

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413625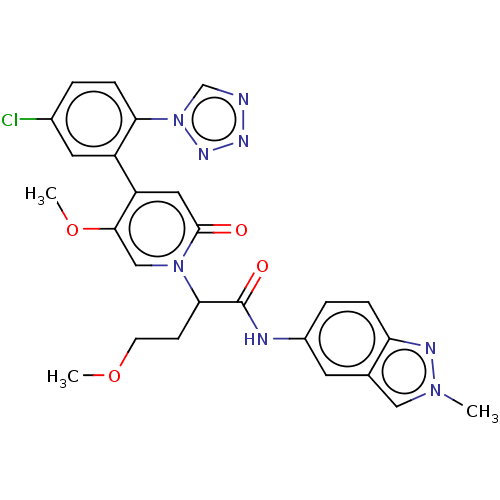 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413725 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(1H-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413625 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413725 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(1H-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246227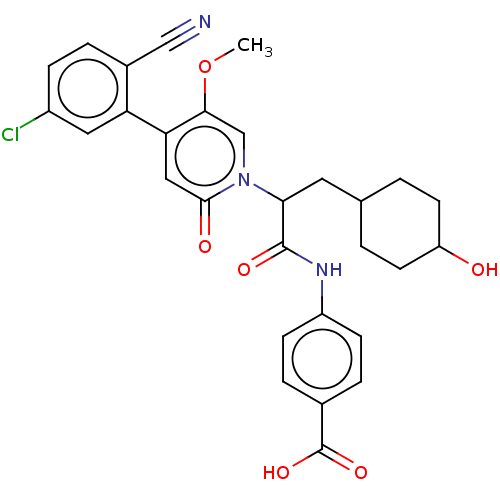 (US10183932, Example 135 | US9434690, 132 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246227 (US10183932, Example 135 | US9434690, 132 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246227 (US10183932, Example 135 | US9434690, 132 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413721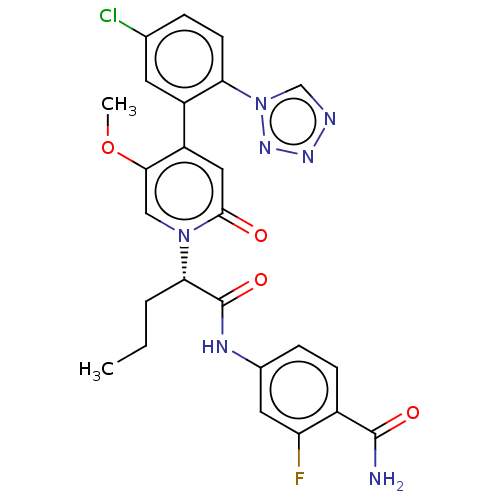 (4-{[(2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413721 (4-{[(2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413626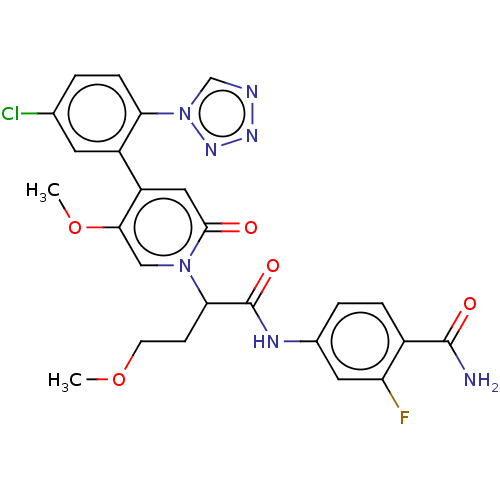 (4-[(2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413727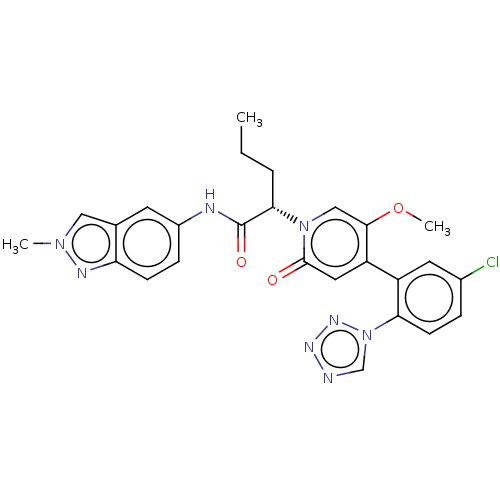 ((2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413626 (4-[(2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413727 ((2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413654 (4-[(2-{4-[5-Chloro-2-(1,2-oxazol-3-yl)phenyl]-5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413719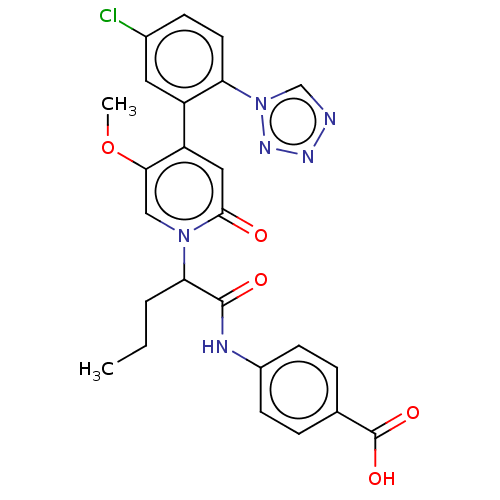 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413745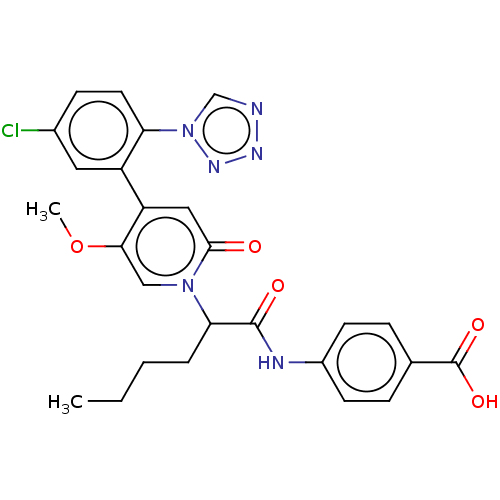 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413654 (4-[(2-{4-[5-Chloro-2-(1,2-oxazol-3-yl)phenyl]-5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413719 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413745 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413610 (4-{[(2S)-2-{4-[5-Chloro-2-(1,3-oxazol-5-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413610 (4-{[(2S)-2-{4-[5-Chloro-2-(1,3-oxazol-5-yl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413722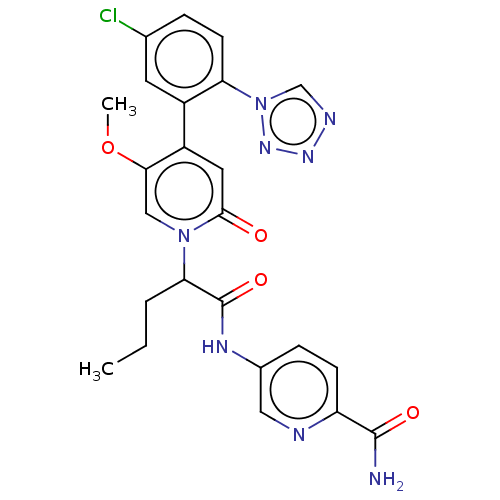 (5-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413722 (5-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413726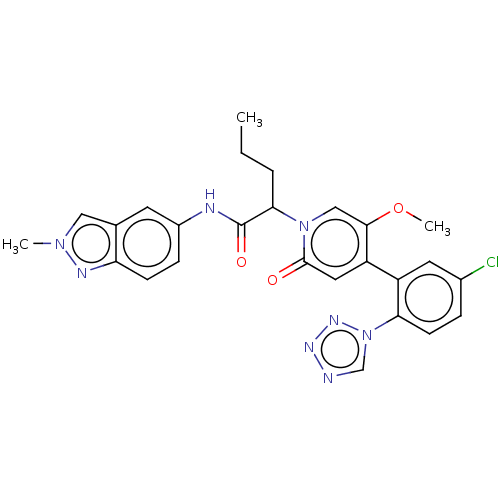 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246196 (US10183932, Example 105 | US9434690, 101 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413726 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM341292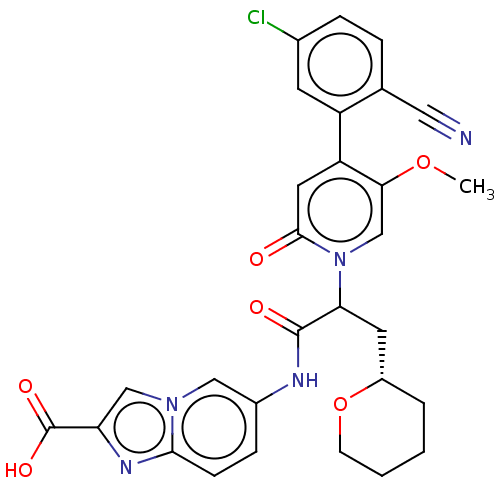 (6-({2-[4-(5-Chloro-2-cyanophenyl)-5-methoxy-2-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9765070 (2017) BindingDB Entry DOI: 10.7270/Q25D8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246196 (US10183932, Example 105 | US9434690, 101 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246196 (US10183932, Example 105 | US9434690, 101 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413652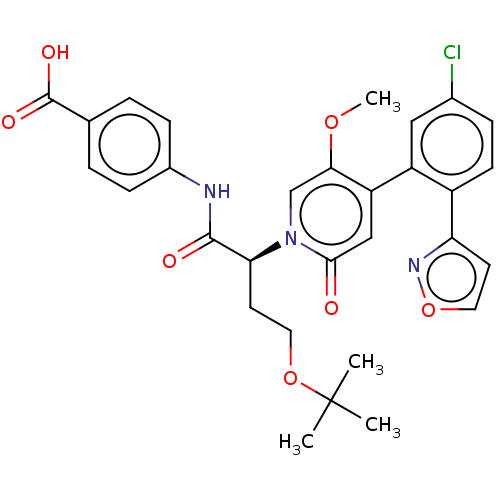 (4-{[(2S)-4-tert-Butoxy-2-{4-[5-chloro-2-(1,2-oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413652 (4-{[(2S)-4-tert-Butoxy-2-{4-[5-chloro-2-(1,2-oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413720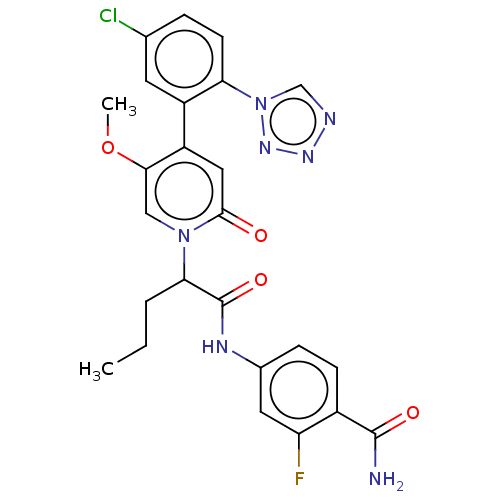 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413720 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413777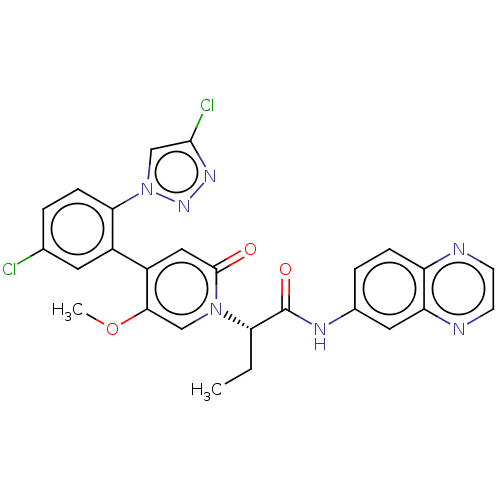 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(4-chlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413777 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(4-chlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413724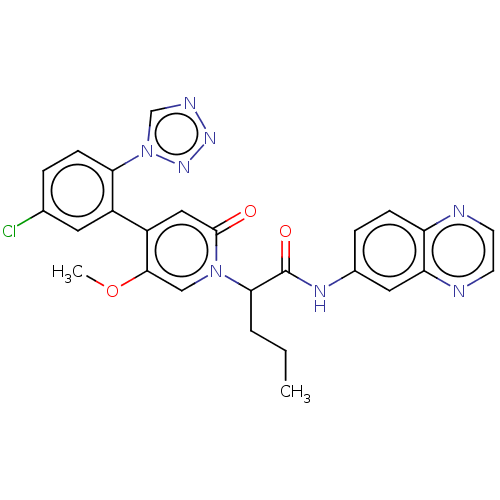 (N-(Quinoxalin-6-yl)-2-{4-[5-chloro-2-(1H-tetrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413724 (N-(Quinoxalin-6-yl)-2-{4-[5-chloro-2-(1H-tetrazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM413725 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(1H-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description To determine the plasma kallikrein inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reac... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM413725 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(1H-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma Kallikrein: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413746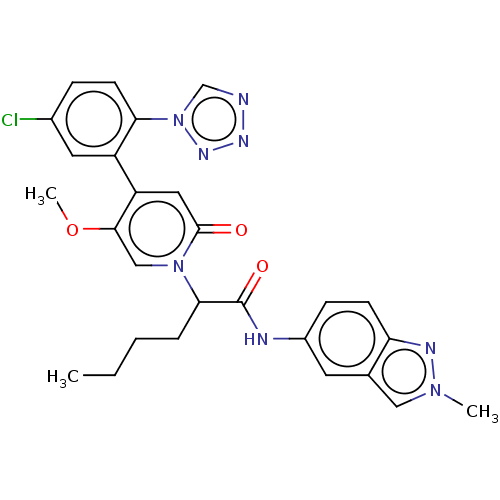 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413746 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246220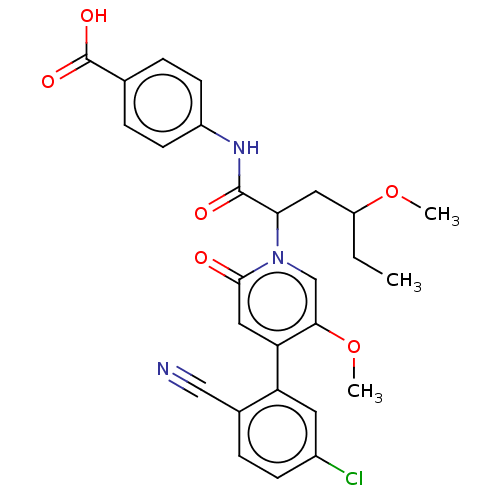 (US10183932, Example 144 | US9434690, 125 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9822102 (2017) BindingDB Entry DOI: 10.7270/Q2FN18G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246220 (US10183932, Example 144 | US9434690, 125 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine the factor XIa inhibition of the substances according to the invention, a biochemical test system is used which utilizes the reaction of... | US Patent US10183932 (2019) BindingDB Entry DOI: 10.7270/Q23T9K99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM246220 (US10183932, Example 144 | US9434690, 125 | US94346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting final con... | US Patent US9434690 (2016) BindingDB Entry DOI: 10.7270/Q2K936FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413773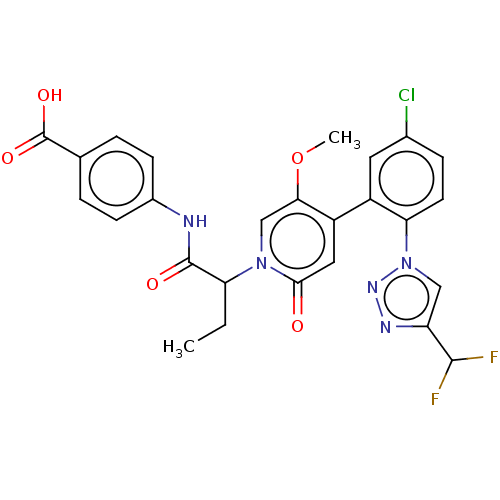 (4-({2-[4-{5-Chloro-2-[4-(difluoromethyl)-1H-1,2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413773 (4-({2-[4-{5-Chloro-2-[4-(difluoromethyl)-1H-1,2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413847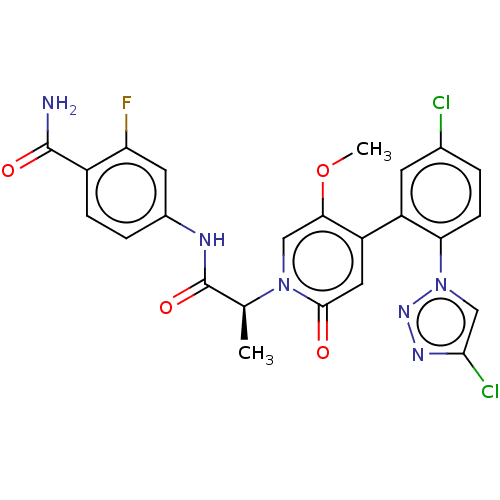 (4-{[(2S)-2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413847 (4-{[(2S)-2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413793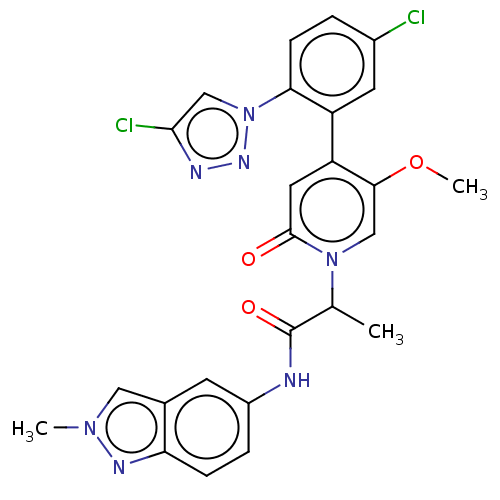 (2-{4-[5-Chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413797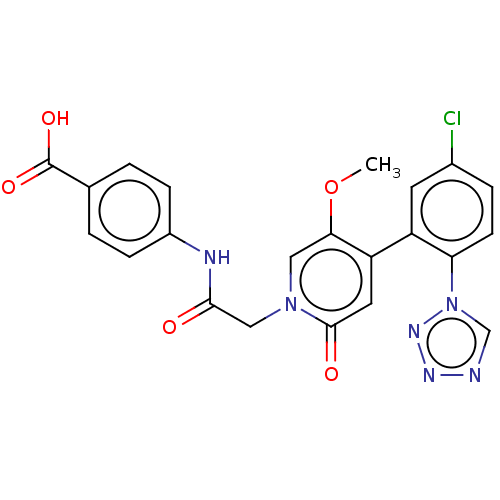 (4-[({4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2315 total ) | Next | Last >> |