 Found 153 hits with Last Name = 'oh' and Initial = 'dc'
Found 153 hits with Last Name = 'oh' and Initial = 'dc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459080
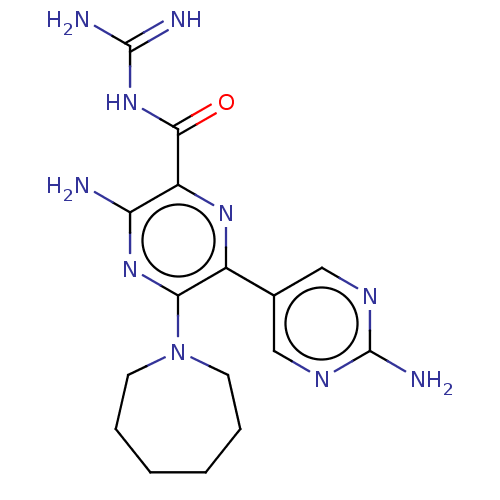
(CHEMBL4211423)Show SMILES NC(=N)NC(=O)c1nc(-c2cnc(N)nc2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C16H22N10O/c17-12-11(14(27)25-15(18)19)23-10(9-7-21-16(20)22-8-9)13(24-12)26-5-3-1-2-4-6-26/h7-8H,1-6H2,(H2,17,24)(H2,20,21,22)(H4,18,19,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459060
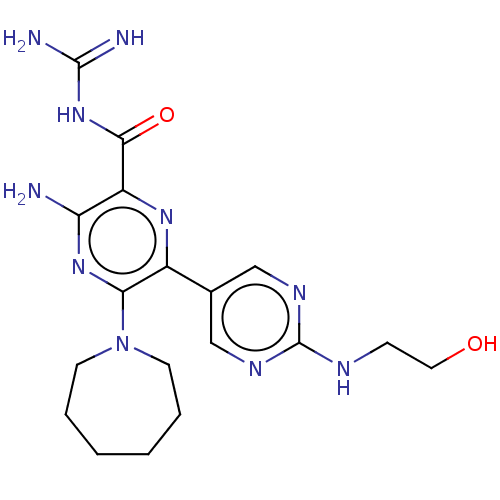
(CHEMBL4208530)Show SMILES NC(=N)NC(=O)c1nc(-c2cnc(NCCO)nc2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C18H26N10O2/c19-14-13(16(30)27-17(20)21)25-12(11-9-23-18(24-10-11)22-5-8-29)15(26-14)28-6-3-1-2-4-7-28/h9-10,29H,1-8H2,(H2,19,26)(H,22,23,24)(H4,20,21,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459079
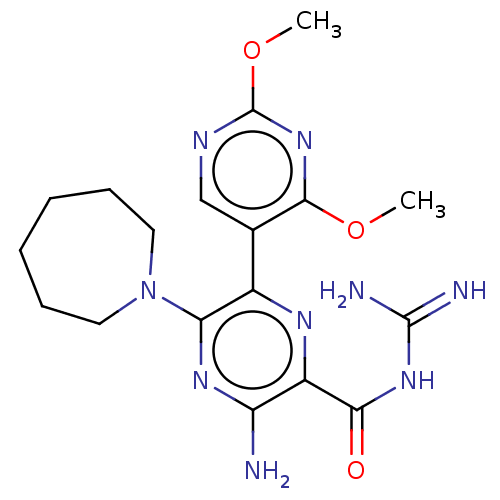
(CHEMBL4206764)Show SMILES COc1ncc(c(OC)n1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C18H25N9O3/c1-29-16-10(9-22-18(26-16)30-2)11-14(27-7-5-3-4-6-8-27)24-13(19)12(23-11)15(28)25-17(20)21/h9H,3-8H2,1-2H3,(H2,19,24)(H4,20,21,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459072
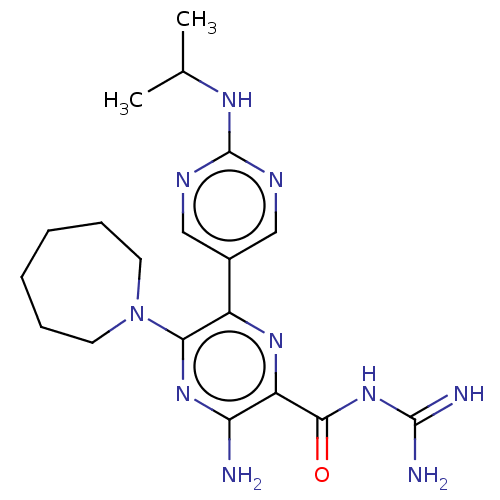
(CHEMBL4215861)Show SMILES CC(C)Nc1ncc(cn1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C19H28N10O/c1-11(2)25-19-23-9-12(10-24-19)13-16(29-7-5-3-4-6-8-29)27-15(20)14(26-13)17(30)28-18(21)22/h9-11H,3-8H2,1-2H3,(H2,20,27)(H,23,24,25)(H4,21,22,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459071
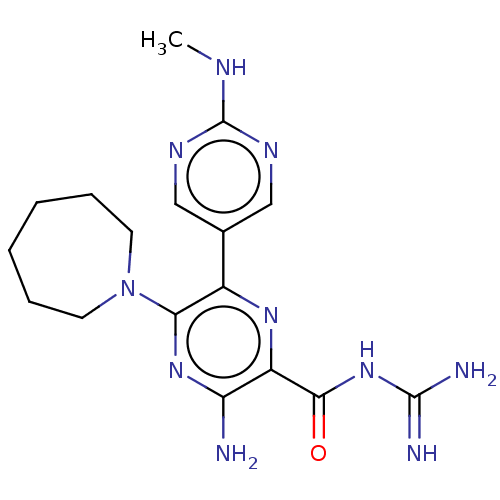
(CHEMBL4204195)Show SMILES CNc1ncc(cn1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C17H24N10O/c1-21-17-22-8-10(9-23-17)11-14(27-6-4-2-3-5-7-27)25-13(18)12(24-11)15(28)26-16(19)20/h8-9H,2-7H2,1H3,(H2,18,25)(H,21,22,23)(H4,19,20,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459059
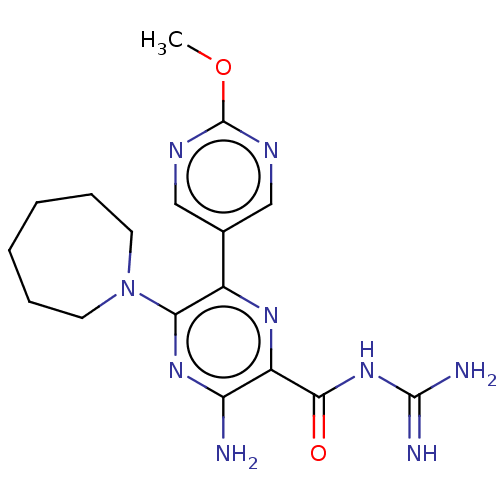
(CHEMBL4217043)Show SMILES COc1ncc(cn1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C17H23N9O2/c1-28-17-21-8-10(9-22-17)11-14(26-6-4-2-3-5-7-26)24-13(18)12(23-11)15(27)25-16(19)20/h8-9H,2-7H2,1H3,(H2,18,24)(H4,19,20,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459055
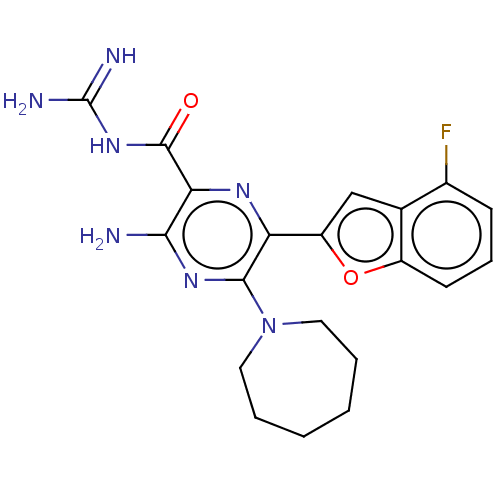
(CHEMBL4207715)Show SMILES NC(=N)NC(=O)c1nc(-c2cc3c(F)cccc3o2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C20H22FN7O2/c21-12-6-5-7-13-11(12)10-14(30-13)15-18(28-8-3-1-2-4-9-28)26-17(22)16(25-15)19(29)27-20(23)24/h5-7,10H,1-4,8-9H2,(H2,22,26)(H4,23,24,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459068
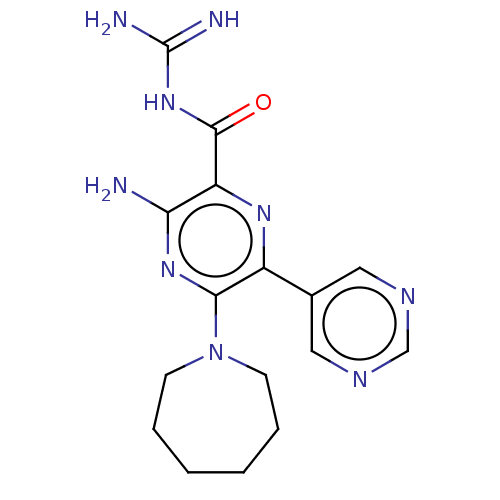
(CHEMBL4212068)Show SMILES NC(=N)NC(=O)c1nc(-c2cncnc2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C16H21N9O/c17-13-12(15(26)24-16(18)19)22-11(10-7-20-9-21-8-10)14(23-13)25-5-3-1-2-4-6-25/h7-9H,1-6H2,(H2,17,23)(H4,18,19,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459053
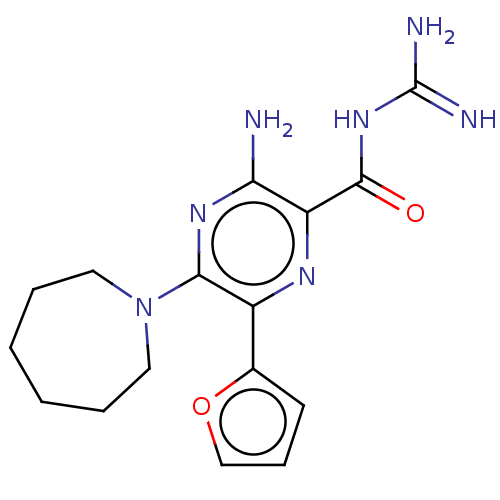
(CHEMBL4208241)Show InChI InChI=1S/C16H21N7O2/c17-13-12(15(24)22-16(18)19)20-11(10-6-5-9-25-10)14(21-13)23-7-3-1-2-4-8-23/h5-6,9H,1-4,7-8H2,(H2,17,21)(H4,18,19,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459078
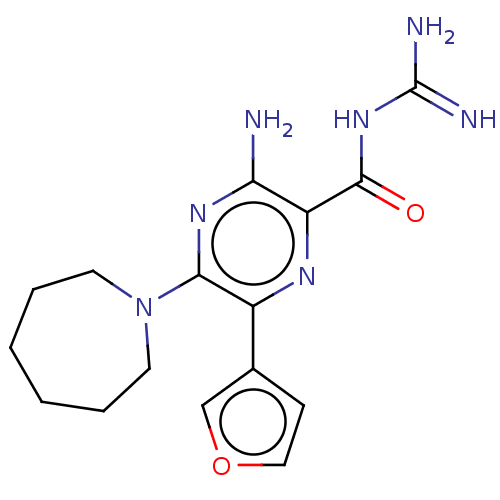
(CHEMBL4216516)Show InChI InChI=1S/C16H21N7O2/c17-13-12(15(24)22-16(18)19)20-11(10-5-8-25-9-10)14(21-13)23-6-3-1-2-4-7-23/h5,8-9H,1-4,6-7H2,(H2,17,21)(H4,18,19,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459054

(CHEMBL4213248)Show SMILES NC(=N)NC(=O)c1nc(-c2cc3ccccc3o2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C20H23N7O2/c21-17-16(19(28)26-20(22)23)24-15(14-11-12-7-3-4-8-13(12)29-14)18(25-17)27-9-5-1-2-6-10-27/h3-4,7-8,11H,1-2,5-6,9-10H2,(H2,21,25)(H4,22,23,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459073
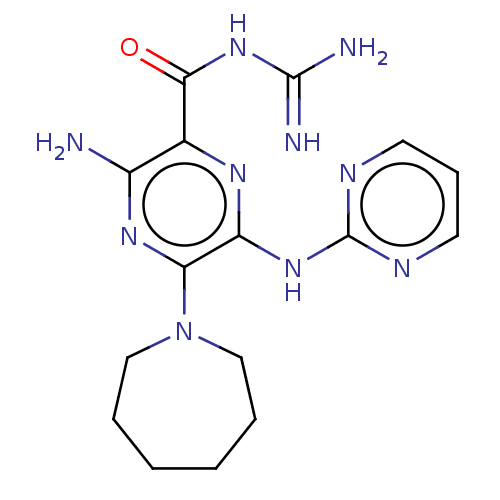
(CHEMBL4203654)Show SMILES NC(=N)NC(=O)c1nc(Nc2ncccn2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C16H22N10O/c17-11-10(14(27)25-15(18)19)22-12(24-16-20-6-5-7-21-16)13(23-11)26-8-3-1-2-4-9-26/h5-7H,1-4,8-9H2,(H2,17,23)(H4,18,19,25,27)(H,20,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459070
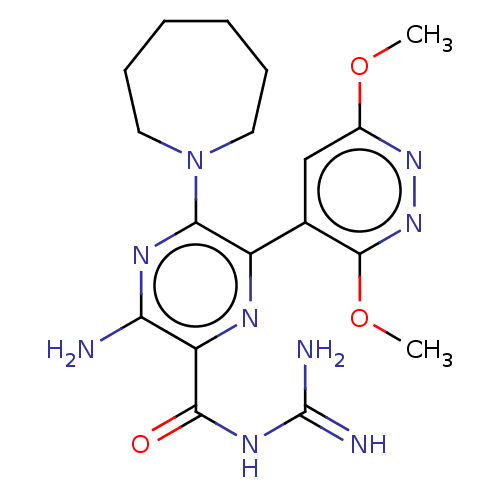
(CHEMBL4216389)Show SMILES COc1cc(c(OC)nn1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C18H25N9O3/c1-29-11-9-10(17(30-2)26-25-11)12-15(27-7-5-3-4-6-8-27)23-14(19)13(22-12)16(28)24-18(20)21/h9H,3-8H2,1-2H3,(H2,19,23)(H4,20,21,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 315 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459074
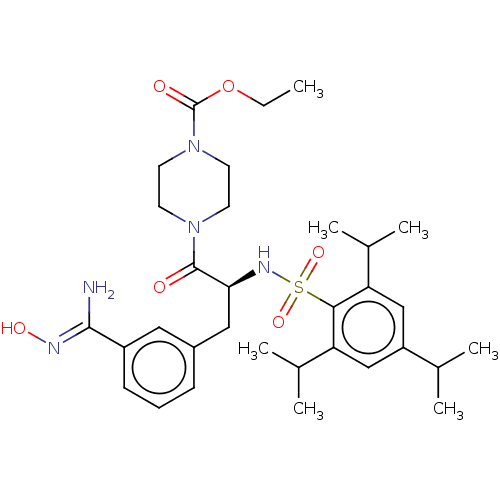
(Upamostat | Wx-671)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(\N)=N\O)NS(=O)(=O)c1c(cc(cc1C(C)C)C(C)C)C(C)C |r| Show InChI InChI=1S/C32H47N5O6S/c1-8-43-32(39)37-14-12-36(13-15-37)31(38)28(17-23-10-9-11-24(16-23)30(33)34-40)35-44(41,42)29-26(21(4)5)18-25(20(2)3)19-27(29)22(6)7/h9-11,16,18-22,28,35,40H,8,12-15,17H2,1-7H3,(H2,33,34)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of uPA (unknown origin) |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459064
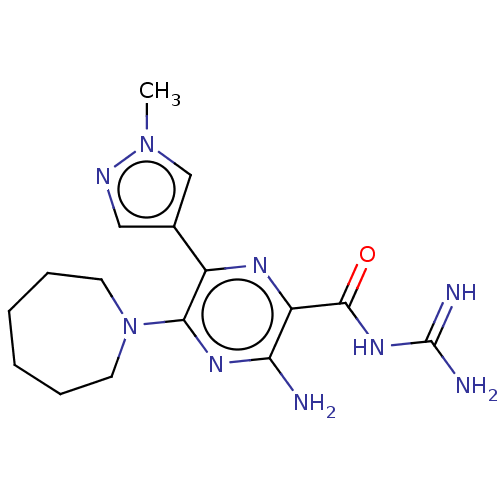
(CHEMBL4203547)Show SMILES Cn1cc(cn1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C16H23N9O/c1-24-9-10(8-20-24)11-14(25-6-4-2-3-5-7-25)22-13(17)12(21-11)15(26)23-16(18)19/h8-9H,2-7H2,1H3,(H2,17,22)(H4,18,19,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 508 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459077
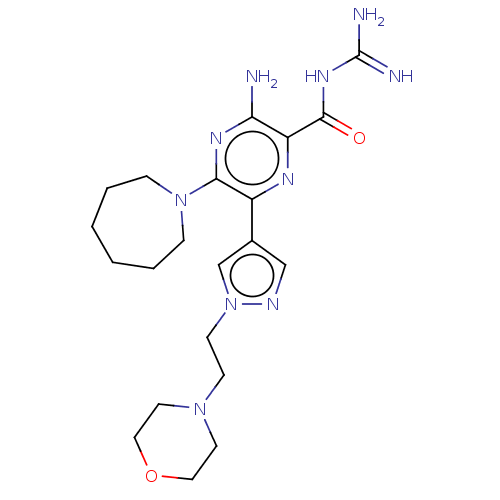
(CHEMBL4210813)Show SMILES NC(=N)NC(=O)c1nc(-c2cnn(CCN3CCOCC3)c2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C21H32N10O2/c22-18-17(20(32)28-21(23)24)26-16(19(27-18)30-5-3-1-2-4-6-30)15-13-25-31(14-15)8-7-29-9-11-33-12-10-29/h13-14H,1-12H2,(H2,22,27)(H4,23,24,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 658 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459052
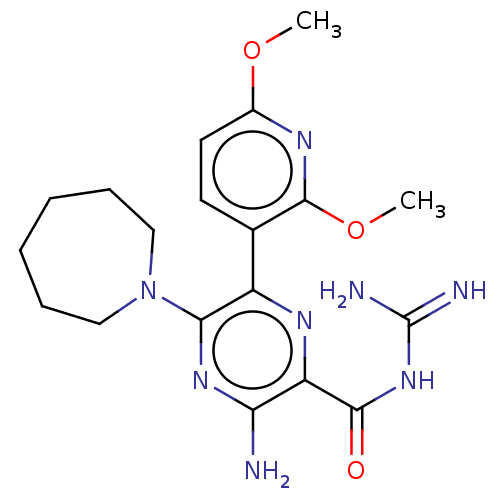
(CHEMBL4211642)Show SMILES COc1ccc(c(OC)n1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C19H26N8O3/c1-29-12-8-7-11(18(23-12)30-2)13-16(27-9-5-3-4-6-10-27)25-15(20)14(24-13)17(28)26-19(21)22/h7-8H,3-6,9-10H2,1-2H3,(H2,20,25)(H4,21,22,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 884 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459063
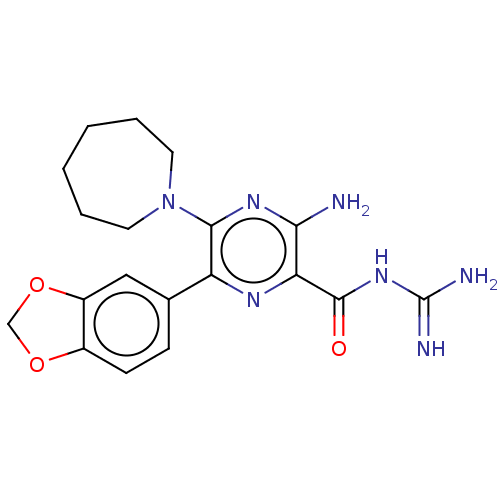
(CHEMBL4218928)Show SMILES NC(=N)NC(=O)c1nc(-c2ccc3OCOc3c2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C19H23N7O3/c20-16-15(18(27)25-19(21)22)23-14(11-5-6-12-13(9-11)29-10-28-12)17(24-16)26-7-3-1-2-4-8-26/h5-6,9H,1-4,7-8,10H2,(H2,20,24)(H4,21,22,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 934 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459057
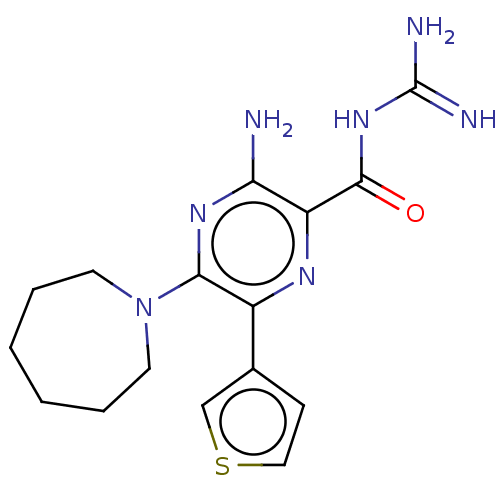
(CHEMBL4203367)Show InChI InChI=1S/C16H21N7OS/c17-13-12(15(24)22-16(18)19)20-11(10-5-8-25-9-10)14(21-13)23-6-3-1-2-4-7-23/h5,8-9H,1-4,6-7H2,(H2,17,21)(H4,18,19,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459058
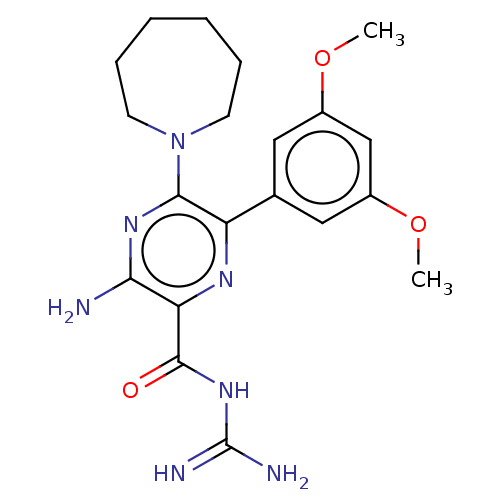
(CHEMBL4207061)Show SMILES COc1cc(OC)cc(c1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C20H27N7O3/c1-29-13-9-12(10-14(11-13)30-2)15-18(27-7-5-3-4-6-8-27)25-17(21)16(24-15)19(28)26-20(22)23/h9-11H,3-8H2,1-2H3,(H2,21,25)(H4,22,23,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81818

(CAS_1794 | CHEMBL1909810 | HMA | NSC_1794)Show InChI InChI=1S/C12H18ClN7O/c13-8-10(20-5-3-1-2-4-6-20)18-9(14)7(17-8)11(21)19-12(15)16/h1-6H2,(H2,14,18)(H4,15,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50459059

(CHEMBL4217043)Show SMILES COc1ncc(cn1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C17H23N9O2/c1-28-17-21-8-10(9-22-17)11-14(26-6-4-2-3-5-7-26)24-13(18)12(23-11)15(27)25-16(19)20/h8-9H,2-7H2,1H3,(H2,18,24)(H4,19,20,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate after 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459067
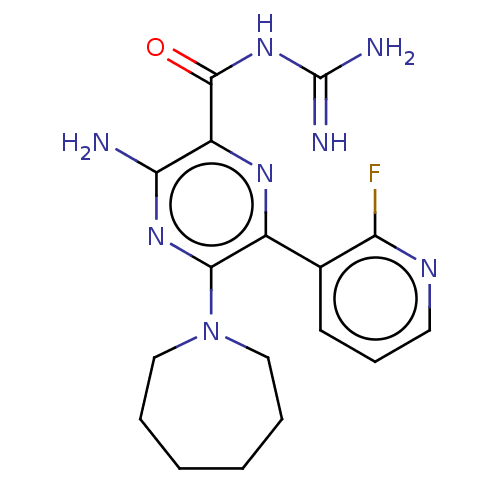
(CHEMBL4204836)Show SMILES NC(=N)NC(=O)c1nc(c(nc1N)N1CCCCCC1)-c1cccnc1F Show InChI InChI=1S/C17H21FN8O/c18-13-10(6-5-7-22-13)11-15(26-8-3-1-2-4-9-26)24-14(19)12(23-11)16(27)25-17(20)21/h5-7H,1-4,8-9H2,(H2,19,24)(H4,20,21,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM50459054

(CHEMBL4213248)Show SMILES NC(=N)NC(=O)c1nc(-c2cc3ccccc3o2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C20H23N7O2/c21-17-16(19(28)26-20(22)23)24-15(14-11-12-7-3-4-8-13(12)29-14)18(25-17)27-9-5-1-2-6-10-27/h3-4,7-8,11H,1-2,5-6,9-10H2,(H2,21,25)(H4,22,23,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate after 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459069
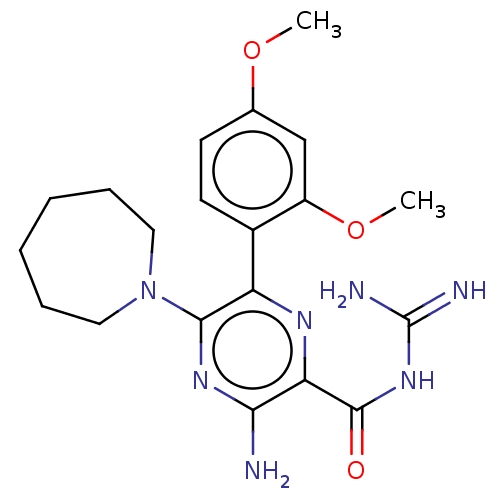
(CHEMBL4211937)Show SMILES COc1ccc(c(OC)c1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C20H27N7O3/c1-29-12-7-8-13(14(11-12)30-2)15-18(27-9-5-3-4-6-10-27)25-17(21)16(24-15)19(28)26-20(22)23/h7-8,11H,3-6,9-10H2,1-2H3,(H2,21,25)(H4,22,23,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM16173
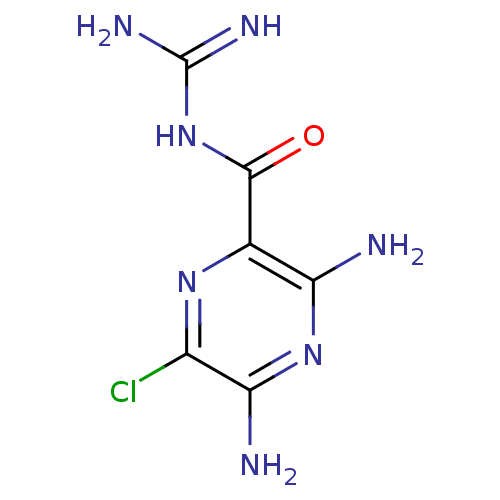
(3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...)Show InChI InChI=1S/C6H8ClN7O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11/h(H4,8,9,13)(H4,10,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate after 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
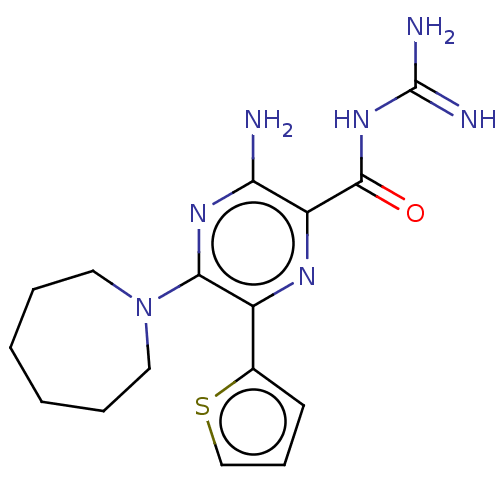
(Homo sapiens (Human)) | BDBM50459066
(CHEMBL4212621)Show InChI InChI=1S/C16H21N7OS/c17-13-12(15(24)22-16(18)19)20-11(10-6-5-9-25-10)14(21-13)23-7-3-1-2-4-8-23/h5-6,9H,1-4,7-8H2,(H2,17,21)(H4,18,19,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16173

(3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...)Show InChI InChI=1S/C6H8ClN7O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11/h(H4,8,9,13)(H4,10,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
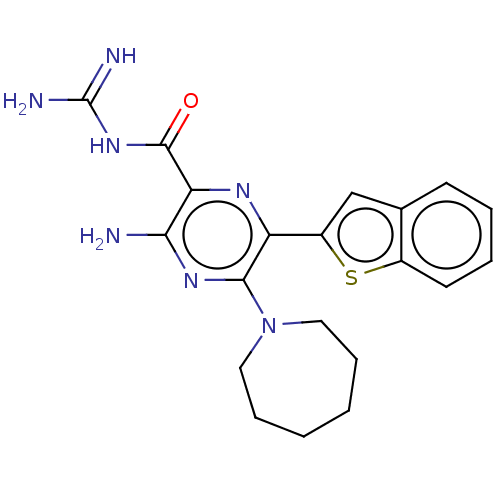
(Homo sapiens (Human)) | BDBM50459075
(CHEMBL4208316)Show SMILES NC(=N)NC(=O)c1nc(-c2cc3ccccc3s2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C20H23N7OS/c21-17-16(19(28)26-20(22)23)24-15(14-11-12-7-3-4-8-13(12)29-14)18(25-17)27-9-5-1-2-6-10-27/h3-4,7-8,11H,1-2,5-6,9-10H2,(H2,21,25)(H4,22,23,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459056
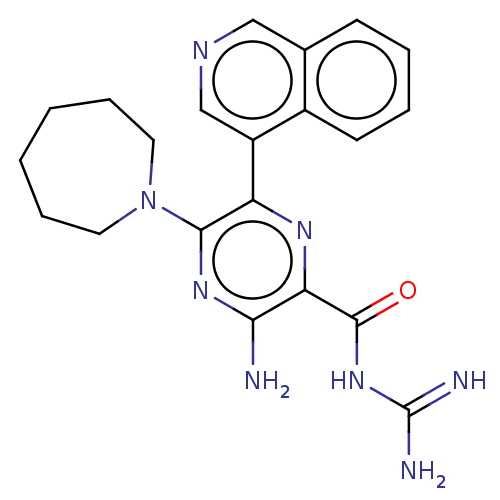
(CHEMBL4215225)Show SMILES NC(=N)NC(=O)c1nc(c(nc1N)N1CCCCCC1)-c1cncc2ccccc12 Show InChI InChI=1S/C21H24N8O/c22-18-17(20(30)28-21(23)24)26-16(19(27-18)29-9-5-1-2-6-10-29)15-12-25-11-13-7-3-4-8-14(13)15/h3-4,7-8,11-12H,1-2,5-6,9-10H2,(H2,22,27)(H4,23,24,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459076
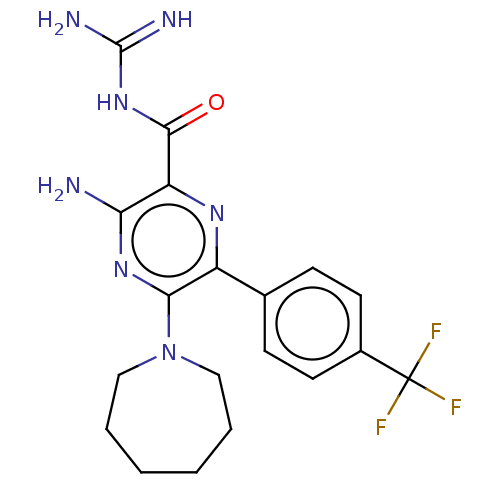
(CHEMBL4203024)Show SMILES NC(=N)NC(=O)c1nc(-c2ccc(cc2)C(F)(F)F)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C19H22F3N7O/c20-19(21,22)12-7-5-11(6-8-12)13-16(29-9-3-1-2-4-10-29)27-15(23)14(26-13)17(30)28-18(24)25/h5-8H,1-4,9-10H2,(H2,23,27)(H4,24,25,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459061
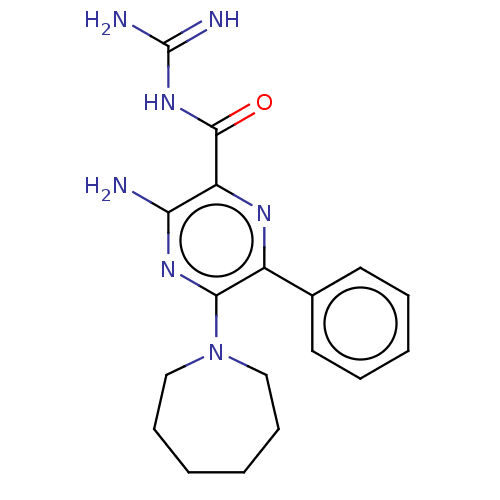
(CHEMBL4207379)Show SMILES NC(=N)NC(=O)c1nc(-c2ccccc2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C18H23N7O/c19-15-14(17(26)24-18(20)21)22-13(12-8-4-3-5-9-12)16(23-15)25-10-6-1-2-7-11-25/h3-5,8-9H,1-2,6-7,10-11H2,(H2,19,23)(H4,20,21,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459065
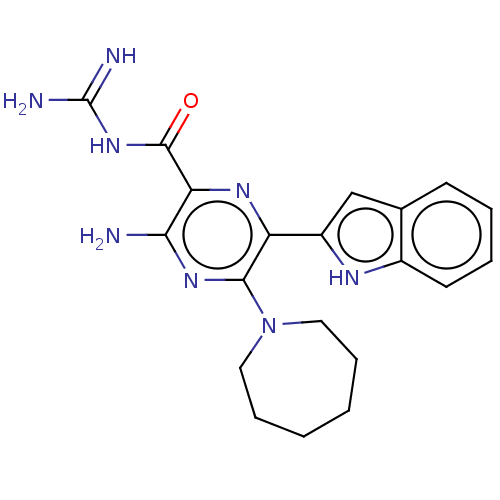
(CHEMBL4215760)Show SMILES NC(=N)NC(=O)c1nc(-c2cc3ccccc3[nH]2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C20H24N8O/c21-17-16(19(29)27-20(22)23)25-15(14-11-12-7-3-4-8-13(12)24-14)18(26-17)28-9-5-1-2-6-10-28/h3-4,7-8,11,24H,1-2,5-6,9-10H2,(H2,21,26)(H4,22,23,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM16173

(3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...)Show InChI InChI=1S/C6H8ClN7O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11/h(H4,8,9,13)(H4,10,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of uPA (unknown origin) using L-pyroglutamyl-glycyl-L-arginine-p-nitro-anilide as susbtrate by Lineweaver-Burk plot analysis |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Mus musculus (Mouse)) | BDBM81818

(CAS_1794 | CHEMBL1909810 | HMA | NSC_1794)Show InChI InChI=1S/C12H18ClN7O/c13-8-10(20-5-3-1-2-4-6-20)18-9(14)7(17-8)11(21)19-12(15)16/h1-6H2,(H2,14,18)(H4,15,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of mouse uPA using Z-Gly-Gly-Arg-AMC as substrate after 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459062
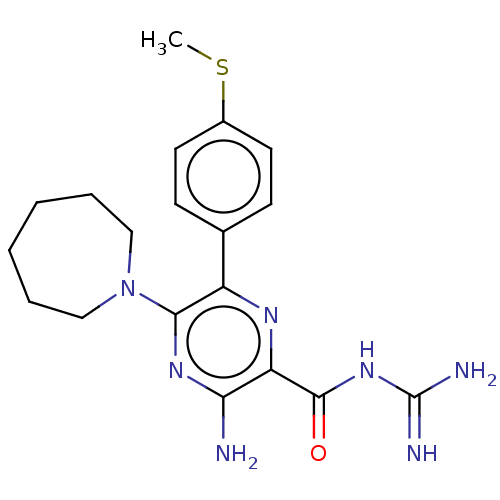
(CHEMBL4212269)Show SMILES CSc1ccc(cc1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C19H25N7OS/c1-28-13-8-6-12(7-9-13)14-17(26-10-4-2-3-5-11-26)24-16(20)15(23-14)18(27)25-19(21)22/h6-9H,2-5,10-11H2,1H3,(H2,20,24)(H4,21,22,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using Z-Gly-Gly-Arg-AMC as substrate measured over 15 mins by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147093
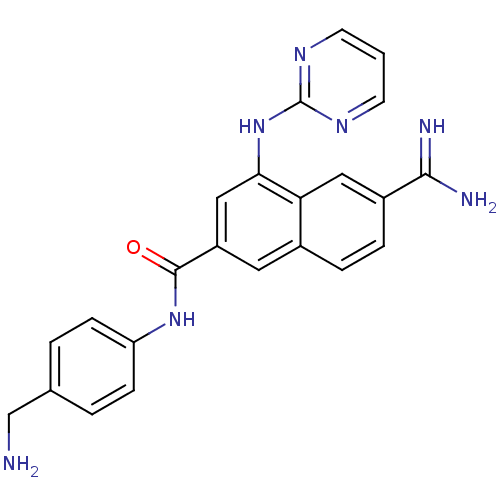
(6-Carbamimidoyl-4-(pyrimidin-2-ylamino)-naphthalen...)Show SMILES NCc1ccc(NC(=O)c2cc(Nc3ncccn3)c3cc(ccc3c2)C(N)=N)cc1 Show InChI InChI=1S/C23H21N7O/c24-13-14-2-6-18(7-3-14)29-22(31)17-10-15-4-5-16(21(25)26)11-19(15)20(12-17)30-23-27-8-1-9-28-23/h1-12H,13,24H2,(H3,25,26)(H,29,31)(H,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of uPA (unknown origin) |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459059

(CHEMBL4217043)Show SMILES COc1ncc(cn1)-c1nc(C(=O)NC(N)=N)c(N)nc1N1CCCCCC1 Show InChI InChI=1S/C17H23N9O2/c1-28-17-21-8-10(9-22-17)11-14(26-6-4-2-3-5-7-26)24-13(18)12(23-11)15(27)25-16(19)20/h8-9H,2-7H2,1H3,(H2,18,24)(H4,19,20,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using chromogenic H-D-Ile-L-Pro-L-Arg-p-nitoraniline as substrate by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50099848
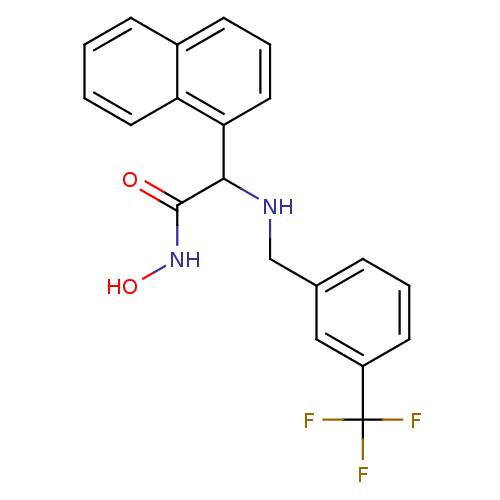
(CHEMBL30051 | N-Hydroxy-2-naphthalen-1-yl-2-(3-tri...)Show SMILES ONC(=O)C(NCc1cccc(c1)C(F)(F)F)c1cccc2ccccc12 Show InChI InChI=1S/C20H17F3N2O2/c21-20(22,23)15-8-3-5-13(11-15)12-24-18(19(26)25-27)17-10-4-7-14-6-1-2-9-16(14)17/h1-11,18,24,27H,12H2,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Peptidyl deformylase (PDF) |
Bioorg Med Chem Lett 11: 1355-8 (2001)
BindingDB Entry DOI: 10.7270/Q2D50M7N |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50099846
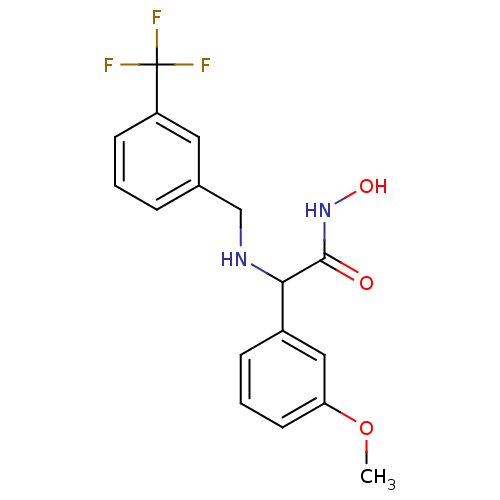
(CHEMBL29911 | N-Hydroxy-2-(3-methoxy-phenyl)-2-(3-...)Show InChI InChI=1S/C17H17F3N2O3/c1-25-14-7-3-5-12(9-14)15(16(23)22-24)21-10-11-4-2-6-13(8-11)17(18,19)20/h2-9,15,21,24H,10H2,1H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Peptidyl deformylase (PDF) |
Bioorg Med Chem Lett 11: 1355-8 (2001)
BindingDB Entry DOI: 10.7270/Q2D50M7N |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50099855
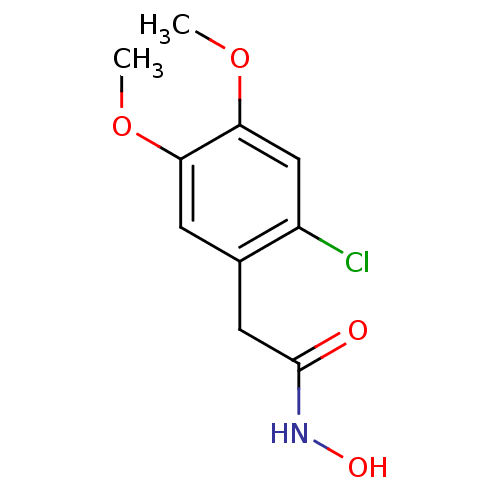
(2-(2-Chloro-4,5-dimethoxy-phenyl)-N-hydroxy-acetam...)Show InChI InChI=1S/C10H12ClNO4/c1-15-8-3-6(4-10(13)12-14)7(11)5-9(8)16-2/h3,5,14H,4H2,1-2H3,(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Peptidyl deformylase (PDF) |
Bioorg Med Chem Lett 11: 1355-8 (2001)
BindingDB Entry DOI: 10.7270/Q2D50M7N |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459055

(CHEMBL4207715)Show SMILES NC(=N)NC(=O)c1nc(-c2cc3c(F)cccc3o2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C20H22FN7O2/c21-12-6-5-7-13-11(12)10-14(30-13)15-18(28-8-3-1-2-4-9-28)26-17(22)16(25-15)19(29)27-20(23)24/h5-7,10H,1-4,8-9H2,(H2,22,26)(H4,23,24,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using chromogenic H-D-Ile-L-Pro-L-Arg-p-nitoraniline as substrate by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50099849
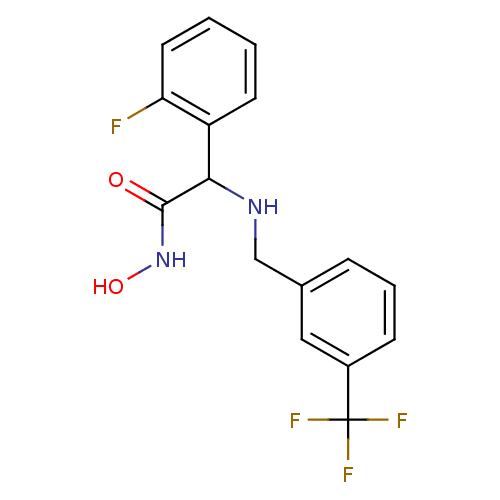
(2-(2-Fluoro-phenyl)-N-hydroxy-2-(3-trifluoromethyl...)Show InChI InChI=1S/C16H14F4N2O2/c17-13-7-2-1-6-12(13)14(15(23)22-24)21-9-10-4-3-5-11(8-10)16(18,19)20/h1-8,14,21,24H,9H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Peptidyl deformylase (PDF) |
Bioorg Med Chem Lett 11: 1355-8 (2001)
BindingDB Entry DOI: 10.7270/Q2D50M7N |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50459054

(CHEMBL4213248)Show SMILES NC(=N)NC(=O)c1nc(-c2cc3ccccc3o2)c(nc1N)N1CCCCCC1 Show InChI InChI=1S/C20H23N7O2/c21-17-16(19(28)26-20(22)23)24-15(14-11-12-7-3-4-8-13(12)29-14)18(25-17)27-9-5-1-2-6-10-27/h3-4,7-8,11H,1-2,5-6,9-10H2,(H2,21,25)(H4,22,23,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using chromogenic H-D-Ile-L-Pro-L-Arg-p-nitoraniline as substrate by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50099847

(2-(3,4-Difluoro-phenyl)-N-hydroxy-2-(3-trifluorome...)Show SMILES ONC(=O)C(NCc1cccc(c1)C(F)(F)F)c1ccc(F)c(F)c1 Show InChI InChI=1S/C16H13F5N2O2/c17-12-5-4-10(7-13(12)18)14(15(24)23-25)22-8-9-2-1-3-11(6-9)16(19,20)21/h1-7,14,22,25H,8H2,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Peptidyl deformylase (PDF) |
Bioorg Med Chem Lett 11: 1355-8 (2001)
BindingDB Entry DOI: 10.7270/Q2D50M7N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was evaluated in vitro against HIV- I reverse transcriptase (RT) |
Bioorg Med Chem Lett 5: 1875-1880 (1995)
Article DOI: 10.1016/0960-894X(95)00311-G
BindingDB Entry DOI: 10.7270/Q2BC3ZGH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50099856

(CHEMBL29912 | N'-hydroxy-N-1-naphthyl-N-{2-[3-(tri...)Show SMILES ONC(=O)N(CCc1cccc(c1)C(F)(F)F)c1cccc2ccccc12 Show InChI InChI=1S/C20H17F3N2O2/c21-20(22,23)16-8-3-5-14(13-16)11-12-25(19(26)24-27)18-10-4-7-15-6-1-2-9-17(15)18/h1-10,13,27H,11-12H2,(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Peptidyl deformylase (PDF) |
Bioorg Med Chem Lett 11: 1355-8 (2001)
BindingDB Entry DOI: 10.7270/Q2D50M7N |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM81818

(CAS_1794 | CHEMBL1909810 | HMA | NSC_1794)Show InChI InChI=1S/C12H18ClN7O/c13-8-10(20-5-3-1-2-4-6-20)18-9(14)7(17-8)11(21)19-12(15)16/h1-6H2,(H2,14,18)(H4,15,16,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of human kidney uPA using chromogenic H-D-Ile-L-Pro-L-Arg-p-nitoraniline as substrate by fluorescence assay |
J Med Chem 61: 8299-8320 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00838
BindingDB Entry DOI: 10.7270/Q2QF8WH4 |
More data for this
Ligand-Target Pair | |
Peptide deformylase
(Staphylococcus aureus (strain Mu50 / ATCC 700699)) | BDBM50099853
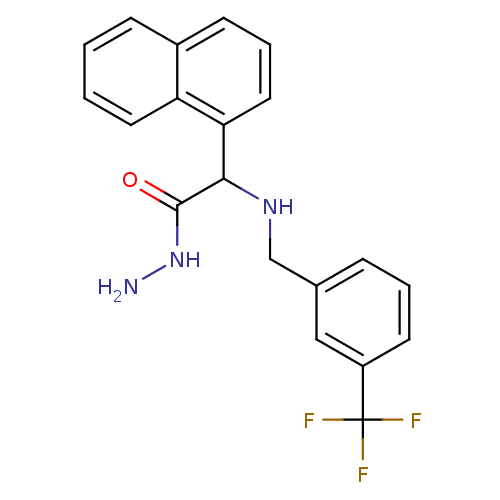
(CHEMBL26420 | Naphthalen-1-yl-(3-trifluoromethyl-b...)Show SMILES NNC(=O)C(NCc1cccc(c1)C(F)(F)F)c1cccc2ccccc12 Show InChI InChI=1S/C20H18F3N3O/c21-20(22,23)15-8-3-5-13(11-15)12-25-18(19(27)26-24)17-10-4-7-14-6-1-2-9-16(14)17/h1-11,18,25H,12,24H2,(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Peptidyl deformylase (PDF) |
Bioorg Med Chem Lett 11: 1355-8 (2001)
BindingDB Entry DOI: 10.7270/Q2D50M7N |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1437
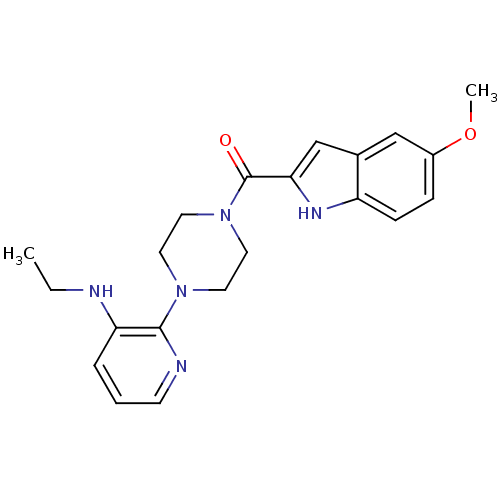
(5-methoxyindole-2-carboxylic acid [N -[3-(aminoeth...)Show SMILES CCNc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(OC)ccc2[nH]1 Show InChI InChI=1S/C21H25N5O2/c1-3-22-18-5-4-8-23-20(18)25-9-11-26(12-10-25)21(27)19-14-15-13-16(28-2)6-7-17(15)24-19/h4-8,13-14,22,24H,3,9-12H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was evaluated in vitro against HIV- I reverse transcriptase (RT) |
Bioorg Med Chem Lett 5: 1875-1880 (1995)
Article DOI: 10.1016/0960-894X(95)00311-G
BindingDB Entry DOI: 10.7270/Q2BC3ZGH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data