 Found 115 hits with Last Name = 'nishio' and Initial = 'k'
Found 115 hits with Last Name = 'nishio' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381600
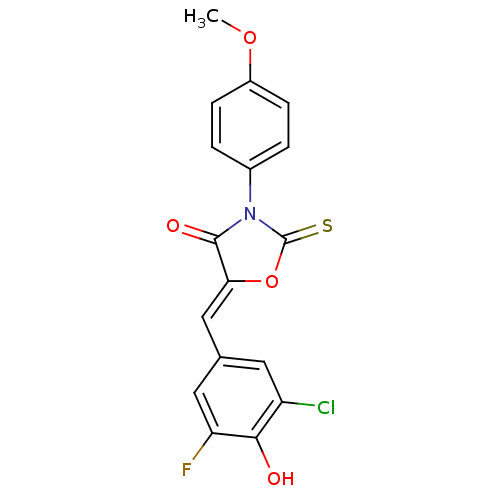
(CHEMBL2018254)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2cc(F)c(O)c(Cl)c2)C1=O Show InChI InChI=1S/C17H11ClFNO4S/c1-23-11-4-2-10(3-5-11)20-16(22)14(24-17(20)25)8-9-6-12(18)15(21)13(19)7-9/h2-8,21H,1H3/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305335

(7-hydroxy-4-((6-methylpyridin-2-ylthio)methyl)-2H-...)Show InChI InChI=1S/C16H13NO3S/c1-10-3-2-4-15(17-10)21-9-11-7-16(19)20-14-8-12(18)5-6-13(11)14/h2-8,18H,9H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381572
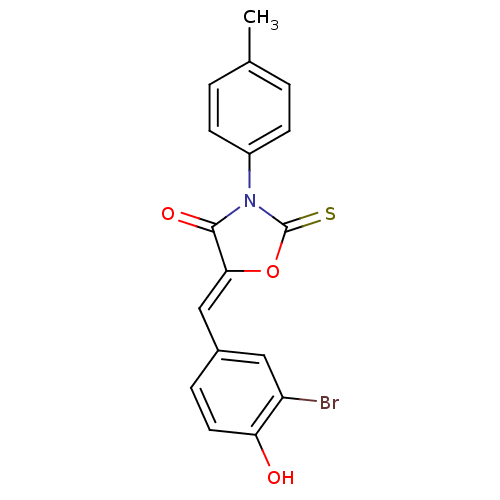
(CHEMBL2018141)Show SMILES Cc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO3S/c1-10-2-5-12(6-3-10)19-16(21)15(22-17(19)23)9-11-4-7-14(20)13(18)8-11/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381596
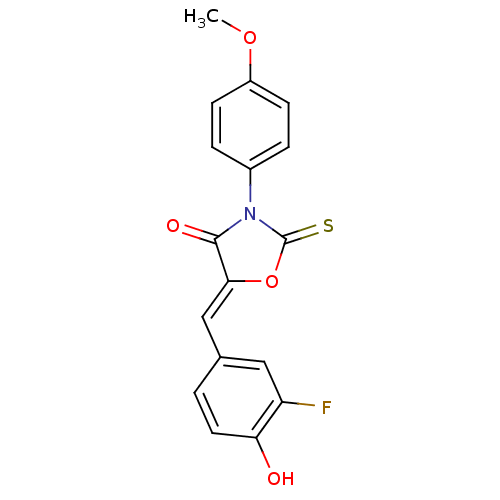
(CHEMBL2018249)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)c(F)c2)C1=O Show InChI InChI=1S/C17H12FNO4S/c1-22-12-5-3-11(4-6-12)19-16(21)15(23-17(19)24)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381570
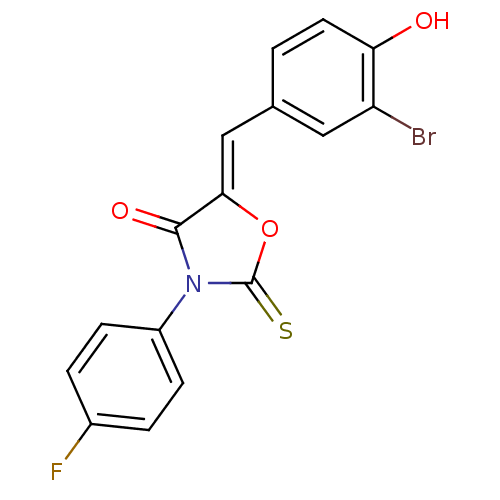
(CHEMBL2018139)Show SMILES Oc1ccc(\C=C2/OC(=S)N(C2=O)c2ccc(F)cc2)cc1Br Show InChI InChI=1S/C16H9BrFNO3S/c17-12-7-9(1-6-13(12)20)8-14-15(21)19(16(23)22-14)11-4-2-10(18)3-5-11/h1-8,20H/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381573

(CHEMBL2018142)Show SMILES Oc1ccc(\C=C2/OC(=S)N(C2=O)c2ccc(cc2)C(F)(F)F)cc1Br Show InChI InChI=1S/C17H9BrF3NO3S/c18-12-7-9(1-6-13(12)23)8-14-15(24)22(16(26)25-14)11-4-2-10(3-5-11)17(19,20)21/h1-8,23H/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381564
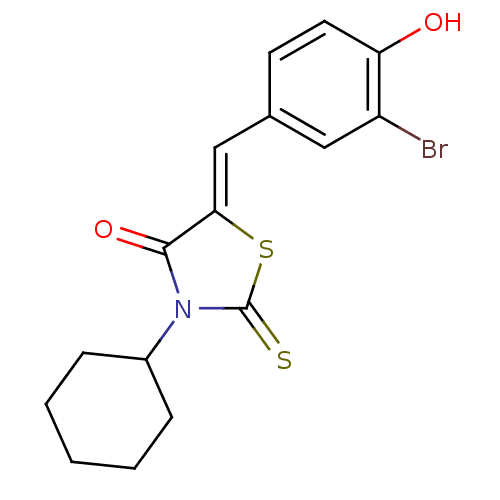
(CHEMBL2018131)Show InChI InChI=1S/C16H16BrNO2S2/c17-12-8-10(6-7-13(12)19)9-14-15(20)18(16(21)22-14)11-4-2-1-3-5-11/h6-9,11,19H,1-5H2/b14-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305325
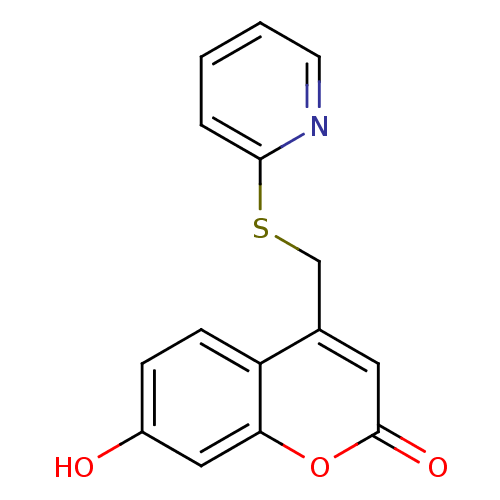
(7-hydroxy-4-((pyridin-2-ylthio)methyl)-2H-chromen-...)Show InChI InChI=1S/C15H11NO3S/c17-11-4-5-12-10(7-15(18)19-13(12)8-11)9-20-14-3-1-2-6-16-14/h1-8,17H,9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381586
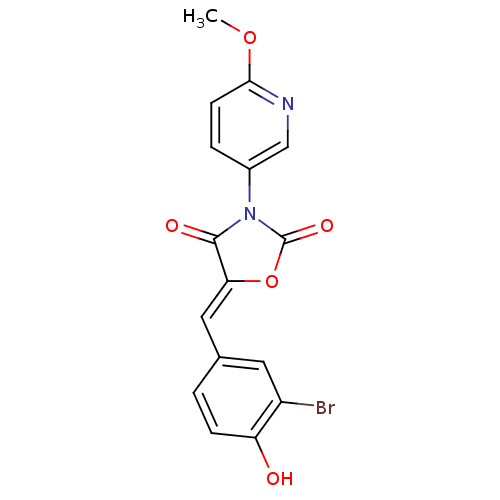
(CHEMBL2018238)Show SMILES COc1ccc(cn1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C16H11BrN2O5/c1-23-14-5-3-10(8-18-14)19-15(21)13(24-16(19)22)7-9-2-4-12(20)11(17)6-9/h2-8,20H,1H3/b13-7- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381590

(CHEMBL2018242)Show SMILES CN(C)c1ccc(cn1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H14BrN3O4/c1-20(2)15-6-4-11(9-19-15)21-16(23)14(25-17(21)24)8-10-3-5-13(22)12(18)7-10/h3-9,22H,1-2H3/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381597
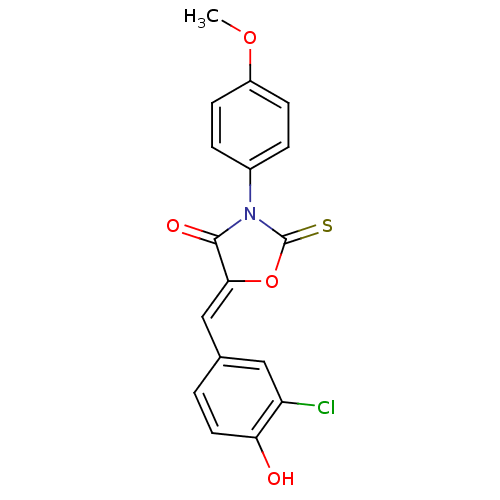
(CHEMBL2018250)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)c(Cl)c2)C1=O Show InChI InChI=1S/C17H12ClNO4S/c1-22-12-5-3-11(4-6-12)19-16(21)15(23-17(19)24)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381598
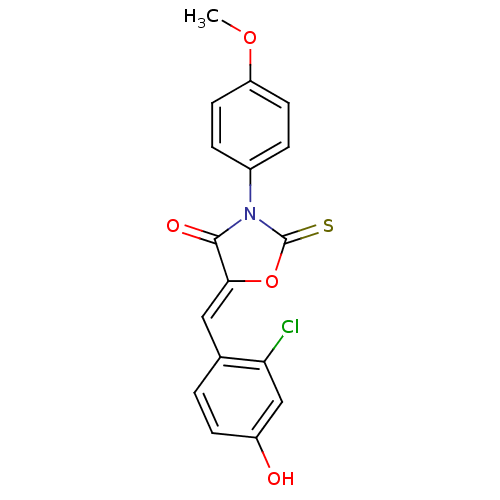
(CHEMBL2018251)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)cc2Cl)C1=O Show InChI InChI=1S/C17H12ClNO4S/c1-22-13-6-3-11(4-7-13)19-16(21)15(23-17(19)24)8-10-2-5-12(20)9-14(10)18/h2-9,20H,1H3/b15-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381559

(CHEMBL2018253)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2cc(F)c(O)c(F)c2)C1=O Show InChI InChI=1S/C17H11F2NO4S/c1-23-11-4-2-10(3-5-11)20-16(22)14(24-17(20)25)8-9-6-12(18)15(21)13(19)7-9/h2-8,21H,1H3/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381566
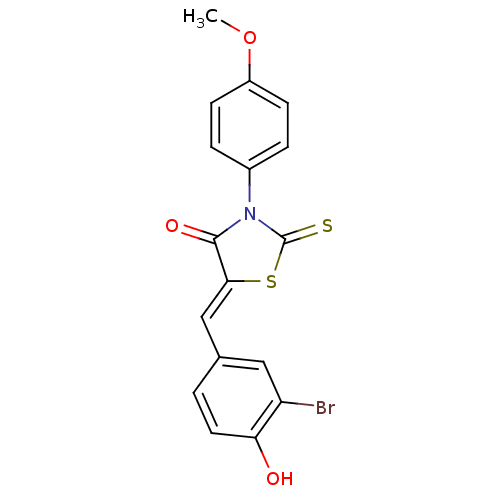
(CHEMBL2018135)Show InChI InChI=1S/C16H10BrNO3S/c17-12-8-10(6-7-13(12)19)9-14-15(20)18(16(22)21-14)11-4-2-1-3-5-11/h1-9,19H/b14-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50360767
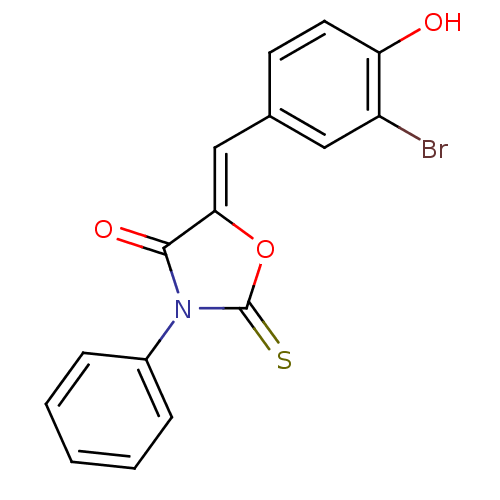
(CHEMBL1934493)Show SMILES COc1ccc(cc1)N1C(=S)S\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO3S2/c1-22-12-5-3-11(4-6-12)19-16(21)15(24-17(19)23)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 in human testes homogenate |
Bioorg Med Chem Lett 22: 504-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.095
BindingDB Entry DOI: 10.7270/Q2BG2PFD |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381571
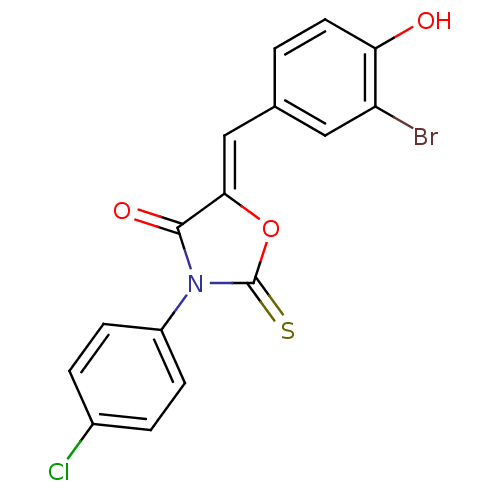
(CHEMBL2018140)Show SMILES Oc1ccc(\C=C2/OC(=S)N(C2=O)c2ccc(Cl)cc2)cc1Br Show InChI InChI=1S/C16H9BrClNO3S/c17-12-7-9(1-6-13(12)20)8-14-15(21)19(16(23)22-14)11-4-2-10(18)3-5-11/h1-8,20H/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381567
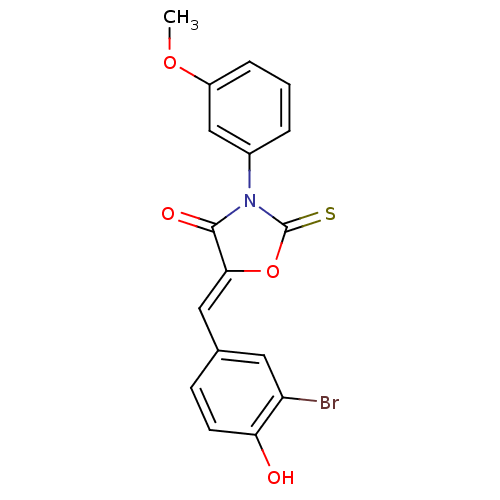
(CHEMBL2018136)Show SMILES COc1cccc(c1)N1C(=S)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO4S/c1-22-12-4-2-3-11(9-12)19-16(21)15(23-17(19)24)8-10-5-6-14(20)13(18)7-10/h2-9,20H,1H3/b15-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305336
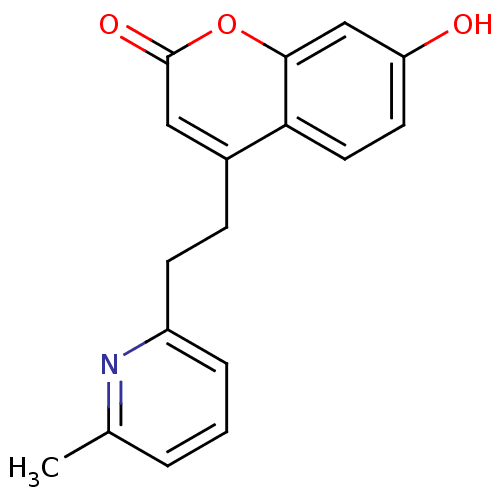
(7-hydroxy-4-(2-(6-methylpyridin-2-yl)ethyl)-2H-chr...)Show InChI InChI=1S/C17H15NO3/c1-11-3-2-4-13(18-11)6-5-12-9-17(20)21-16-10-14(19)7-8-15(12)16/h2-4,7-10,19H,5-6H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305330
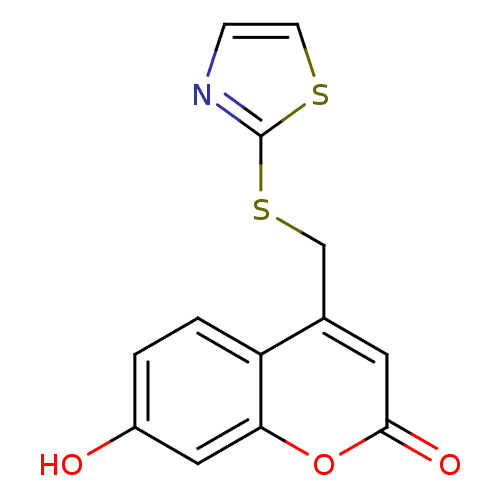
(7-hydroxy-4-((thiazol-2-ylthio)methyl)-2H-chromen-...)Show InChI InChI=1S/C13H9NO3S2/c15-9-1-2-10-8(5-12(16)17-11(10)6-9)7-19-13-14-3-4-18-13/h1-6,15H,7H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381569
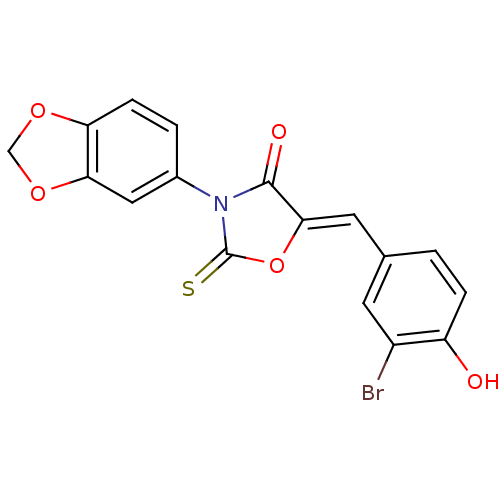
(CHEMBL2018138)Show SMILES Oc1ccc(\C=C2/OC(=S)N(C2=O)c2ccc3OCOc3c2)cc1Br Show InChI InChI=1S/C17H10BrNO5S/c18-11-5-9(1-3-12(11)20)6-15-16(21)19(17(25)24-15)10-2-4-13-14(7-10)23-8-22-13/h1-7,20H,8H2/b15-6- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381599

(CHEMBL2018252)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2cc(Cl)c(O)c(Cl)c2)C1=O Show InChI InChI=1S/C17H11Cl2NO4S/c1-23-11-4-2-10(3-5-11)20-16(22)14(24-17(20)25)8-9-6-12(18)15(21)13(19)7-9/h2-8,21H,1H3/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50360766
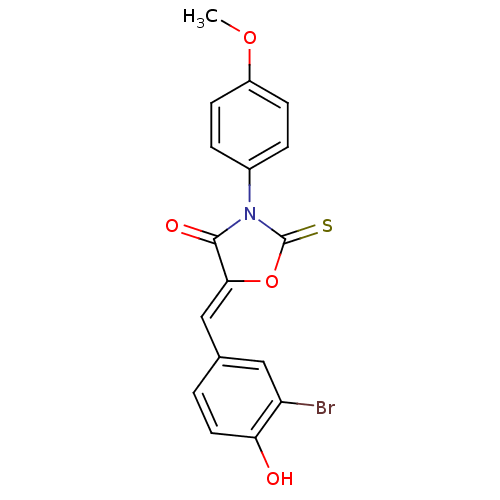
(CHEMBL1934492)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO4S/c1-22-12-5-3-11(4-6-12)19-16(21)15(23-17(19)24)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50360766

(CHEMBL1934492)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO4S/c1-22-12-5-3-11(4-6-12)19-16(21)15(23-17(19)24)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells assessed as conversion of 4-androstene-3,17-dione to testosterone |
Bioorg Med Chem Lett 22: 504-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.095
BindingDB Entry DOI: 10.7270/Q2BG2PFD |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50360767

(CHEMBL1934493)Show SMILES COc1ccc(cc1)N1C(=S)S\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO3S2/c1-22-12-5-3-11(4-6-12)19-16(21)15(24-17(19)23)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells assessed as conversion of 4-androstene-3,17-dione to testosterone |
Bioorg Med Chem Lett 22: 504-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.095
BindingDB Entry DOI: 10.7270/Q2BG2PFD |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50360767

(CHEMBL1934493)Show SMILES COc1ccc(cc1)N1C(=S)S\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO3S2/c1-22-12-5-3-11(4-6-12)19-16(21)15(24-17(19)23)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381574
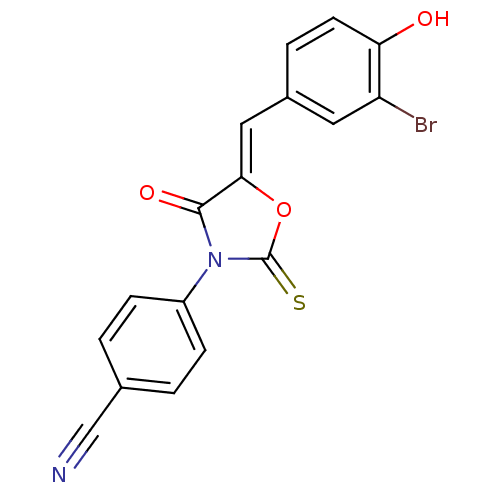
(CHEMBL2018223)Show SMILES Oc1ccc(\C=C2/OC(=S)N(C2=O)c2ccc(cc2)C#N)cc1Br Show InChI InChI=1S/C17H9BrN2O3S/c18-13-7-11(3-6-14(13)21)8-15-16(22)20(17(24)23-15)12-4-1-10(9-19)2-5-12/h1-8,21H/b15-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381589
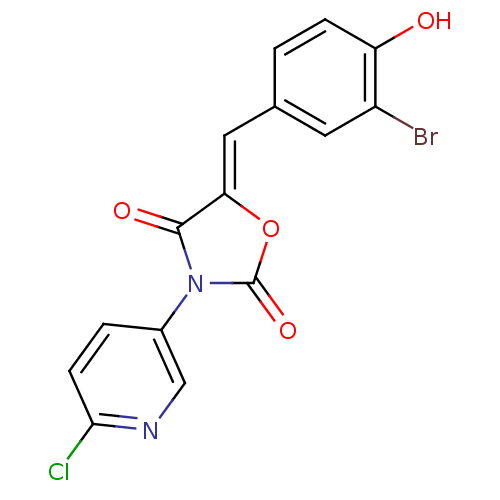
(CHEMBL2018241)Show SMILES Oc1ccc(\C=C2/OC(=O)N(C2=O)c2ccc(Cl)nc2)cc1Br Show InChI InChI=1S/C15H8BrClN2O4/c16-10-5-8(1-3-11(10)20)6-12-14(21)19(15(22)23-12)9-2-4-13(17)18-7-9/h1-7,20H/b12-6- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305320
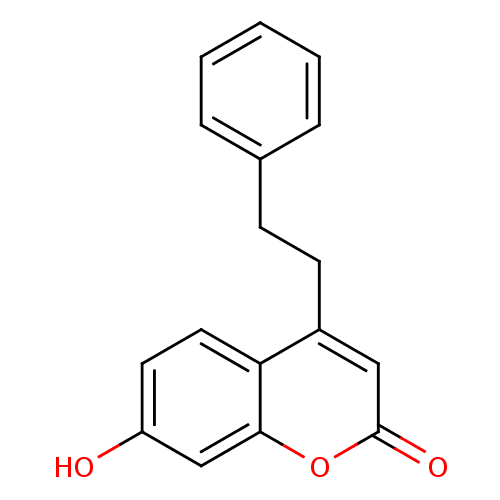
(7-hydroxy-4-phenethyl-2H-chromen-2-one | CHEMBL592...)Show InChI InChI=1S/C17H14O3/c18-14-8-9-15-13(10-17(19)20-16(15)11-14)7-6-12-4-2-1-3-5-12/h1-5,8-11,18H,6-7H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381594
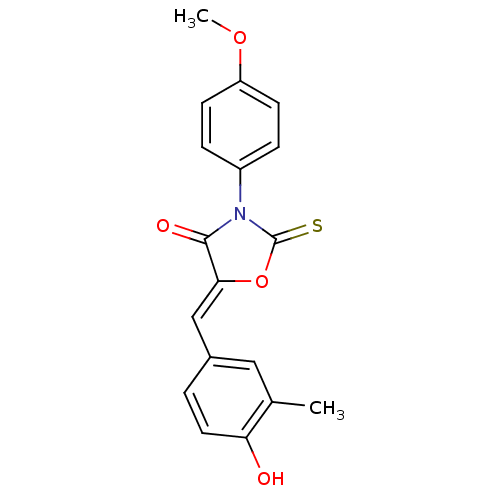
(CHEMBL2018247)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)c(C)c2)C1=O Show InChI InChI=1S/C18H15NO4S/c1-11-9-12(3-8-15(11)20)10-16-17(21)19(18(24)23-16)13-4-6-14(22-2)7-5-13/h3-10,20H,1-2H3/b16-10- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381588
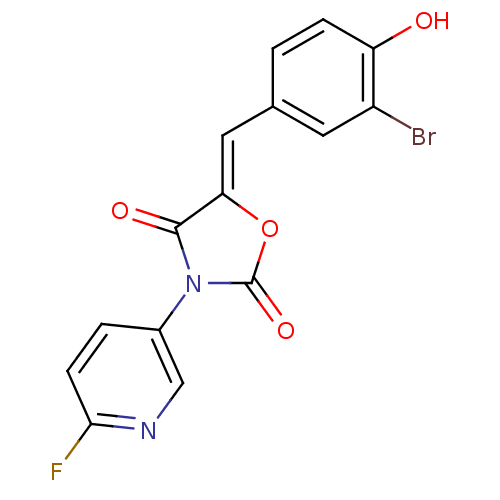
(CHEMBL2018240)Show SMILES Oc1ccc(\C=C2/OC(=O)N(C2=O)c2ccc(F)nc2)cc1Br Show InChI InChI=1S/C15H8BrFN2O4/c16-10-5-8(1-3-11(10)20)6-12-14(21)19(15(22)23-12)9-2-4-13(17)18-7-9/h1-7,20H/b12-6- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381558
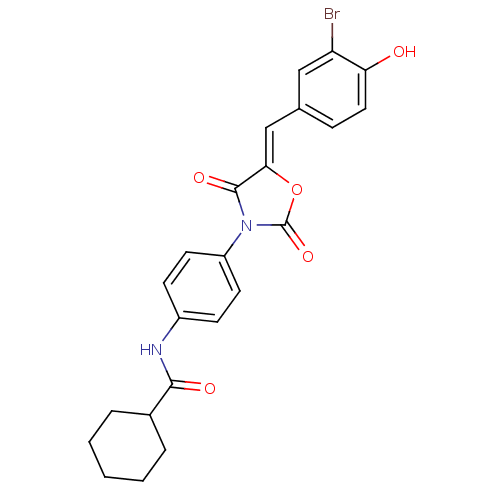
(CHEMBL2018232)Show SMILES Oc1ccc(\C=C2/OC(=O)N(C2=O)c2ccc(NC(=O)C3CCCCC3)cc2)cc1Br Show InChI InChI=1S/C23H21BrN2O5/c24-18-12-14(6-11-19(18)27)13-20-22(29)26(23(30)31-20)17-9-7-16(8-10-17)25-21(28)15-4-2-1-3-5-15/h6-13,15,27H,1-5H2,(H,25,28)/b20-13- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381585

(CHEMBL2018237)Show InChI InChI=1S/C15H9BrN2O4/c16-11-6-9(3-4-12(11)19)7-13-14(20)18(15(21)22-13)10-2-1-5-17-8-10/h1-8,19H/b13-7- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50360765
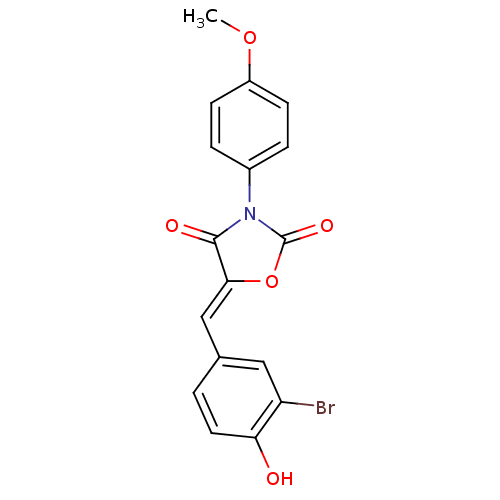
(CHEMBL1934491)Show SMILES COc1ccc(cc1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO5/c1-23-12-5-3-11(4-6-12)19-16(21)15(24-17(19)22)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50360765

(CHEMBL1934491)Show SMILES COc1ccc(cc1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO5/c1-23-12-5-3-11(4-6-12)19-16(21)15(24-17(19)22)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells assessed as conversion of 4-androstene-3,17-dione to testosterone |
Bioorg Med Chem Lett 22: 504-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.095
BindingDB Entry DOI: 10.7270/Q2BG2PFD |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381575
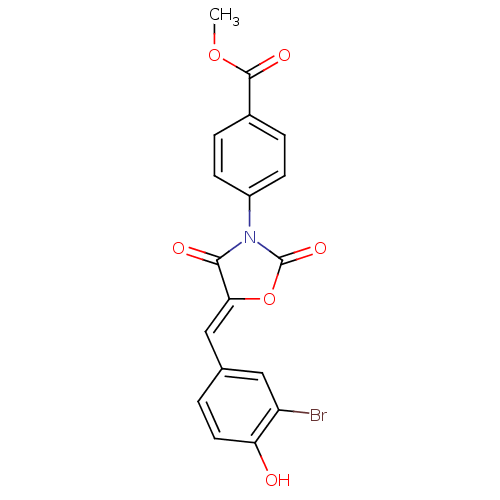
(CHEMBL2018225)Show SMILES COC(=O)c1ccc(cc1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C18H12BrNO6/c1-25-17(23)11-3-5-12(6-4-11)20-16(22)15(26-18(20)24)9-10-2-7-14(21)13(19)8-10/h2-9,21H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381580
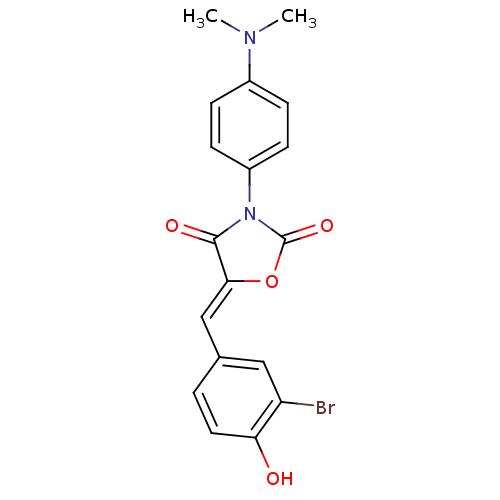
(CHEMBL2018230)Show SMILES CN(C)c1ccc(cc1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C18H15BrN2O4/c1-20(2)12-4-6-13(7-5-12)21-17(23)16(25-18(21)24)10-11-3-8-15(22)14(19)9-11/h3-10,22H,1-2H3/b16-10- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305338
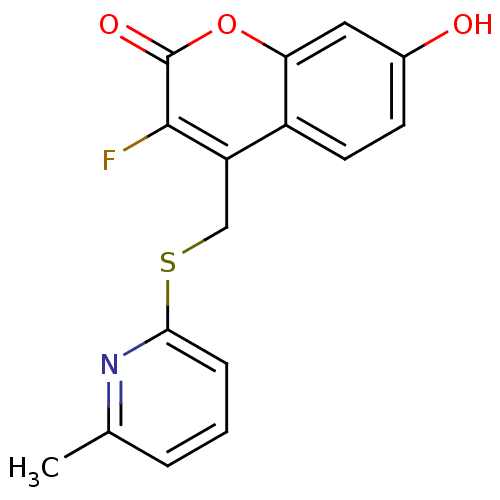
(3-fluoro-7-hydroxy-4-((6-methylpyridin-2-ylthio)me...)Show InChI InChI=1S/C16H12FNO3S/c1-9-3-2-4-14(18-9)22-8-12-11-6-5-10(19)7-13(11)21-16(20)15(12)17/h2-7,19H,8H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305316
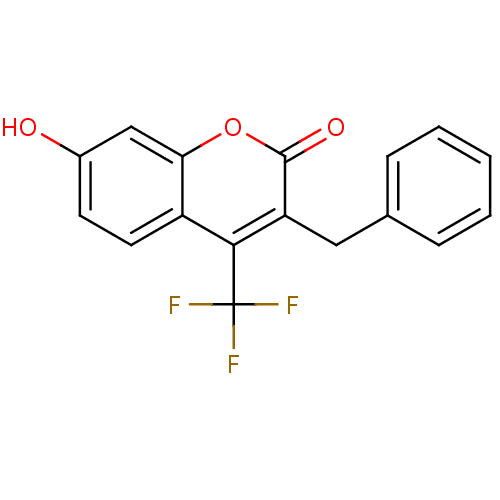
(3-benzyl-7-hydroxy-4-(trifluoromethyl)-2H-chromen-...)Show InChI InChI=1S/C17H11F3O3/c18-17(19,20)15-12-7-6-11(21)9-14(12)23-16(22)13(15)8-10-4-2-1-3-5-10/h1-7,9,21H,8H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Mus musculus) | BDBM50360767

(CHEMBL1934493)Show SMILES COc1ccc(cc1)N1C(=S)S\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO3S2/c1-22-12-5-3-11(4-6-12)19-16(21)15(24-17(19)23)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 in mouse testes homogenate |
Bioorg Med Chem Lett 22: 504-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.095
BindingDB Entry DOI: 10.7270/Q2BG2PFD |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381578
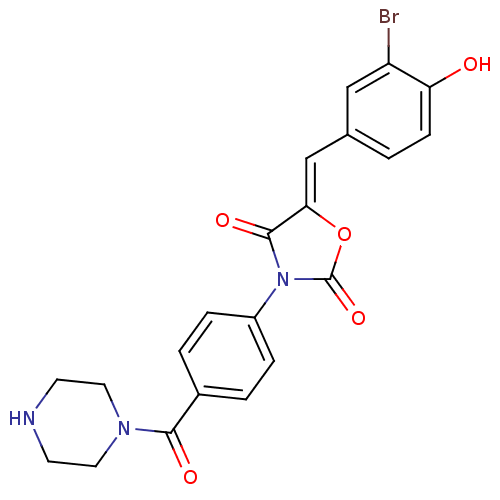
(CHEMBL2018228)Show SMILES Oc1ccc(\C=C2/OC(=O)N(C2=O)c2ccc(cc2)C(=O)N2CCNCC2)cc1Br Show InChI InChI=1S/C21H18BrN3O5/c22-16-11-13(1-6-17(16)26)12-18-20(28)25(21(29)30-18)15-4-2-14(3-5-15)19(27)24-9-7-23-8-10-24/h1-6,11-12,23,26H,7-10H2/b18-12- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381602
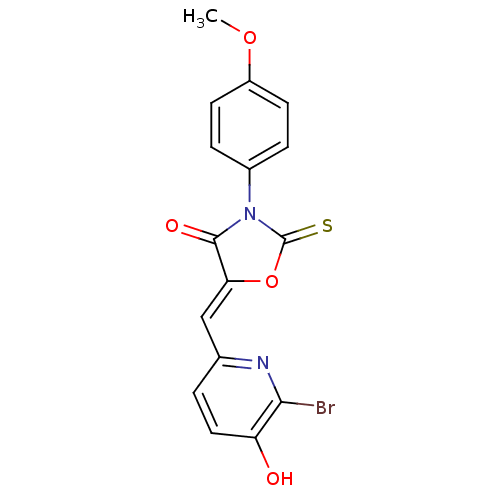
(CHEMBL2018260)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)c(Br)n2)C1=O Show InChI InChI=1S/C16H11BrN2O4S/c1-22-11-5-3-10(4-6-11)19-15(21)13(23-16(19)24)8-9-2-7-12(20)14(17)18-9/h2-8,20H,1H3/b13-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305339
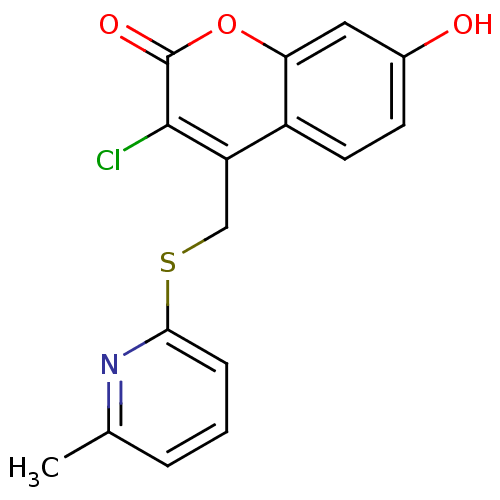
(3-chloro-7-hydroxy-4-((6-methylpyridin-2-ylthio)me...)Show InChI InChI=1S/C16H12ClNO3S/c1-9-3-2-4-14(18-9)22-8-12-11-6-5-10(19)7-13(11)21-16(20)15(12)17/h2-7,19H,8H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381576
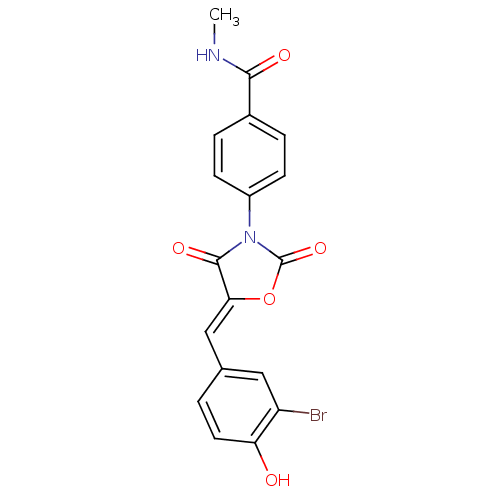
(CHEMBL2018226)Show SMILES CNC(=O)c1ccc(cc1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C18H13BrN2O5/c1-20-16(23)11-3-5-12(6-4-11)21-17(24)15(26-18(21)25)9-10-2-7-14(22)13(19)8-10/h2-9,22H,1H3,(H,20,23)/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381581
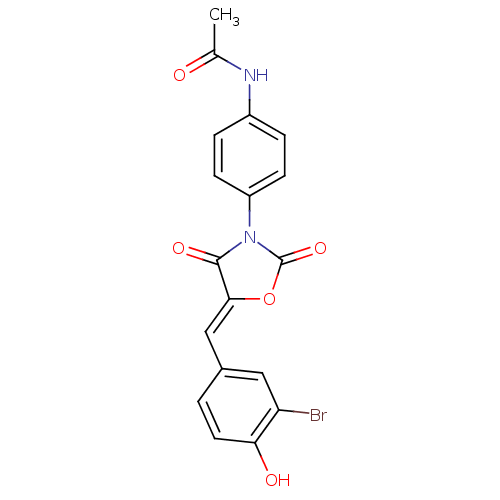
(CHEMBL2018231)Show SMILES CC(=O)Nc1ccc(cc1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C18H13BrN2O5/c1-10(22)20-12-3-5-13(6-4-12)21-17(24)16(26-18(21)25)9-11-2-7-15(23)14(19)8-11/h2-9,23H,1H3,(H,20,22)/b16-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305309
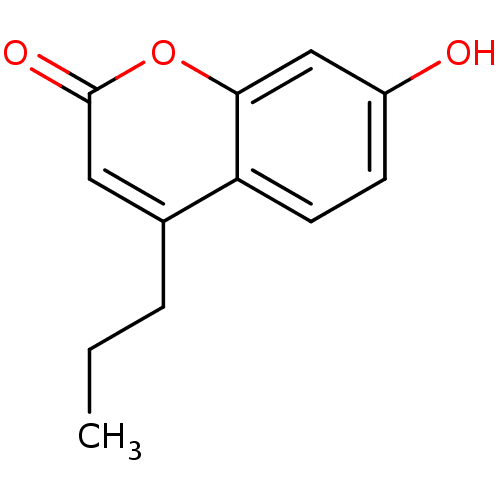
(7-hydroxy-4-propyl-2H-chromen-2-one | CHEMBL605407)Show InChI InChI=1S/C12H12O3/c1-2-3-8-6-12(14)15-11-7-9(13)4-5-10(8)11/h4-7,13H,2-3H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381568

(CHEMBL2018137)Show SMILES COc1ccccc1N1C(=S)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO4S/c1-22-14-5-3-2-4-12(14)19-16(21)15(23-17(19)24)9-10-6-7-13(20)11(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
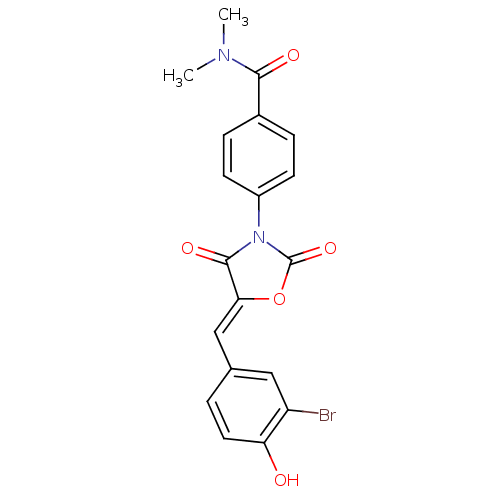
(Homo sapiens (Human)) | BDBM50381577
(CHEMBL2018227)Show SMILES CN(C)C(=O)c1ccc(cc1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C19H15BrN2O5/c1-21(2)17(24)12-4-6-13(7-5-12)22-18(25)16(27-19(22)26)10-11-3-8-15(23)14(20)9-11/h3-10,23H,1-2H3/b16-10- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
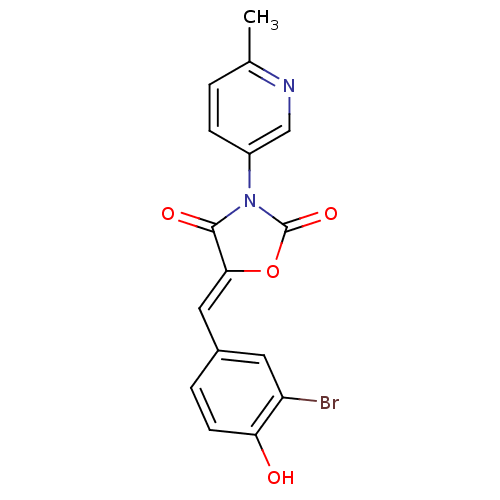
(Homo sapiens (Human)) | BDBM50381587
(CHEMBL2018239)Show SMILES Cc1ccc(cn1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C16H11BrN2O4/c1-9-2-4-11(8-18-9)19-15(21)14(23-16(19)22)7-10-3-5-13(20)12(17)6-10/h2-8,20H,1H3/b14-7- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381584

(CHEMBL2018236)Show InChI InChI=1S/C15H9BrN2O4/c16-10-7-9(4-5-11(10)19)8-12-14(20)18(15(21)22-12)13-3-1-2-6-17-13/h1-8,19H/b12-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381565
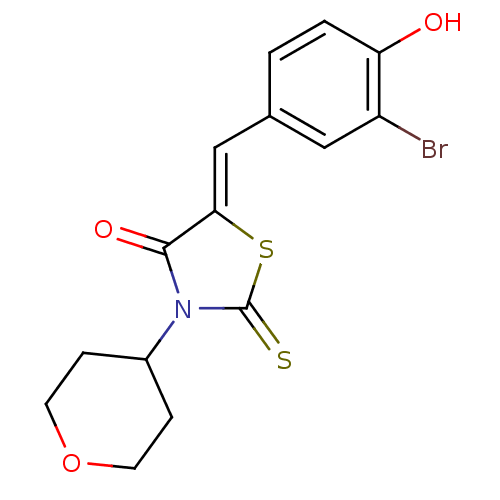
(CHEMBL2018132)Show InChI InChI=1S/C15H14BrNO3S2/c16-11-7-9(1-2-12(11)18)8-13-14(19)17(15(21)22-13)10-3-5-20-6-4-10/h1-2,7-8,10,18H,3-6H2/b13-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data