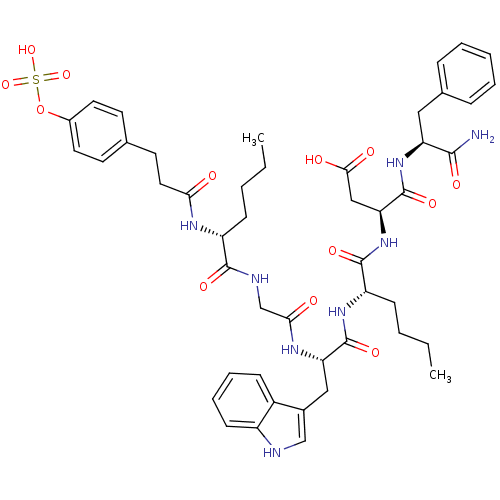 (CCK7 analogue | CHEMBL98446) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its binding affinity towards Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004481 (CCK7 analogue | CHEMBL99499) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004482 (CCK7 analogue | CHEMBL263742 | N-(1-Carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040524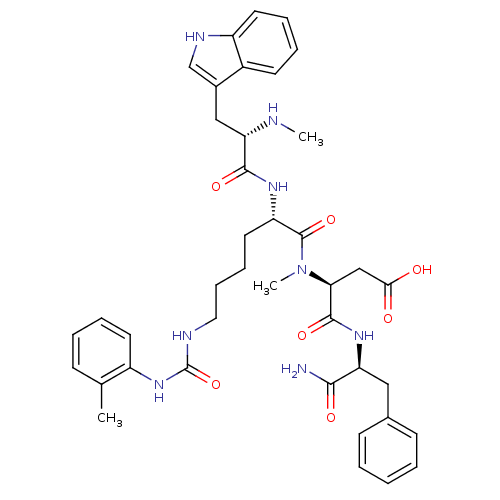 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{[(S)-2-[...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004492 (CCK7 analogue | CHEMBL98720) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004486 (CCK7 analogue | CHEMBL316709) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50455609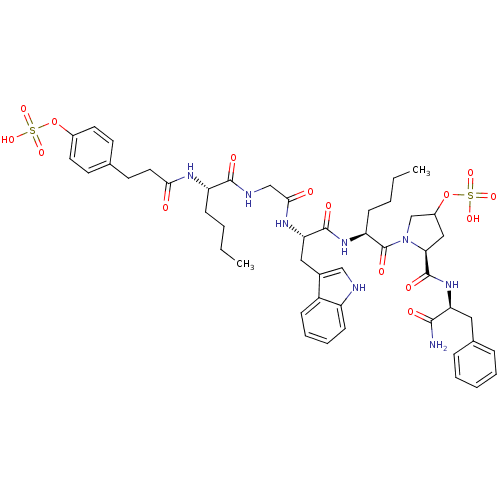 (CHEMBL2310857) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040522 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-[(S)-2-[(...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040518 (3-{2-[2-(2-{2-[2-(tert-Butoxycarbonyl-methyl-amino...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]propionyl-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004478 (CCK7 analogue | CHEMBL98332) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004488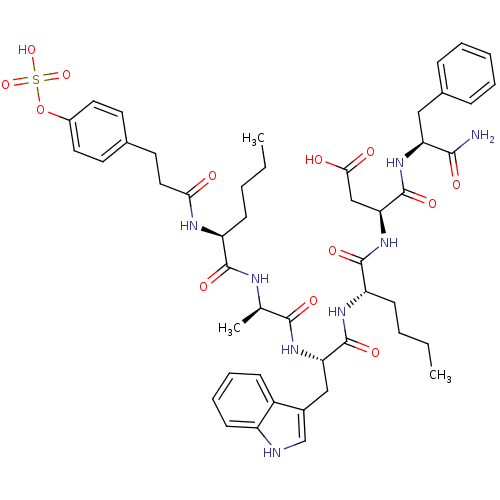 (CCK7 analogue | CHEMBL414793) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50041659 ((S)-3-[(S)-2-[2-tert-Butoxycarbonylamino-3-(1H-ind...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding for half maximal inhibition of [125I]- Bolton-Hunter-CCK-8 in guinea pig pancreas | J Med Chem 37: 1569-71 (1994) BindingDB Entry DOI: 10.7270/Q2JD4XF7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004487 (CCK7 analogue | CHEMBL408286) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040528 ((S)-3-[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004486 (CCK7 analogue | CHEMBL316709) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004488 (CCK7 analogue | CHEMBL414793) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040521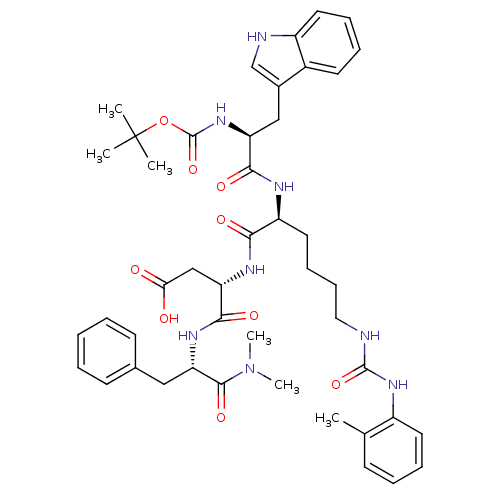 ((S)-3-[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004492 (CCK7 analogue | CHEMBL98720) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004490 (CCK7 analogue | CHEMBL412238 | N-(1-Carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [125I]Bolton-Hunter-CCK-8 binding to Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 37: 630-5 (1994) BindingDB Entry DOI: 10.7270/Q23X878C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50455609 (CHEMBL2310857) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type B receptor of guinea pig cortex | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004490 (CCK7 analogue | CHEMBL412238 | N-(1-Carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Evaluated in vitro for its binding affinity towards cholecystokinin type A receptor of guinea pig pancreas | J Med Chem 35: 2919-28 (1992) BindingDB Entry DOI: 10.7270/Q2W66JRH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 83 total ) | Next | Last >> |