 Found 138 hits with Last Name = 'nolt' and Initial = 'mb'
Found 138 hits with Last Name = 'nolt' and Initial = 'mb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208973

(US9266881, 12-1)Show SMILES Cn1cc(nc1CCn1nc2cccc(N3CCOCC3)n2c1=O)-c1ccccc1 Show InChI InChI=1S/C22H24N6O2/c1-25-16-18(17-6-3-2-4-7-17)23-19(25)10-11-27-22(29)28-20(24-27)8-5-9-21(28)26-12-14-30-15-13-26/h2-9,16H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.410 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208974
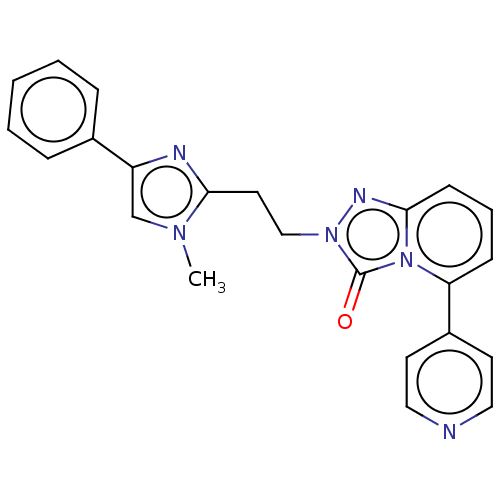
(US9266881, B-1)Show SMILES Cn1cc(nc1CCn1nc2cccc(-c3ccncc3)n2c1=O)-c1ccccc1 Show InChI InChI=1S/C23H20N6O/c1-27-16-19(17-6-3-2-4-7-17)25-21(27)12-15-28-23(30)29-20(8-5-9-22(29)26-28)18-10-13-24-14-11-18/h2-11,13-14,16H,12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208959
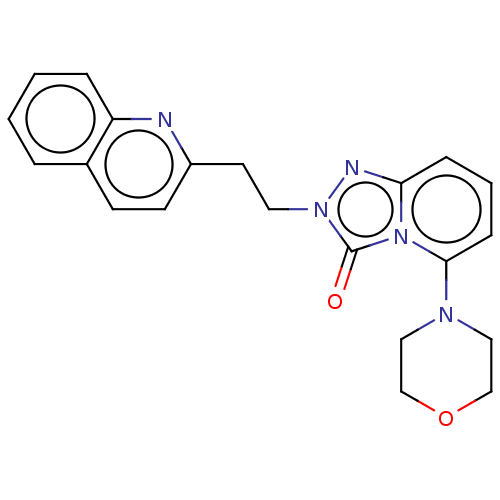
(US9266881, 6-1)Show SMILES O=c1n(CCc2ccc3ccccc3n2)nc2cccc(N3CCOCC3)n12 Show InChI InChI=1S/C21H21N5O2/c27-21-25(11-10-17-9-8-16-4-1-2-5-18(16)22-17)23-19-6-3-7-20(26(19)21)24-12-14-28-15-13-24/h1-9H,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.5 | -49.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208958

(US9266881, 5-1)Show SMILES O=c1n(CCc2ccc3ccccc3n2)nc2cccc(-c3ccncc3)n12 Show InChI InChI=1S/C22H17N5O/c28-22-26(15-12-18-9-8-16-4-1-2-5-19(16)24-18)25-21-7-3-6-20(27(21)22)17-10-13-23-14-11-17/h1-11,13-14H,12,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.40 | -47.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208972
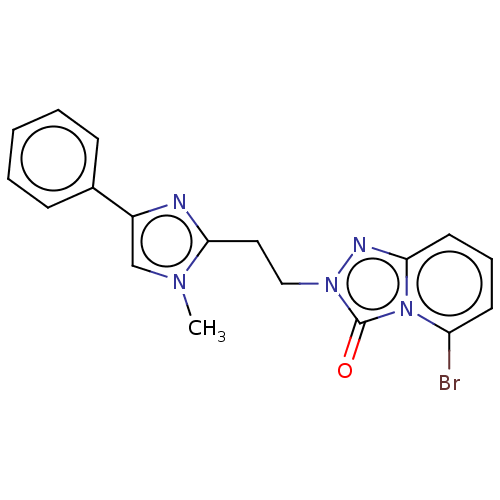
(US9266881, 11-4)Show InChI InChI=1S/C18H16BrN5O/c1-22-12-14(13-6-3-2-4-7-13)20-16(22)10-11-23-18(25)24-15(19)8-5-9-17(24)21-23/h2-9,12H,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 7.20 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208964

(US9266881, A-1)Show SMILES O=c1n(CCc2ccc3ccccc3n2)nc2cccc(-c3cccnc3)n12 Show InChI InChI=1S/C22H17N5O/c28-22-26(14-12-18-11-10-16-5-1-2-7-19(16)24-18)25-21-9-3-8-20(27(21)22)17-6-4-13-23-15-17/h1-11,13,15H,12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208960

(US9266881, 7-2)Show SMILES COc1ccc(CC(C)(O)c2cccc3nn(CCc4ccc5ccccc5n4)c(=O)n23)cc1 Show InChI InChI=1S/C27H26N4O3/c1-27(33,18-19-10-14-22(34-2)15-11-19)24-8-5-9-25-29-30(26(32)31(24)25)17-16-21-13-12-20-6-3-4-7-23(20)28-21/h3-15,33H,16-18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 11 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208970

(US9266881, A-7)Show SMILES O=c1n(CCc2ccc3ccccc3n2)nc2cccc(NCc3cccnc3)n12 Show InChI InChI=1S/C23H20N6O/c30-23-28(14-12-19-11-10-18-6-1-2-7-20(18)26-19)27-22-9-3-8-21(29(22)23)25-16-17-5-4-13-24-15-17/h1-11,13,15,25H,12,14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208966

(US9266881, A-3)Show SMILES Cc1ccc(cc1)-c1cccc2nn(CCc3ccc4ccccc4n3)c(=O)n12 Show InChI InChI=1S/C24H20N4O/c1-17-9-11-19(12-10-17)22-7-4-8-23-26-27(24(29)28(22)23)16-15-20-14-13-18-5-2-3-6-21(18)25-20/h2-14H,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 17 | -44.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208969
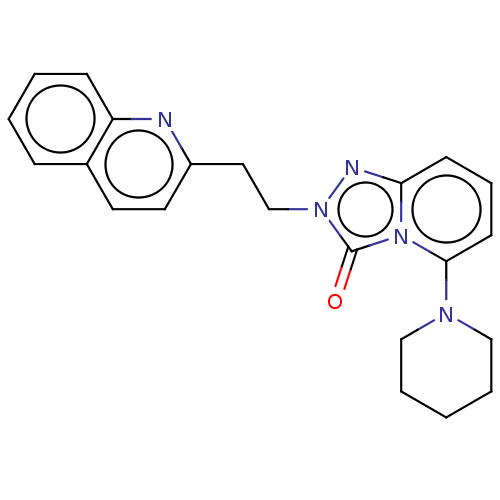
(US9266881, A-6)Show SMILES O=c1n(CCc2ccc3ccccc3n2)nc2cccc(N3CCCCC3)n12 Show InChI InChI=1S/C22H23N5O/c28-22-26(16-13-18-12-11-17-7-2-3-8-19(17)23-18)24-20-9-6-10-21(27(20)22)25-14-4-1-5-15-25/h2-3,6-12H,1,4-5,13-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 19 | -44.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208968
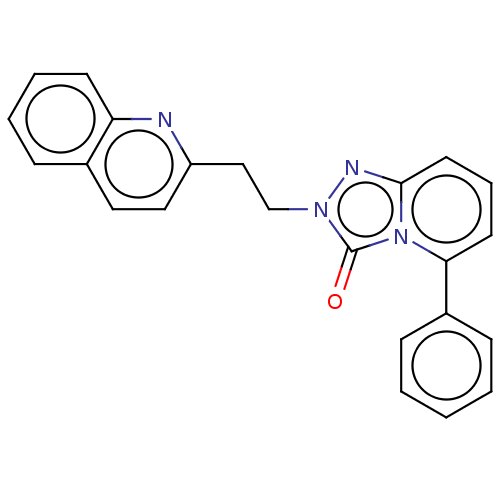
(US9266881, A-5)Show SMILES O=c1n(CCc2ccc3ccccc3n2)nc2cccc(-c3ccccc3)n12 Show InChI InChI=1S/C23H18N4O/c28-23-26(16-15-19-14-13-17-7-4-5-10-20(17)24-19)25-22-12-6-11-21(27(22)23)18-8-2-1-3-9-18/h1-14H,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208957
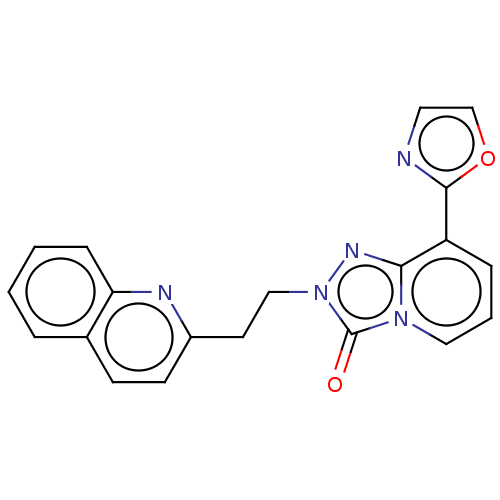
(US9266881, 4-1)Show SMILES O=c1n(CCc2ccc3ccccc3n2)nc2c(cccn12)-c1ncco1 Show InChI InChI=1S/C20H15N5O2/c26-20-24-11-3-5-16(19-21-10-13-27-19)18(24)23-25(20)12-9-15-8-7-14-4-1-2-6-17(14)22-15/h1-8,10-11,13H,9,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 22 | -43.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208967
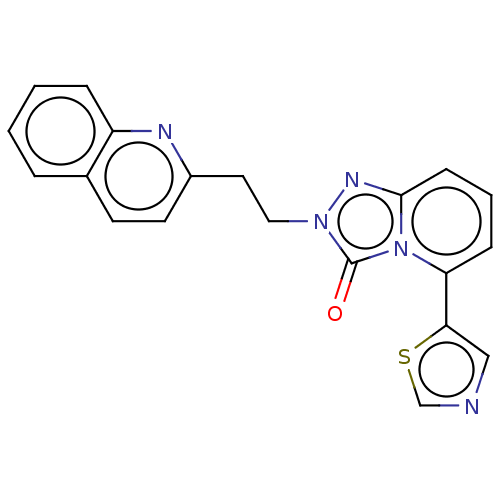
(US9266881, A-4)Show SMILES O=c1n(CCc2ccc3ccccc3n2)nc2cccc(-c3cncs3)n12 Show InChI InChI=1S/C20H15N5OS/c26-20-24(11-10-15-9-8-14-4-1-2-5-16(14)22-15)23-19-7-3-6-17(25(19)20)18-12-21-13-27-18/h1-9,12-13H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 23 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208954
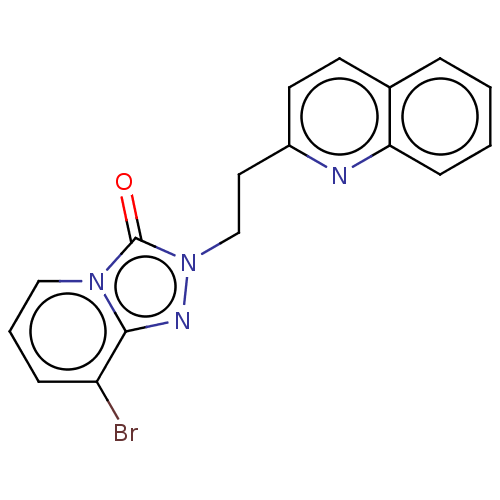
(US9266881, 1-6)Show InChI InChI=1S/C17H13BrN4O/c18-14-5-3-10-21-16(14)20-22(17(21)23)11-9-13-8-7-12-4-1-2-6-15(12)19-13/h1-8,10H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 37 | -42.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208955

(US9266881, 2-4)Show InChI InChI=1S/C17H13BrN4O/c18-15-6-3-7-16-20-21(17(23)22(15)16)11-10-13-9-8-12-4-1-2-5-14(12)19-13/h1-9H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 59 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208971
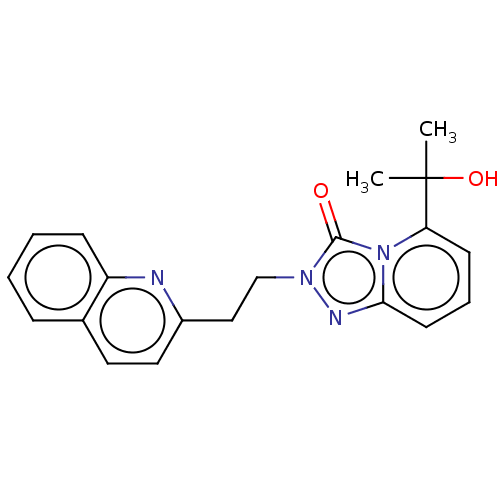
(US9266881, A-8)Show SMILES CC(C)(O)c1cccc2nn(CCc3ccc4ccccc4n3)c(=O)n12 Show InChI InChI=1S/C20H20N4O2/c1-20(2,26)17-8-5-9-18-22-23(19(25)24(17)18)13-12-15-11-10-14-6-3-4-7-16(14)21-15/h3-11,26H,12-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 72 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208965

(US9266881, A-2)Show InChI InChI=1S/C18H16N4O/c1-13-5-4-11-21-17(13)20-22(18(21)23)12-10-15-9-8-14-6-2-3-7-16(14)19-15/h2-9,11H,10,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 74 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208961
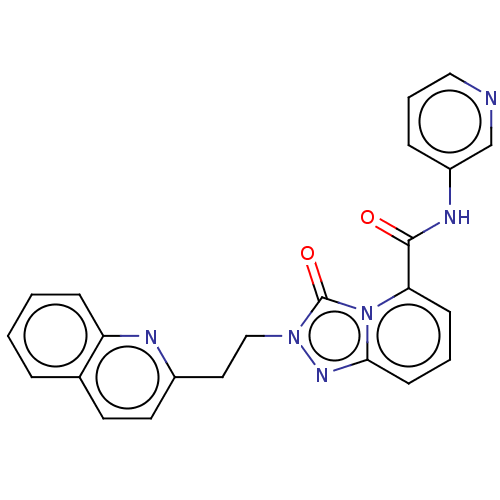
(US9266881, 8-2)Show SMILES O=C(Nc1cccnc1)c1cccc2nn(CCc3ccc4ccccc4n3)c(=O)n12 Show InChI InChI=1S/C23H18N6O2/c30-22(26-18-6-4-13-24-15-18)20-8-3-9-21-27-28(23(31)29(20)21)14-12-17-11-10-16-5-1-2-7-19(16)25-17/h1-11,13,15H,12,14H2,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 97 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208963

(US9266881, 10-4)Show SMILES O=c1n(CCc2ccc3ncccc3n2)nc2c(cccn12)-c1ccncc1 Show InChI InChI=1S/C21H16N6O/c28-21-26-13-2-3-17(15-7-11-22-12-8-15)20(26)25-27(21)14-9-16-5-6-18-19(24-16)4-1-10-23-18/h1-8,10-13H,9,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 103 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208956

(US9266881, 3-4)Show InChI InChI=1S/C17H13BrN4O/c18-13-6-8-16-20-22(17(23)21(16)11-13)10-9-14-7-5-12-3-1-2-4-15(12)19-14/h1-8,11H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 155 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208962

(US9266881, 9-1)Show SMILES O=c1n(CCc2ccc3ccccc3n2)nc2ccc(cn12)-c1cccnc1 Show InChI InChI=1S/C22H17N5O/c28-22-26-15-18(17-5-3-12-23-14-17)8-10-21(26)25-27(22)13-11-19-9-7-16-4-1-2-6-20(16)24-19/h1-10,12,14-15H,11,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 510 | -35.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM208975
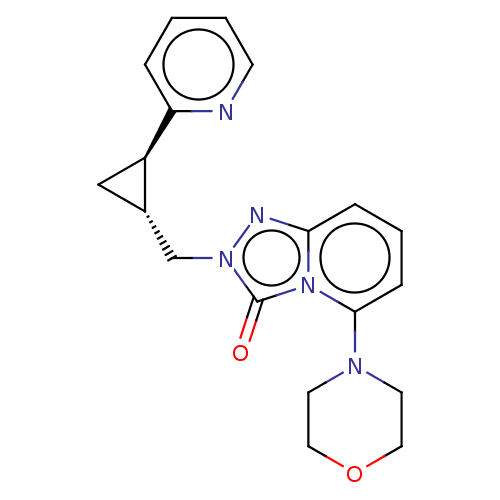
(US9266881, 13-6)Show SMILES O=c1n(C[C@@H]2C[C@H]2c2ccccn2)nc2cccc(N3CCOCC3)n12 |r| Show InChI InChI=1S/C19H21N5O2/c25-19-23(13-14-12-15(14)16-4-1-2-7-20-16)21-17-5-3-6-18(24(17)19)22-8-10-26-11-9-22/h1-7,14-15H,8-13H2/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 546 | -35.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunn... |
US Patent US9266881 (2016)
BindingDB Entry DOI: 10.7270/Q2154FVR |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235240
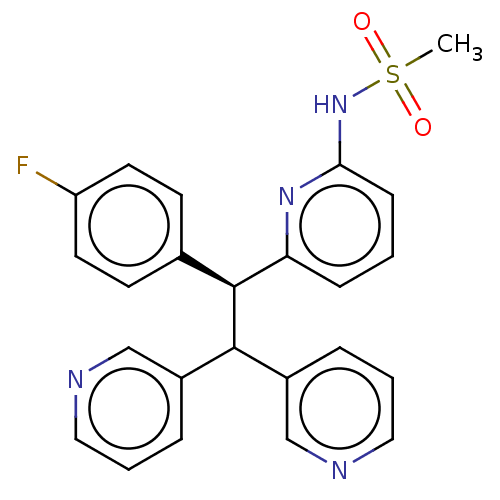
(CHEMBL4065169)Show SMILES CS(=O)(=O)Nc1cccc(n1)[C@@H](C(c1cccnc1)c1cccnc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H21FN4O2S/c1-32(30,31)29-22-8-2-7-21(28-22)24(17-9-11-20(25)12-10-17)23(18-5-3-13-26-15-18)19-6-4-14-27-16-19/h2-16,23-24H,1H3,(H,28,29)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonistic activity towards LTB4 receptor was evaluated by inhibition of binding of [3H]LTB4 to human neutrophils |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235240

(CHEMBL4065169)Show SMILES CS(=O)(=O)Nc1cccc(n1)[C@@H](C(c1cccnc1)c1cccnc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H21FN4O2S/c1-32(30,31)29-22-8-2-7-21(28-22)24(17-9-11-20(25)12-10-17)23(18-5-3-13-26-15-18)19-6-4-14-27-16-19/h2-16,23-24H,1H3,(H,28,29)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 mediated ultra-rapid delayed rectifier current Ikur in human atrial myocytes by voltage-patch clamp electrophysiology method |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235250

(CHEMBL4104525)Show SMILES O[C@H](C(c1cccnc1)c1cccnc1)c1cccnc1-c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C23H17Cl2N3O/c24-18-10-17(11-19(25)12-18)22-20(6-3-9-28-22)23(29)21(15-4-1-7-26-13-15)16-5-2-8-27-14-16/h1-14,21,23,29H/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 mediated ultra-rapid delayed rectifier current Ikur in human atrial myocytes by voltage-patch clamp electrophysiology method |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235255

(CHEMBL4085436)Show InChI InChI=1S/C23H18ClN3/c24-22-10-2-1-9-20(22)23-17(6-5-13-27-23)14-21(18-7-3-11-25-15-18)19-8-4-12-26-16-19/h1-13,15-16,21H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235259
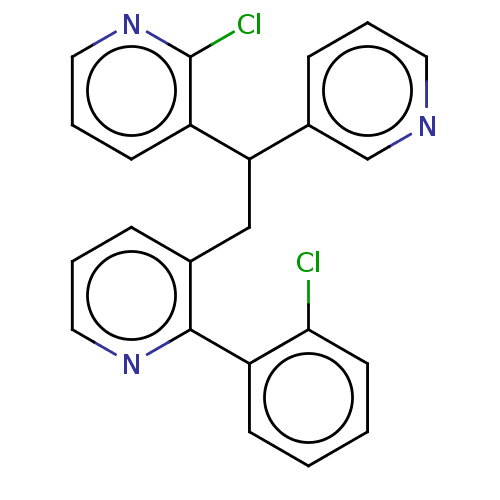
(CHEMBL4105245)Show InChI InChI=1S/C23H17Cl2N3/c24-21-10-2-1-8-19(21)22-16(6-4-12-27-22)14-20(17-7-3-11-26-15-17)18-9-5-13-28-23(18)25/h1-13,15,20H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235250

(CHEMBL4104525)Show SMILES O[C@H](C(c1cccnc1)c1cccnc1)c1cccnc1-c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C23H17Cl2N3O/c24-18-10-17(11-19(25)12-18)22-20(6-3-9-28-22)23(29)21(15-4-1-7-26-13-15)16-5-2-8-27-14-16/h1-14,21,23,29H/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235275
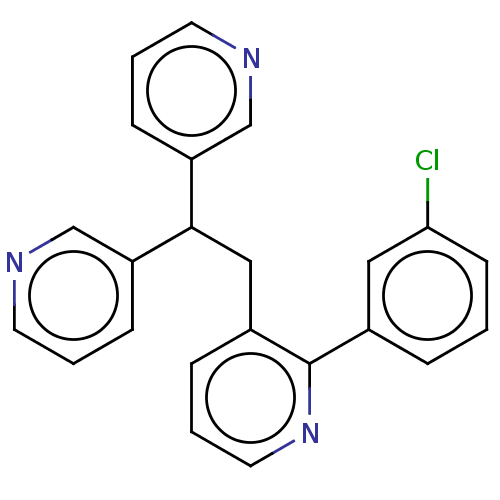
(CHEMBL4062996)Show InChI InChI=1S/C23H18ClN3/c24-21-9-1-5-17(13-21)23-18(6-4-12-27-23)14-22(19-7-2-10-25-15-19)20-8-3-11-26-16-20/h1-13,15-16,22H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235253
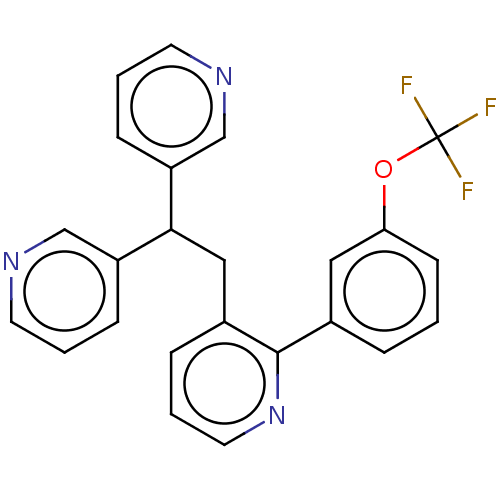
(CHEMBL4075394)Show SMILES FC(F)(F)Oc1cccc(c1)-c1ncccc1CC(c1cccnc1)c1cccnc1 Show InChI InChI=1S/C24H18F3N3O/c25-24(26,27)31-21-9-1-5-17(13-21)23-18(6-4-12-30-23)14-22(19-7-2-10-28-15-19)20-8-3-11-29-16-20/h1-13,15-16,22H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50194539

(4-(3-(2-fluorophenyl)propanamido)-N,N-diisopropyl-...)Show SMILES CC(C)N(C(C)C)C(=O)C(C(CNC(=O)CCc1ccccc1F)c1ccccc1)c1cccnc1 Show InChI InChI=1S/C30H36FN3O2/c1-21(2)34(22(3)4)30(36)29(25-14-10-18-32-19-25)26(23-11-6-5-7-12-23)20-33-28(35)17-16-24-13-8-9-15-27(24)31/h5-15,18-19,21-22,26,29H,16-17,20H2,1-4H3,(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 |
Bioorg Med Chem Lett 16: 5897-901 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.054
BindingDB Entry DOI: 10.7270/Q2Z60NP6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235254

(CHEMBL4095750)Show InChI InChI=1S/C24H21N3O/c1-28-22-10-2-6-18(14-22)24-19(7-5-13-27-24)15-23(20-8-3-11-25-16-20)21-9-4-12-26-17-21/h2-14,16-17,23H,15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235260

(CHEMBL4088894)Show SMILES Nc1cccc(n1)C(Cc1cccnc1-c1cccc(c1)C(F)(F)F)c1cccnc1 Show InChI InChI=1S/C24H19F3N4/c25-24(26,27)19-8-1-5-16(13-19)23-17(6-4-12-30-23)14-20(18-7-3-11-29-15-18)21-9-2-10-22(28)31-21/h1-13,15,20H,14H2,(H2,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235272

(CHEMBL4067665)Show SMILES Nc1cccc(n1)C(Cc1cccnc1-c1cc(Cl)cc(Cl)c1)c1cccnc1 Show InChI InChI=1S/C23H18Cl2N4/c24-18-10-17(11-19(25)13-18)23-15(4-3-9-28-23)12-20(16-5-2-8-27-14-16)21-6-1-7-22(26)29-21/h1-11,13-14,20H,12H2,(H2,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235246
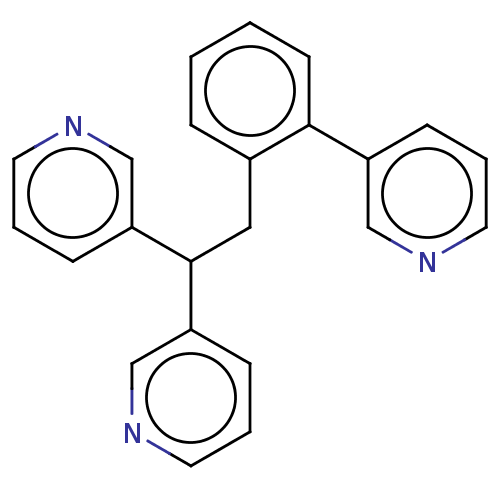
(CHEMBL4097667)Show InChI InChI=1S/C23H19N3/c1-2-10-22(19-7-3-11-24-15-19)18(6-1)14-23(20-8-4-12-25-16-20)21-9-5-13-26-17-21/h1-13,15-17,23H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235243
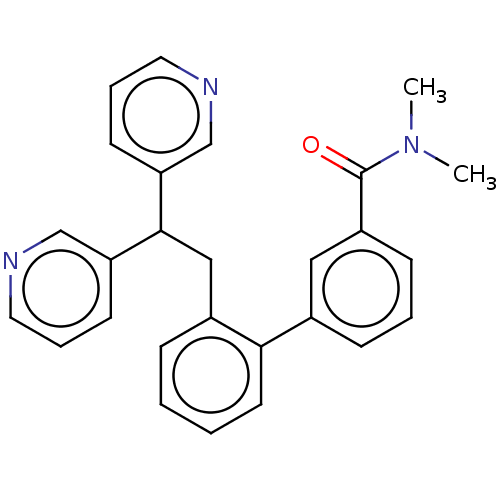
(CHEMBL4100226)Show SMILES CN(C)C(=O)c1cccc(c1)-c1ccccc1CC(c1cccnc1)c1cccnc1 Show InChI InChI=1S/C27H25N3O/c1-30(2)27(31)22-10-5-9-20(16-22)25-13-4-3-8-21(25)17-26(23-11-6-14-28-18-23)24-12-7-15-29-19-24/h3-16,18-19,26H,17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235271

(CHEMBL4094177)Show SMILES OC(Cc1cccnc1-c1cc(Cl)cc(Cl)c1)(c1cccnc1)c1cccnc1 Show InChI InChI=1S/C23H17Cl2N3O/c24-20-10-17(11-21(25)12-20)22-16(4-1-9-28-22)13-23(29,18-5-2-7-26-14-18)19-6-3-8-27-15-19/h1-12,14-15,29H,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235264

(CHEMBL4100098)Show SMILES FC(F)(F)c1cccc(c1)-c1ncccc1CC(c1cccnc1)c1cccnc1 Show InChI InChI=1S/C24H18F3N3/c25-24(26,27)21-9-1-5-17(13-21)23-18(6-4-12-30-23)14-22(19-7-2-10-28-15-19)20-8-3-11-29-16-20/h1-13,15-16,22H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235241
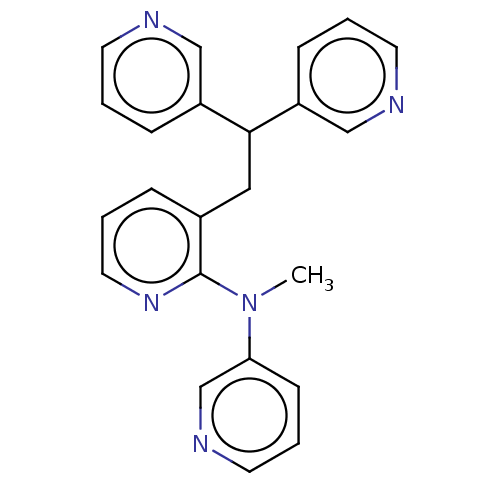
(CHEMBL4080304)Show InChI InChI=1S/C23H21N5/c1-28(21-9-5-12-26-17-21)23-18(6-4-13-27-23)14-22(19-7-2-10-24-15-19)20-8-3-11-25-16-20/h2-13,15-17,22H,14H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50194512

(CHEMBL216955 | N,N-diisopropyl-3-phenyl-4-(3-pheny...)Show SMILES CC(C)N(C(C)C)C(=O)C(C(CNC(=O)CC(C)c1ccccc1)c1ccccc1)c1cccnc1 Show InChI InChI=1S/C31H39N3O2/c1-22(2)34(23(3)4)31(36)30(27-17-12-18-32-20-27)28(26-15-10-7-11-16-26)21-33-29(35)19-24(5)25-13-8-6-9-14-25/h6-18,20,22-24,28,30H,19,21H2,1-5H3,(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 |
Bioorg Med Chem Lett 16: 5897-901 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.054
BindingDB Entry DOI: 10.7270/Q2Z60NP6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50194549
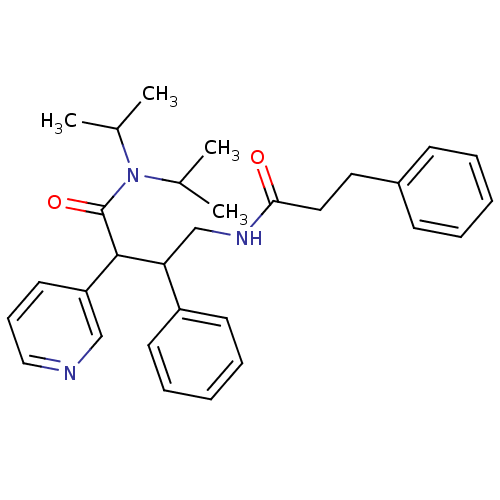
(CHEMBL217222 | N,N-diisopropyl-3-phenyl-4-(3-pheny...)Show SMILES CC(C)N(C(C)C)C(=O)C(C(CNC(=O)CCc1ccccc1)c1ccccc1)c1cccnc1 Show InChI InChI=1S/C30H37N3O2/c1-22(2)33(23(3)4)30(35)29(26-16-11-19-31-20-26)27(25-14-9-6-10-15-25)21-32-28(34)18-17-24-12-7-5-8-13-24/h5-16,19-20,22-23,27,29H,17-18,21H2,1-4H3,(H,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 |
Bioorg Med Chem Lett 16: 5897-901 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.054
BindingDB Entry DOI: 10.7270/Q2Z60NP6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235265
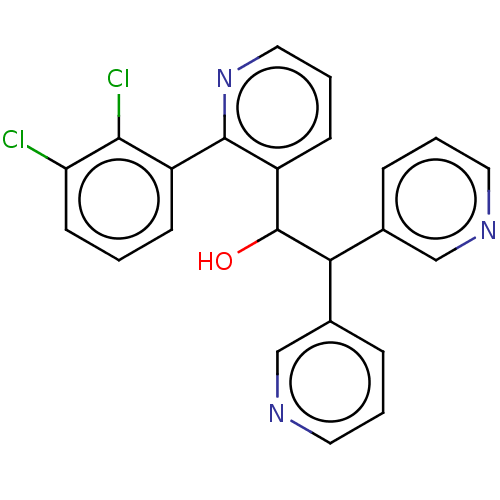
(CHEMBL4097545)Show SMILES OC(C(c1cccnc1)c1cccnc1)c1cccnc1-c1cccc(Cl)c1Cl Show InChI InChI=1S/C23H17Cl2N3O/c24-19-9-1-7-17(21(19)25)22-18(8-4-12-28-22)23(29)20(15-5-2-10-26-13-15)16-6-3-11-27-14-16/h1-14,20,23,29H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50194510

(CHEMBL386441 | methyl 5-(diisopropylamino)-3-(3-(3...)Show SMILES COC(=O)CC(C(C(=O)N(C(C)C)C(C)C)c1cccnc1)c1cccc(c1)-c1c(C)noc1C Show InChI InChI=1S/C28H35N3O4/c1-17(2)31(18(3)4)28(33)27(23-12-9-13-29-16-23)24(15-25(32)34-7)21-10-8-11-22(14-21)26-19(5)30-35-20(26)6/h8-14,16-18,24,27H,15H2,1-7H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 |
Bioorg Med Chem Lett 16: 5897-901 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.054
BindingDB Entry DOI: 10.7270/Q2Z60NP6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235249

(CHEMBL4094185)Show SMILES CN(C)C(=O)c1cccc(c1)-c1ccc(F)cc1CC(c1cccnc1)c1cccnc1 Show InChI InChI=1S/C27H24FN3O/c1-31(2)27(32)20-7-3-6-19(14-20)25-11-10-24(28)15-23(25)16-26(21-8-4-12-29-17-21)22-9-5-13-30-18-22/h3-15,17-18,26H,16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235247
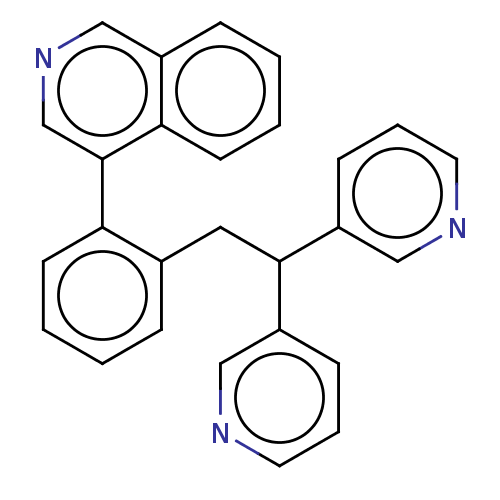
(CHEMBL4079636)Show SMILES C(C(c1cccnc1)c1cccnc1)c1ccccc1-c1cncc2ccccc12 Show InChI InChI=1S/C27H21N3/c1-3-11-24(27-19-30-18-21-8-2-4-12-25(21)27)20(7-1)15-26(22-9-5-13-28-16-22)23-10-6-14-29-17-23/h1-14,16-19,26H,15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235266
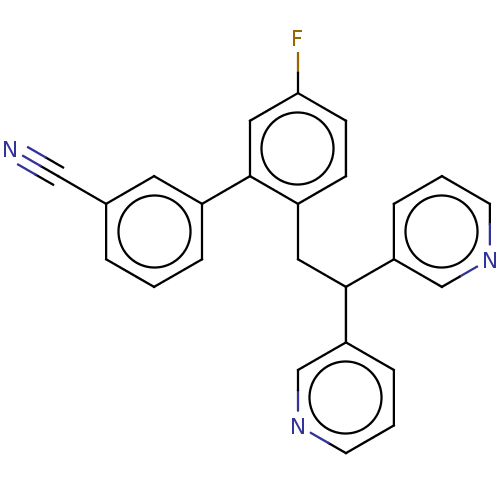
(CHEMBL4068841)Show SMILES Fc1ccc(CC(c2cccnc2)c2cccnc2)c(c1)-c1cccc(c1)C#N Show InChI InChI=1S/C25H18FN3/c26-23-9-8-20(25(14-23)19-5-1-4-18(12-19)15-27)13-24(21-6-2-10-28-16-21)22-7-3-11-29-17-22/h1-12,14,16-17,24H,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235269
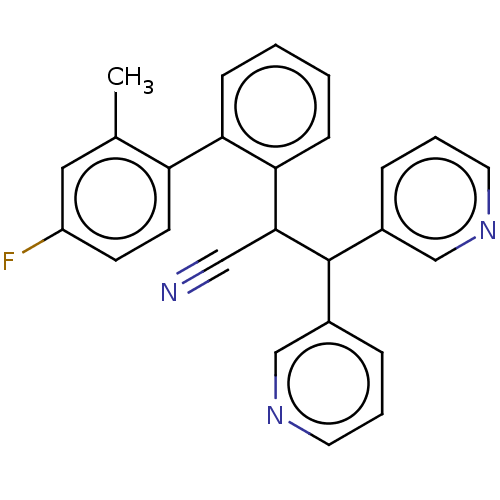
(CHEMBL4072707)Show SMILES Cc1cc(F)ccc1-c1ccccc1C(C#N)C(c1cccnc1)c1cccnc1 Show InChI InChI=1S/C26H20FN3/c1-18-14-21(27)10-11-22(18)23-8-2-3-9-24(23)25(15-28)26(19-6-4-12-29-16-19)20-7-5-13-30-17-20/h2-14,16-17,25-26H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50194540
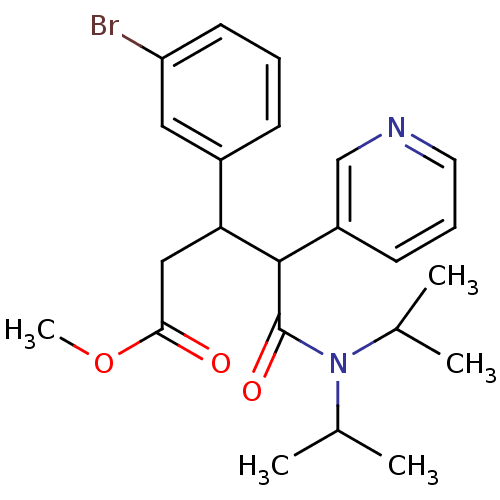
(CHEMBL217011 | methyl 3-(3-bromophenyl)-5-(diisopr...)Show SMILES COC(=O)CC(C(C(=O)N(C(C)C)C(C)C)c1cccnc1)c1cccc(Br)c1 Show InChI InChI=1S/C23H29BrN2O3/c1-15(2)26(16(3)4)23(28)22(18-9-7-11-25-14-18)20(13-21(27)29-5)17-8-6-10-19(24)12-17/h6-12,14-16,20,22H,13H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 |
Bioorg Med Chem Lett 16: 5897-901 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.054
BindingDB Entry DOI: 10.7270/Q2Z60NP6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50194514
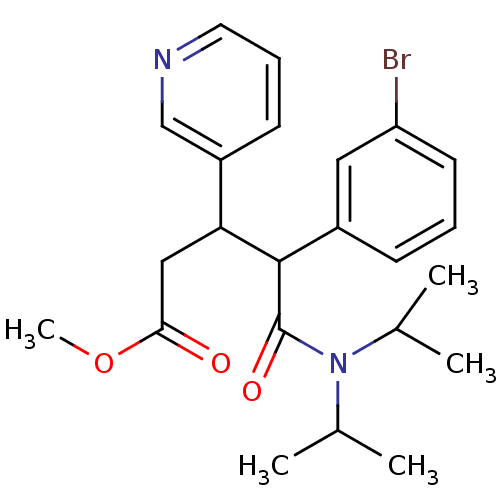
(CHEMBL217921 | methyl 4-(3-bromophenyl)-5-(diisopr...)Show SMILES COC(=O)CC(C(C(=O)N(C(C)C)C(C)C)c1cccc(Br)c1)c1cccnc1 Show InChI InChI=1S/C23H29BrN2O3/c1-15(2)26(16(3)4)23(28)22(17-8-6-10-19(24)12-17)20(13-21(27)29-5)18-9-7-11-25-14-18/h6-12,14-16,20,22H,13H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 |
Bioorg Med Chem Lett 16: 5897-901 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.054
BindingDB Entry DOI: 10.7270/Q2Z60NP6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily A member 5
(Homo sapiens (Human)) | BDBM50235258

(CHEMBL4081003)Show SMILES CC(=O)NC(C(c1cccnc1)c1cccnc1)c1ccccc1-c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C26H21Cl2N3O/c1-17(32)31-26(25(18-6-4-10-29-15-18)19-7-5-11-30-16-19)24-9-3-2-8-23(24)20-12-21(27)14-22(28)13-20/h2-16,25-26H,1H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Kv1.5 (unknown origin) |
Bioorg Med Chem Lett 27: 1062-1069 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.054
BindingDB Entry DOI: 10.7270/Q2DV1N5F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data