

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147785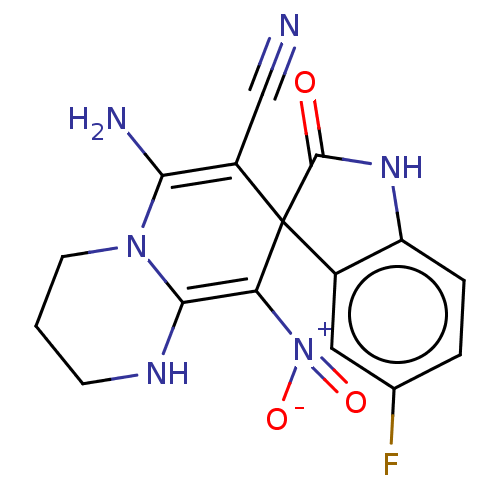 (CHEMBL3764376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147774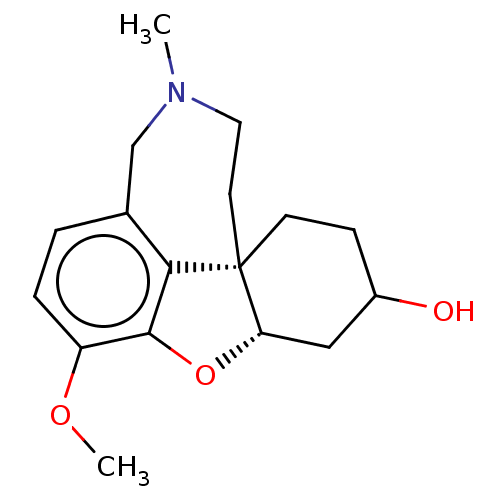 (CHEMBL3764305) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147768 (CHEMBL3764100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147772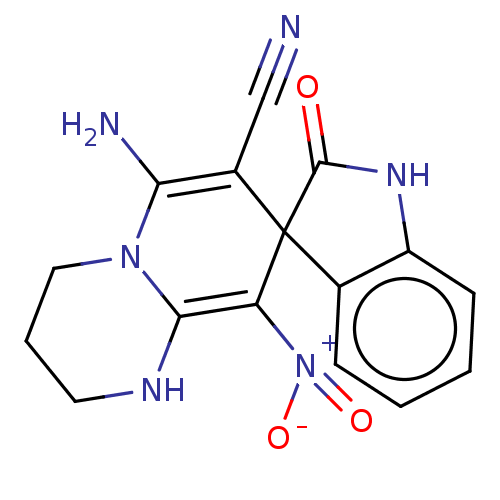 (CHEMBL3763203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147775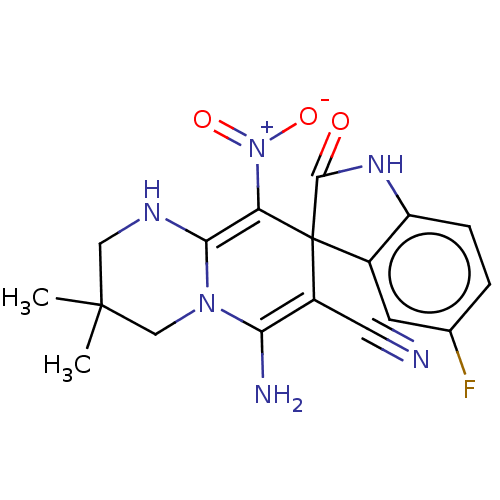 (CHEMBL3763232) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147796 (CHEMBL3765697) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by ... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147771 (CHEMBL3763778) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147885 (CHEMBL3765093) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147794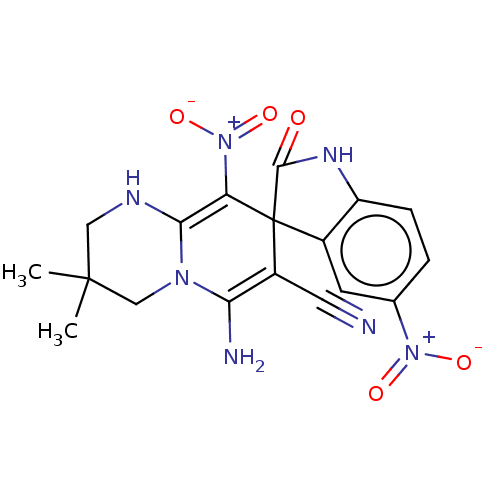 (CHEMBL3763820) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147827 (CHEMBL3765569) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147786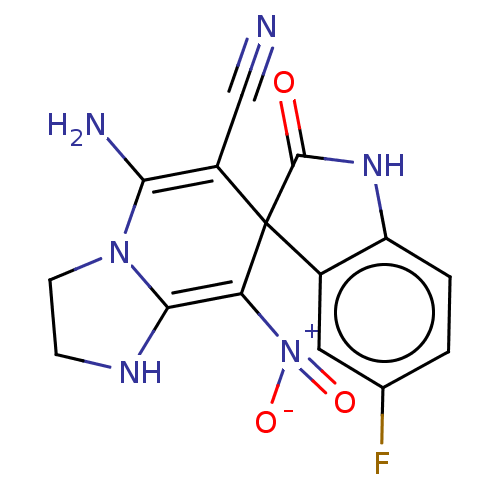 (CHEMBL3763609) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147787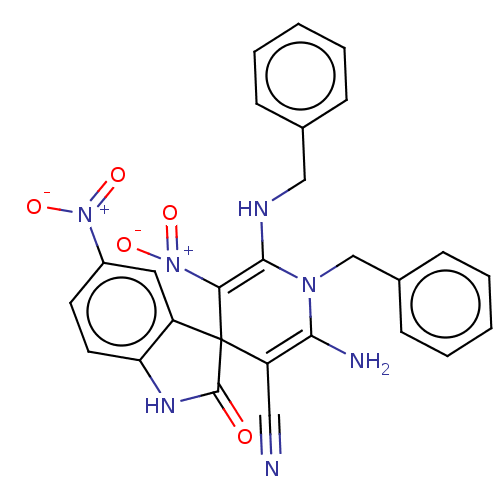 (CHEMBL3765476) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147776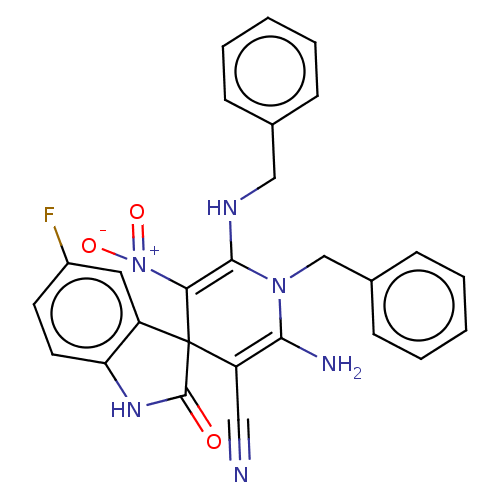 (CHEMBL3763973) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236974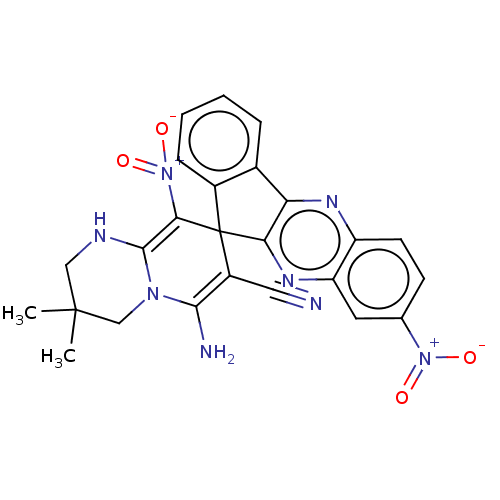 (CHEMBL4073501) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147882 (CHEMBL3763482) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147769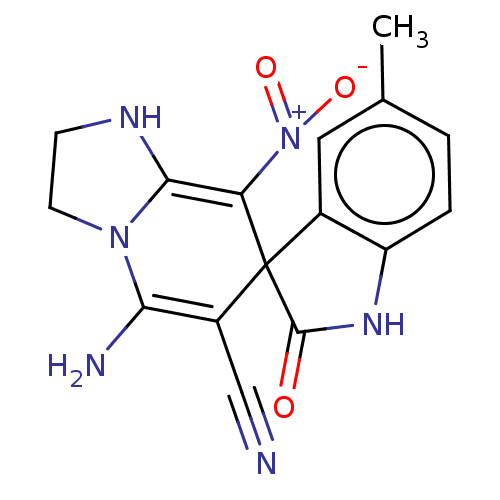 (CHEMBL3764679) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236975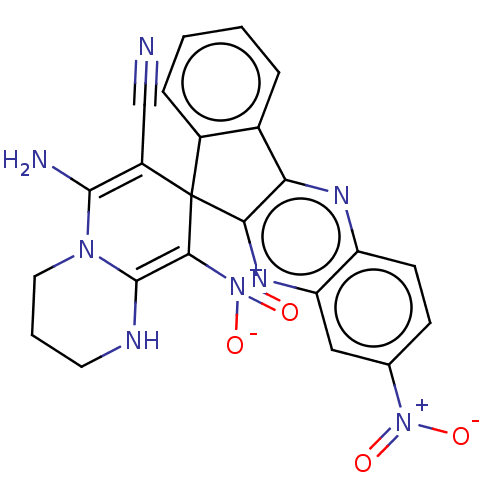 (CHEMBL4092602) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147770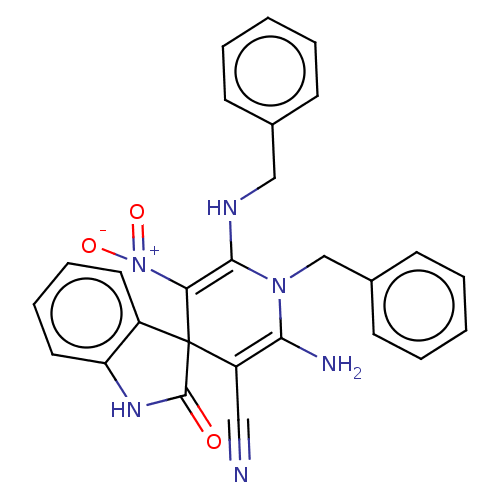 (CHEMBL3765496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236973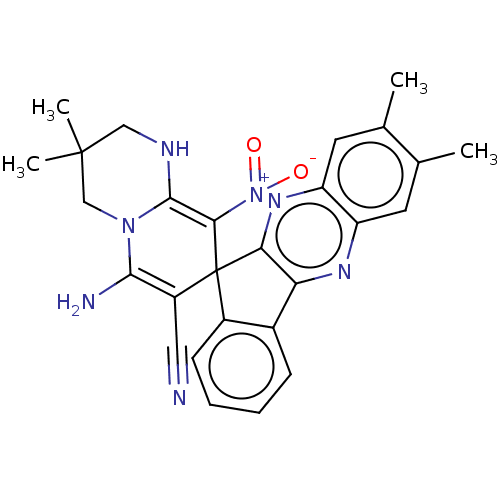 (CHEMBL4100338) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236708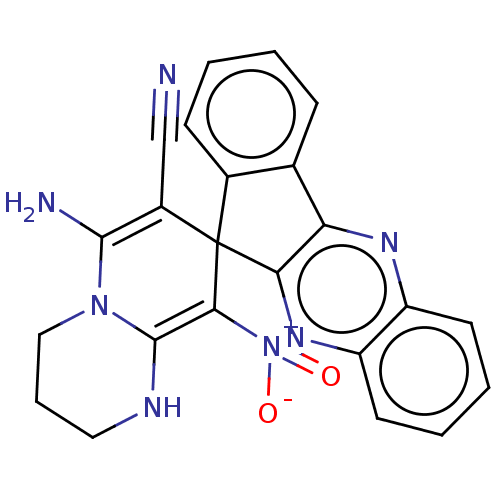 (CHEMBL4070769) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236979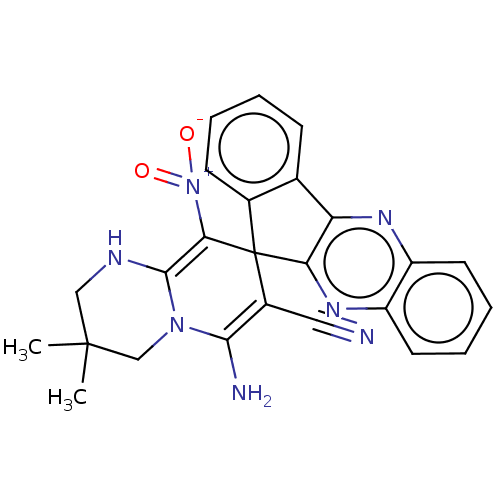 (CHEMBL4064904) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236976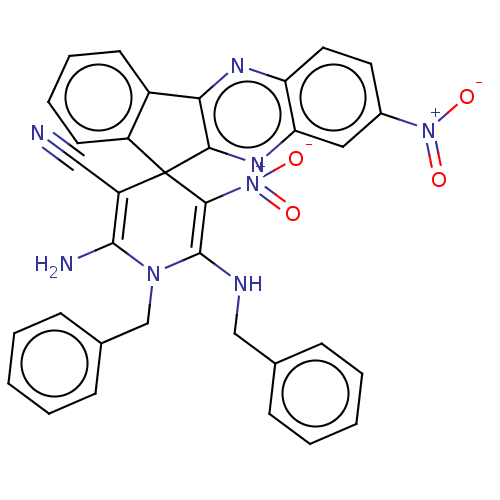 (CHEMBL4060800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147773 (CHEMBL3765162) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236971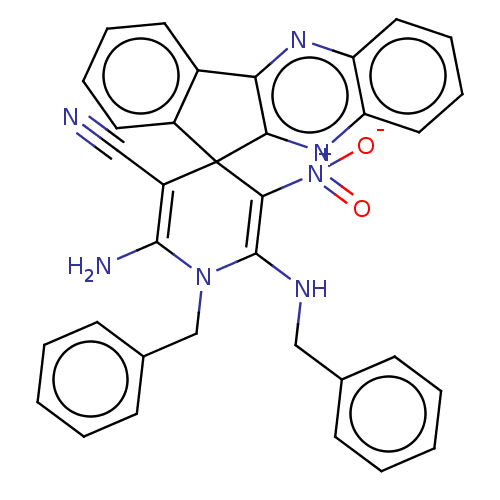 (CHEMBL4074566) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236978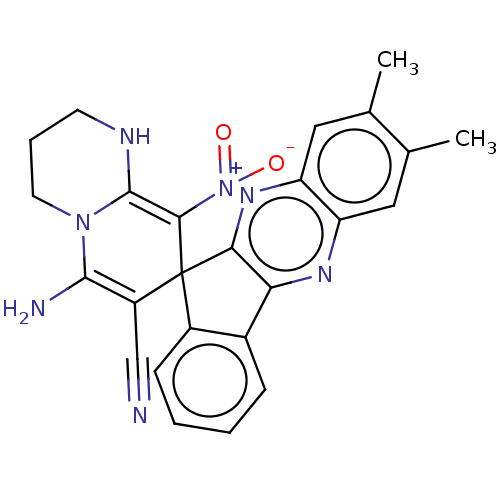 (CHEMBL4091651) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236972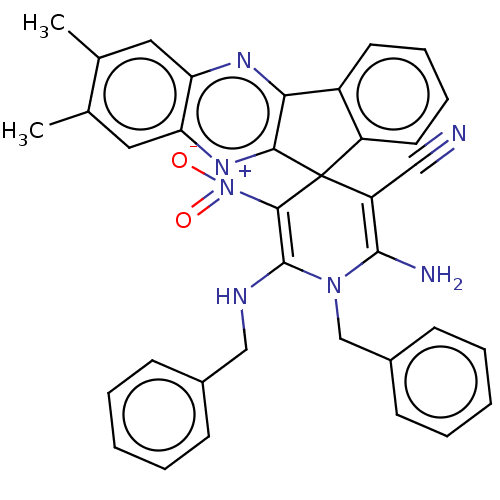 (CHEMBL4095032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147780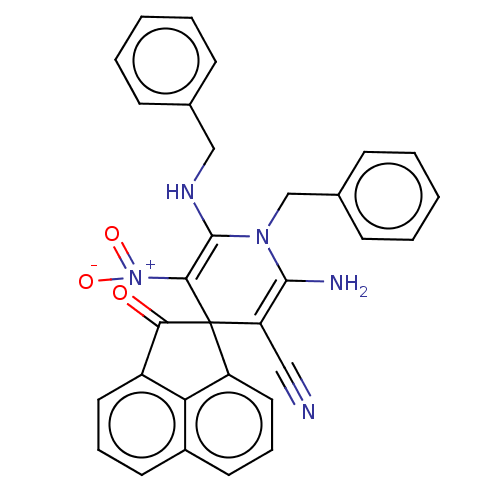 (CHEMBL3763417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147783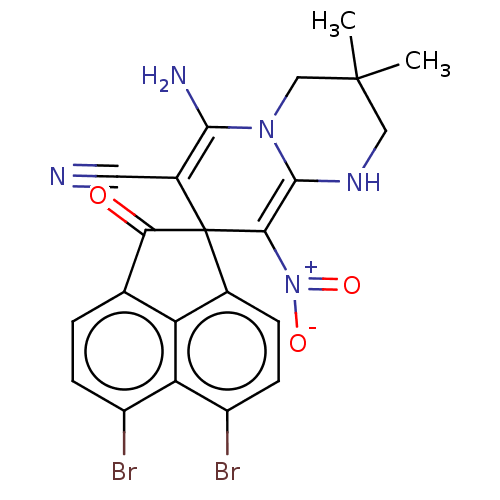 (CHEMBL3763832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147774 (CHEMBL3764305) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147784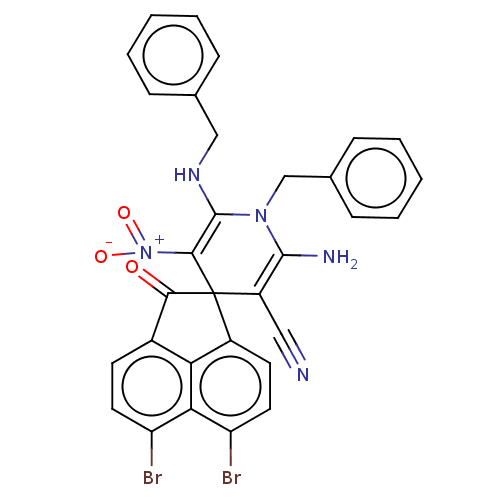 (CHEMBL3765098) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147781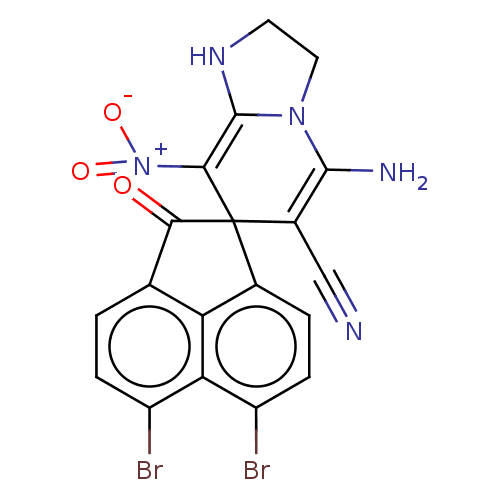 (CHEMBL3764065) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236706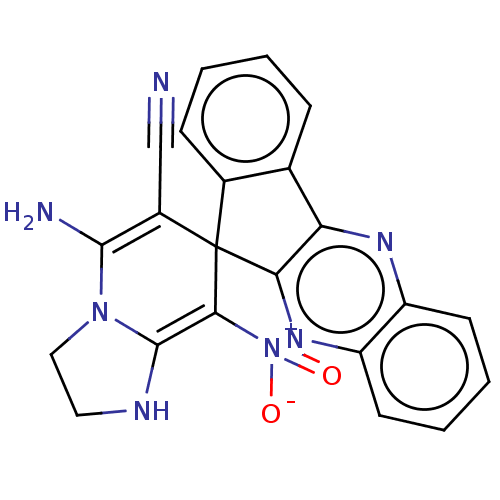 (CHEMBL4099598) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236977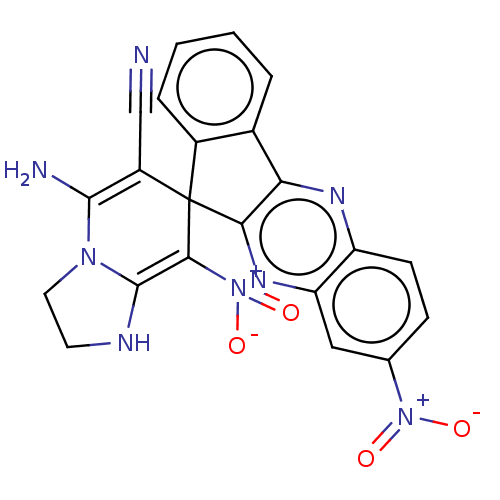 (CHEMBL4083875) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147778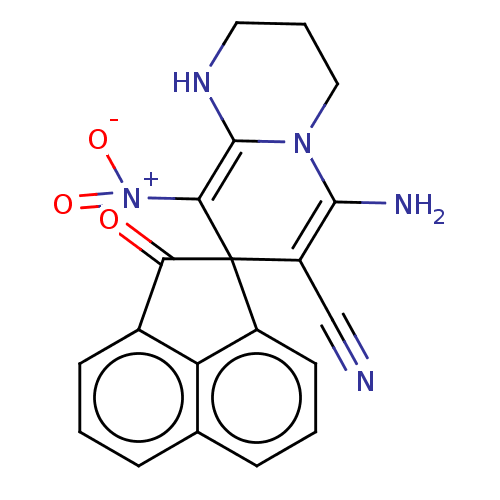 (CHEMBL3764900) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50236707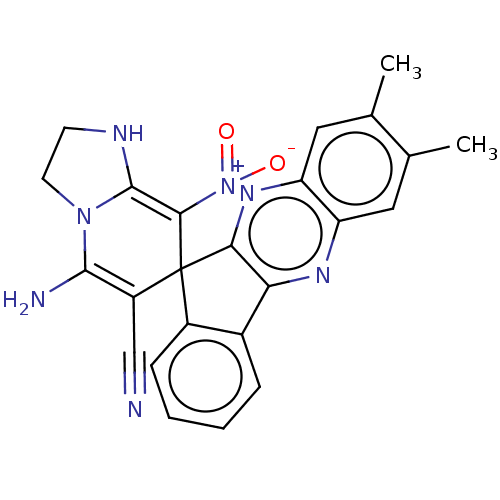 (CHEMBL4062146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by E... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147782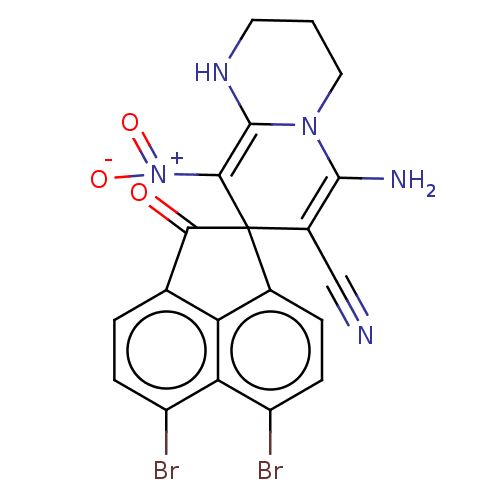 (CHEMBL3764488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147777 (CHEMBL3764433) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50236707 (CHEMBL4062146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by ... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50147779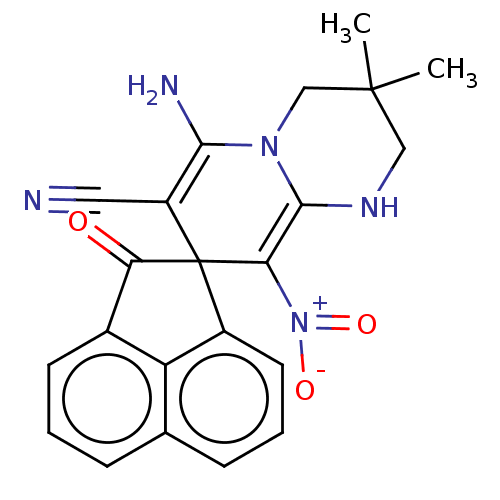 (CHEMBL3764714) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50236706 (CHEMBL4099598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by ... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50236971 (CHEMBL4074566) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by ... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50236973 (CHEMBL4100338) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by ... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50236972 (CHEMBL4095032) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibitory activity against histone deacetylase enzyme derived from partially purified extracts of Eimeria tenella protozoa using [3H]11 as radioliga... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50147772 (CHEMBL3763203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of BChE (unknown origin) using acetylthiocholine as substrate assessed as reduction of DTNB to TNB preincubated for 20 mins followed by ad... | Bioorg Med Chem 24: 1408-17 (2016) Article DOI: 10.1016/j.bmc.2016.02.019 BindingDB Entry DOI: 10.7270/Q2028TDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50236976 (CHEMBL4060800) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by ... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50236978 (CHEMBL4091651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by ... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50236977 (CHEMBL4083875) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by ... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50236975 (CHEMBL4092602) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Persian Gulf University Curated by ChEMBL | Assay Description Inhibition of butyrylcholinesterase (unknown origin) using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by ... | Bioorg Med Chem 25: 2057-2064 (2017) Article DOI: 10.1016/j.bmc.2017.02.017 BindingDB Entry DOI: 10.7270/Q2WM1GP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |