

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50428854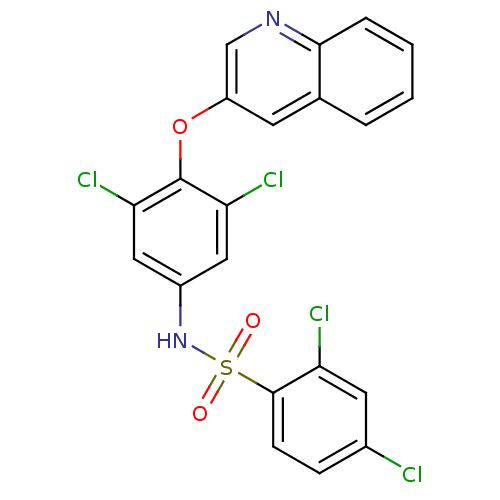 (CHEMBL1236924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Displacement of [3H]rosiglitazone from PPARgamma (unknown origin) | Bioorg Med Chem 24: 5455-5461 (2016) Article DOI: 10.1016/j.bmc.2016.08.067 BindingDB Entry DOI: 10.7270/Q21V5JGB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247432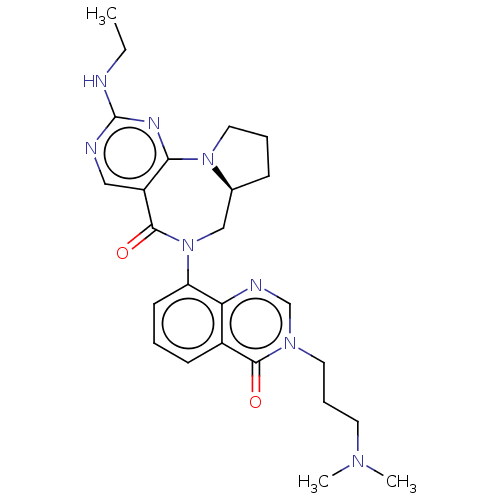 (US9453021, 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247431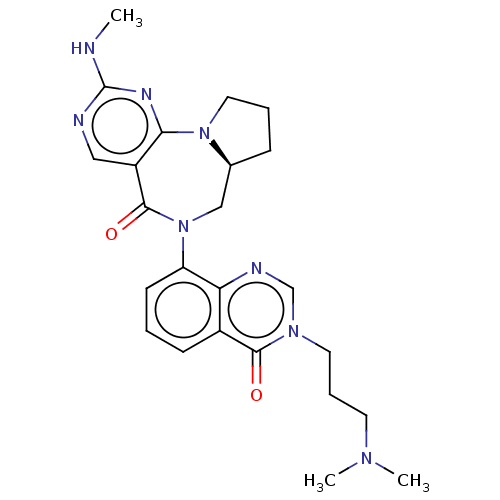 (US9453021, 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50180193 (AZD-2624 | AZD2624) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]His3-MePhe7)-NKB from human NK3R expressed in CHO cell membranes by topcounting method | Bioorg Med Chem 24: 3494-500 (2016) Article DOI: 10.1016/j.bmc.2016.05.054 BindingDB Entry DOI: 10.7270/Q2RR216W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247426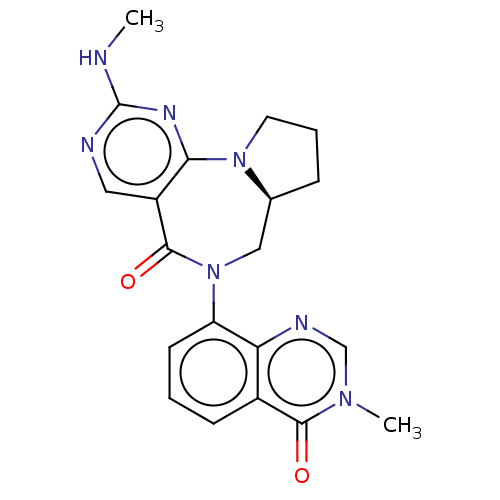 (US9453021, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247427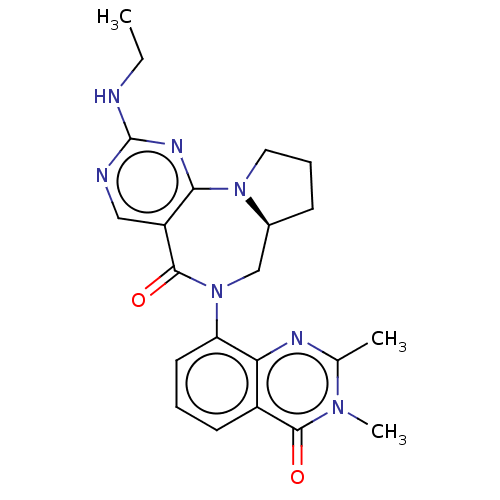 (US9453021, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247439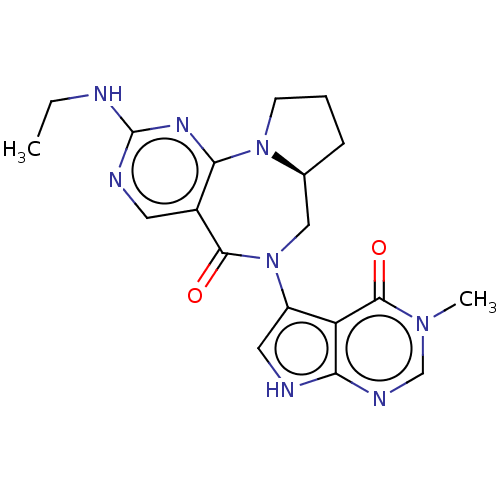 (US9453021, 113) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247424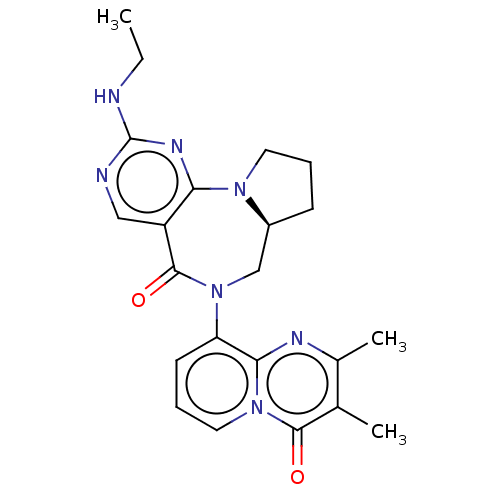 (US9453021, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247436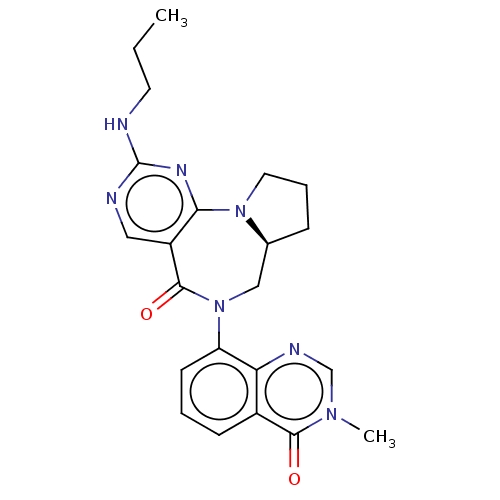 (US9453021, 71) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247437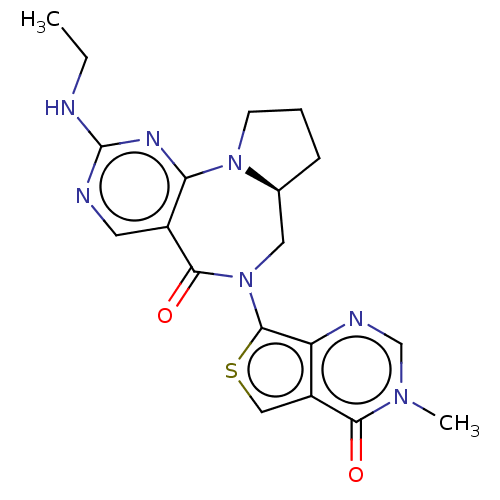 (US9453021, 97) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50430145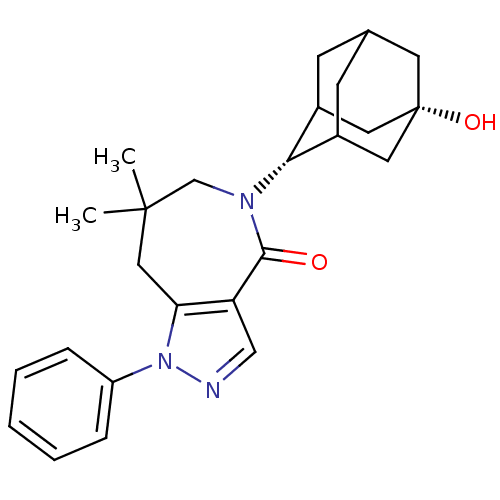 (CHEMBL2338266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247438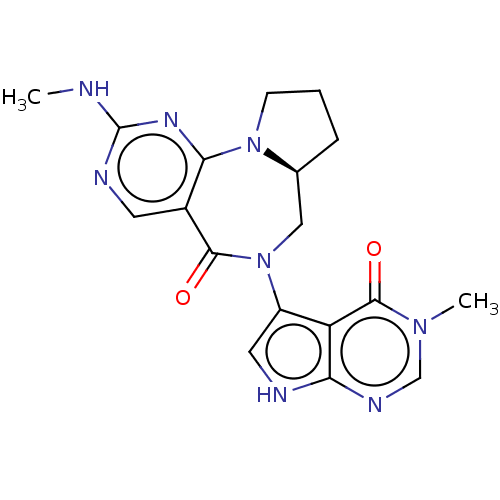 (US9453021, 112) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247434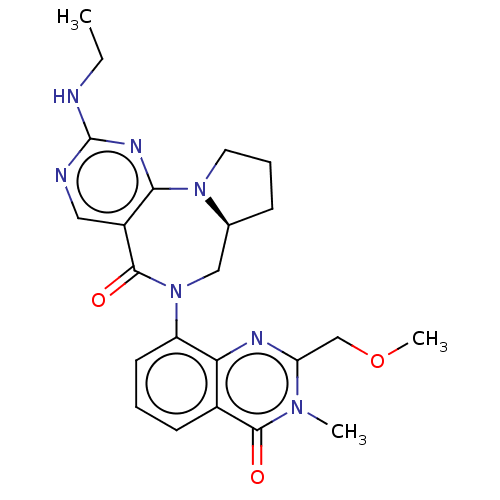 (US9453021, 65) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50430135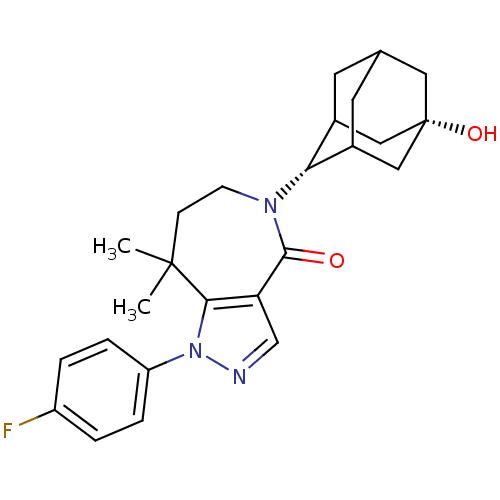 (CHEMBL2338251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50180171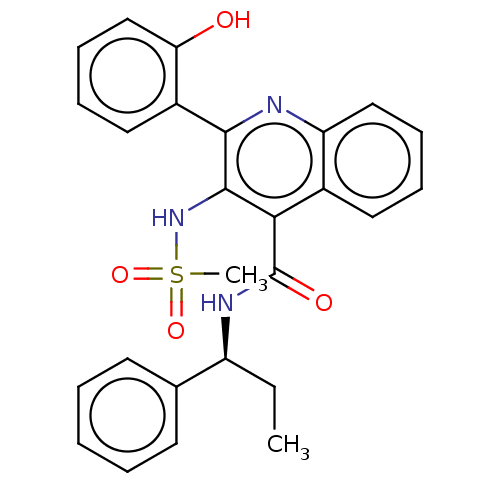 (CHEMBL3813763) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]His3-MePhe7)-NKB from human NK3R expressed in CHO cell membranes by topcounting method | Bioorg Med Chem 24: 3494-500 (2016) Article DOI: 10.1016/j.bmc.2016.05.054 BindingDB Entry DOI: 10.7270/Q2RR216W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247429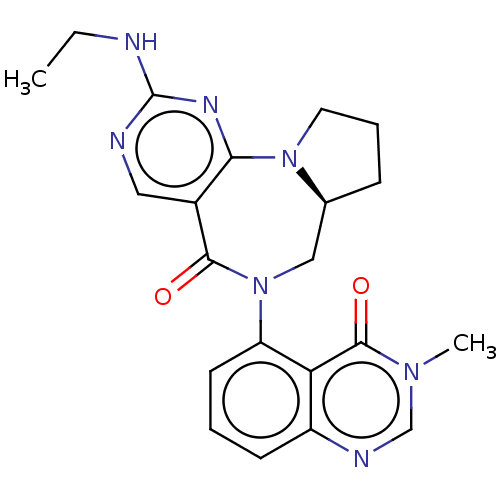 (US9453021, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247428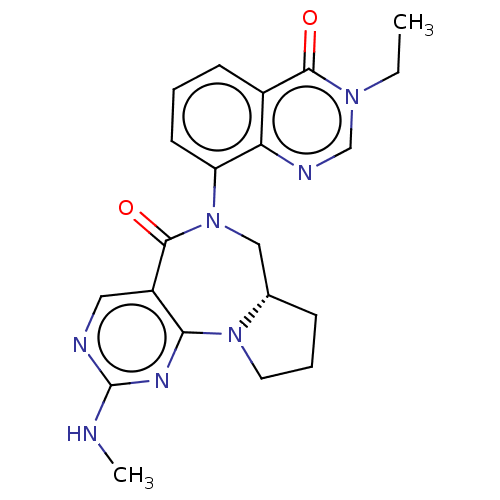 (US9453021, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247435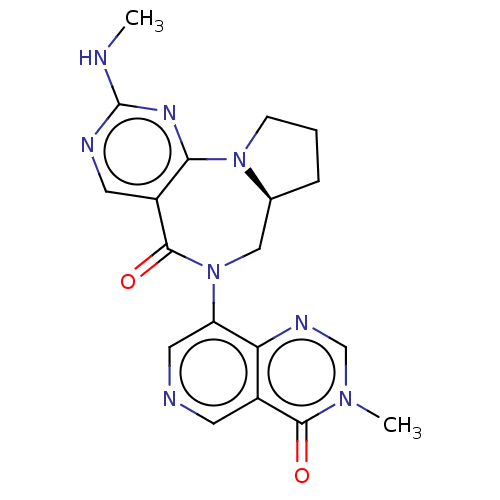 (US9453021, 68) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50144654 (CHEMBL3760043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247425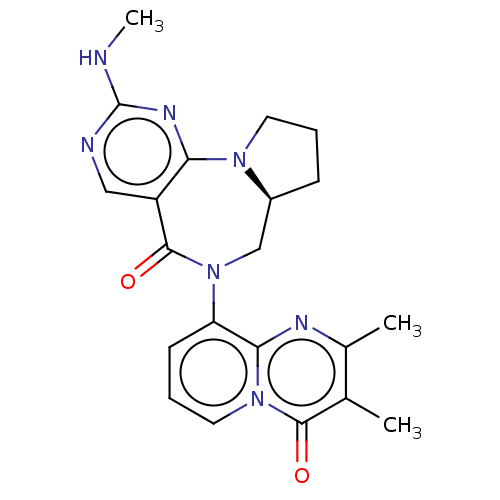 (US9453021, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247433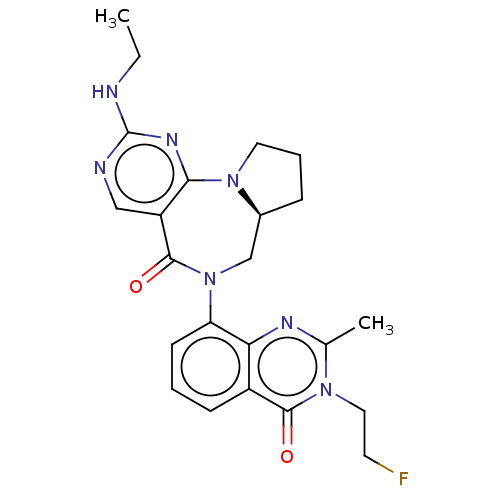 (US9453021, 59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247430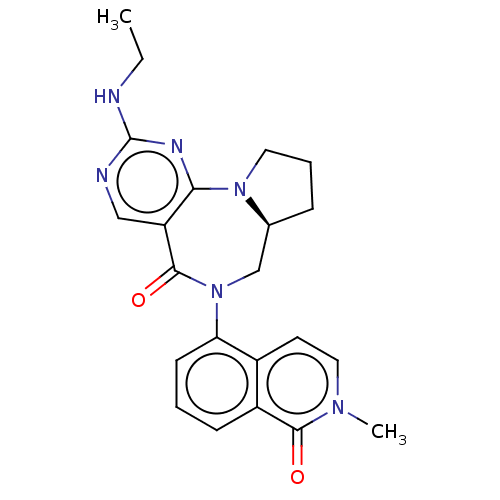 (US9453021, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-2/delta (Rattus norvegicus (Rat)) | BDBM247423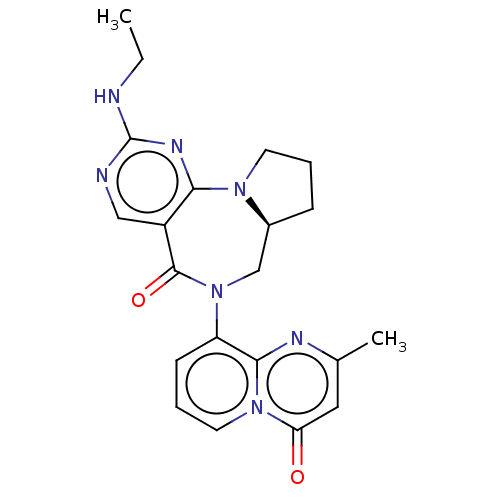 (US9453021, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Kyowa Hakko Kirin Co., Ltd. US Patent | Assay Description A test compound (20 μL) diluted to 5 times of the final concentration with Binding buffer, [3H]-gabapentin diluted to 100 nmol/L with binding buff... | US Patent US9453021 (2016) BindingDB Entry DOI: 10.7270/Q2251H4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50430139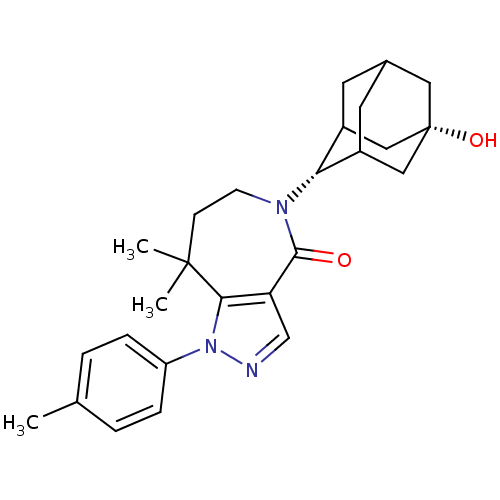 (CHEMBL2338247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50180169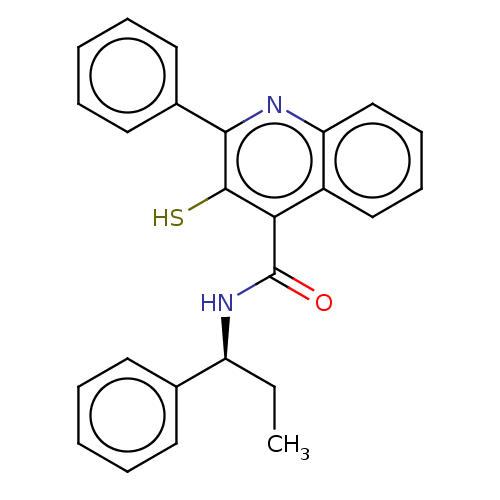 (CHEMBL3814787) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]His3-MePhe7)-NKB from human NK3R expressed in CHO cell membranes by topcounting method | Bioorg Med Chem 24: 3494-500 (2016) Article DOI: 10.1016/j.bmc.2016.05.054 BindingDB Entry DOI: 10.7270/Q2RR216W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50180174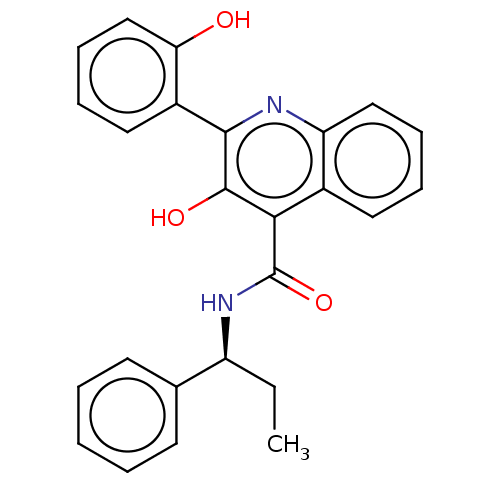 (CHEMBL3813719) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]His3-MePhe7)-NKB from human NK3R expressed in CHO cell membranes by topcounting method | Bioorg Med Chem 24: 3494-500 (2016) Article DOI: 10.1016/j.bmc.2016.05.054 BindingDB Entry DOI: 10.7270/Q2RR216W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50430136 (CHEMBL2338250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50430140 (CHEMBL2338246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50430141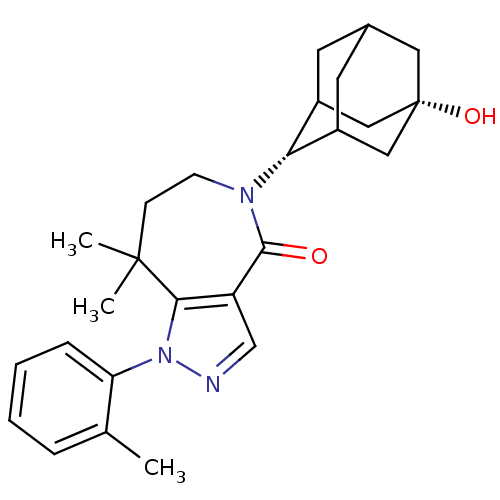 (CHEMBL2338245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50430132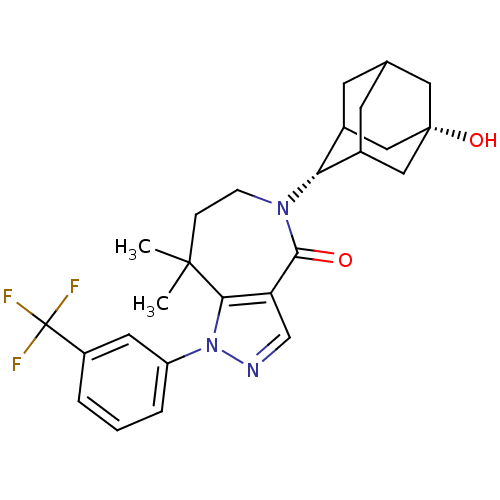 (CHEMBL2338254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50051293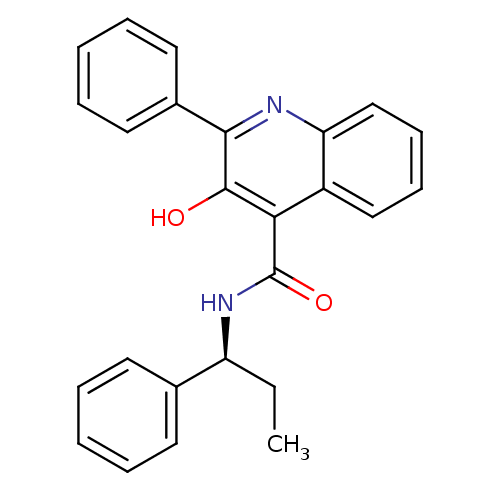 ((S)-(-)-N-(R-ethylbenzyl)-3-hydroxy-2-phenylquinol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Displacement of [125I]His3-MePhe7)-NKB from human NK3R expressed in CHO cell membranes by topcounting method | Bioorg Med Chem 24: 3494-500 (2016) Article DOI: 10.1016/j.bmc.2016.05.054 BindingDB Entry DOI: 10.7270/Q2RR216W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50144679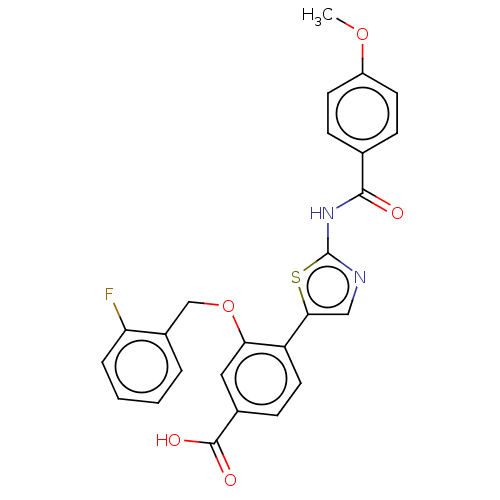 (CHEMBL3759871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50430147 (CHEMBL2338264) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50144655 (CHEMBL3758606) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50144688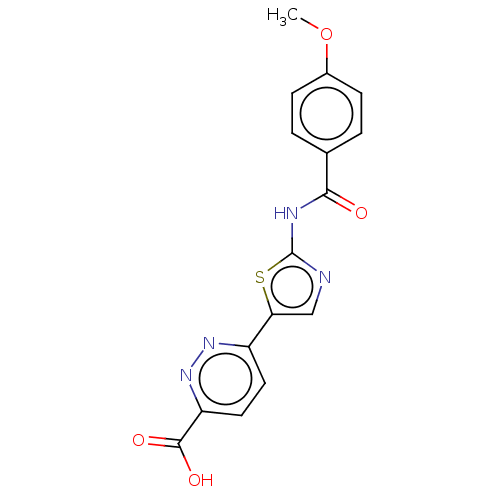 (CHEMBL3758317) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50430147 (CHEMBL2338264) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50144682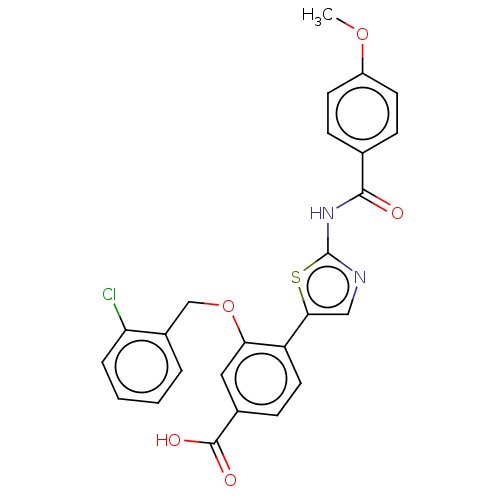 (CHEMBL3759512) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50430134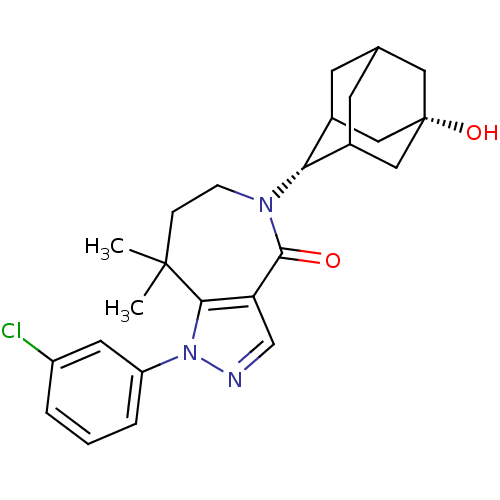 (CHEMBL2338252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50430142 (CHEMBL2338244) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50144689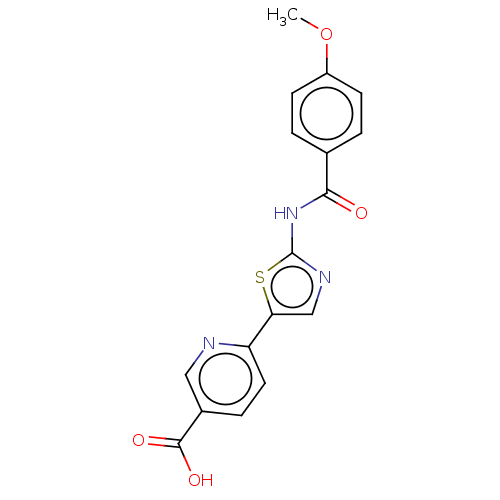 (CHEMBL3759755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50144686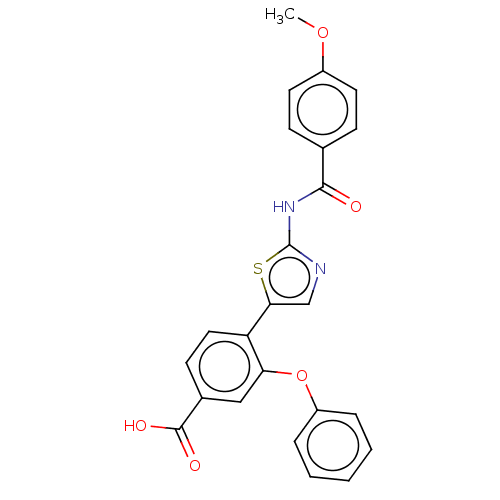 (CHEMBL3758519) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50430137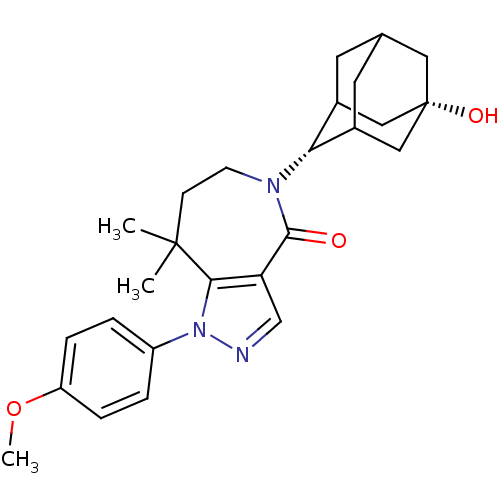 (CHEMBL2338249) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50430138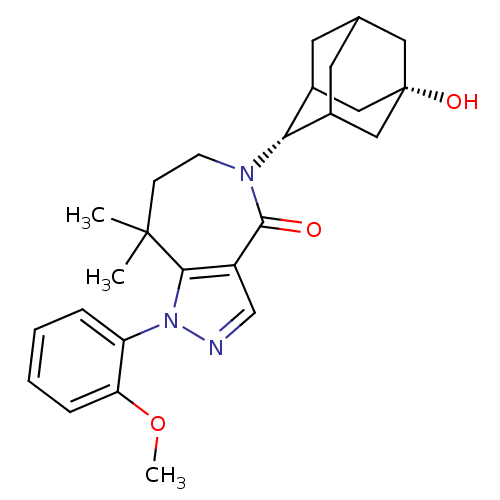 (CHEMBL2338248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50189627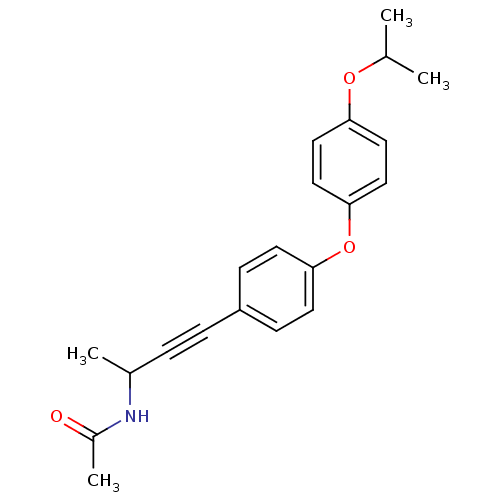 (CHEMBL212460 | N-{3-[4-(4-isopropoxyphenoxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-tagged human ACC2 expressed in baculovirus infected sf9 cells using acetyl-CoA as substrate afte... | Bioorg Med Chem 24: 5258-5269 (2016) Article DOI: 10.1016/j.bmc.2016.08.045 BindingDB Entry DOI: 10.7270/Q2S46TXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50420563 (CHEMBL2087023) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50335638 (5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50144683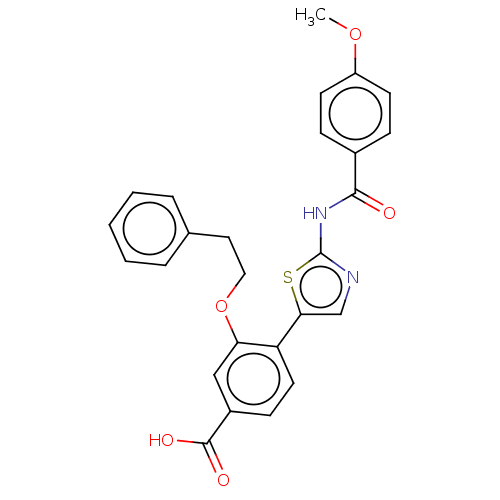 (CHEMBL3760009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50144670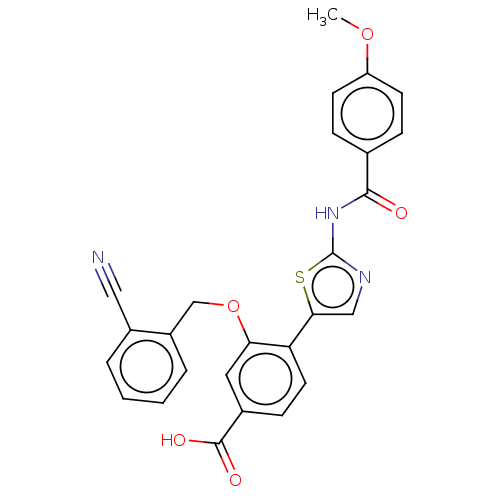 (CHEMBL3759349) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50430148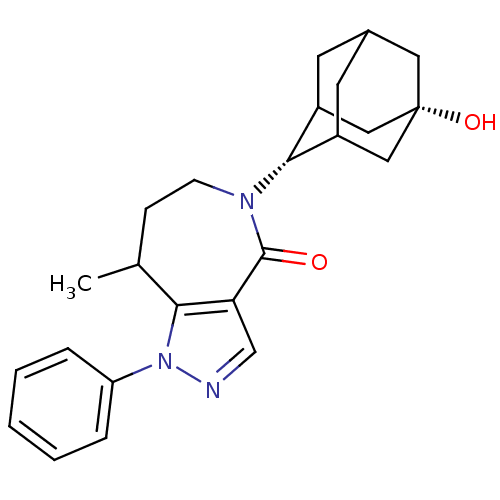 (CHEMBL2338263) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells using cortisone and NADPH as substrate by HTRF assay | Bioorg Med Chem Lett 23: 1617-21 (2013) Article DOI: 10.1016/j.bmcl.2013.01.090 BindingDB Entry DOI: 10.7270/Q27P90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM50144685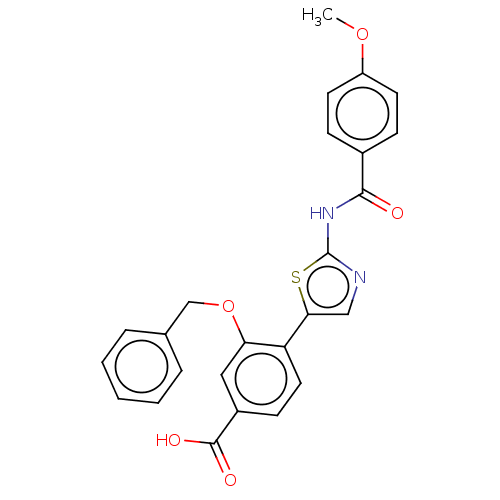 (CHEMBL3758270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of GST-tagged human CK2alpha' (1 to 350 residues) using CK2tide as substrate incubated for 1 hr by off-chip mobility shift assay | Bioorg Med Chem 24: 1136-41 (2016) Article DOI: 10.1016/j.bmc.2016.01.043 BindingDB Entry DOI: 10.7270/Q2BK1F7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 181 total ) | Next | Last >> |