 Found 74 hits with Last Name = 'okonogi' and Initial = 't'
Found 74 hits with Last Name = 'okonogi' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50005414
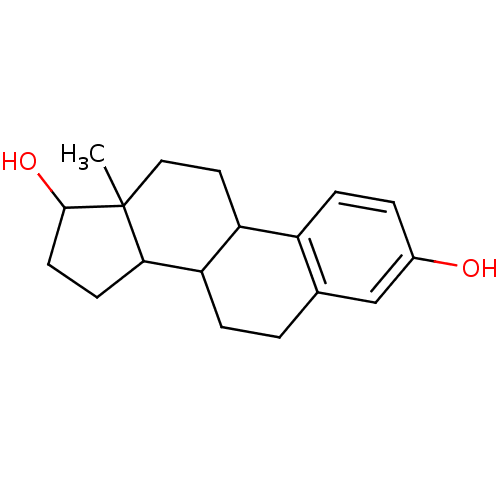
((estradiol)13-Methyl-7,8,9,11,12,13,14,15,16,17-de...)Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM50025452

((testosterone)17-Hydroxy-10,13-dimethyl-1,2,6,7,8,...)Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM86680

(CAS_4042 | Mpa | NSC_4042)Show SMILES CC1CC2C3CCC(OC(C)=O)(C(C)=O)C3(C)CCC2C2(C)CCC(=O)C=C12 |t:28| Show InChI InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM86680

(CAS_4042 | Mpa | NSC_4042)Show SMILES CC1CC2C3CCC(OC(C)=O)(C(C)=O)C3(C)CCC2C2(C)CCC(=O)C=C12 |t:28| Show InChI InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM86678

(CP8816)Show SMILES COC1C=C2C=C3N(C)C(=O)C(C)=C3[C@@H](C)[C@]2(C)CC1OC(=O)N(C)C |r,c:12,t:3,5| Show InChI InChI=1S/C20H28N2O4/c1-11-17-12(2)20(3)10-16(26-19(24)21(4)5)15(25-7)9-13(20)8-14(17)22(6)18(11)23/h8-9,12,15-16H,10H2,1-7H3/t12-,15?,16?,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM86679
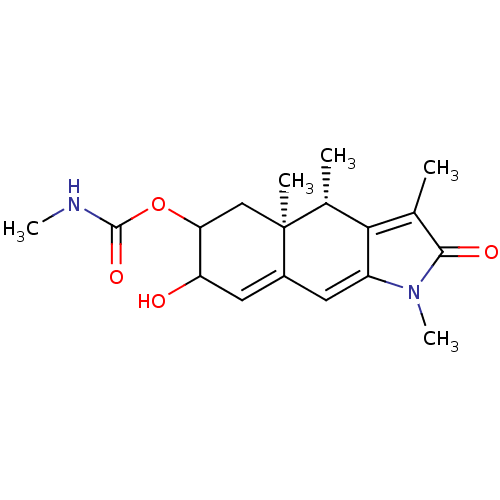
(CP8863)Show SMILES CNC(=O)OC1C[C@@]2(C)[C@H](C)C3=C(C)C(=O)N(C)C3=CC2=CC1O |r,c:11,19,22| Show InChI InChI=1S/C18H24N2O4/c1-9-15-10(2)18(3)8-14(24-17(23)19-4)13(21)7-11(18)6-12(15)20(5)16(9)22/h6-7,10,13-14,21H,8H2,1-5H3,(H,19,23)/t10-,13?,14?,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM86680

(CAS_4042 | Mpa | NSC_4042)Show SMILES CC1CC2C3CCC(OC(C)=O)(C(C)=O)C3(C)CCC2C2(C)CCC(=O)C=C12 |t:28| Show InChI InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM86678

(CP8816)Show SMILES COC1C=C2C=C3N(C)C(=O)C(C)=C3[C@@H](C)[C@]2(C)CC1OC(=O)N(C)C |r,c:12,t:3,5| Show InChI InChI=1S/C20H28N2O4/c1-11-17-12(2)20(3)10-16(26-19(24)21(4)5)15(25-7)9-13(20)8-14(17)22(6)18(11)23/h8-9,12,15-16H,10H2,1-7H3/t12-,15?,16?,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Rattus norvegicus (Rat)) | BDBM86679

(CP8863)Show SMILES CNC(=O)OC1C[C@@]2(C)[C@H](C)C3=C(C)C(=O)N(C)C3=CC2=CC1O |r,c:11,19,22| Show InChI InChI=1S/C18H24N2O4/c1-9-15-10(2)18(3)8-14(24-17(23)19-4)13(21)7-11(18)6-12(15)20(5)16(9)22/h6-7,10,13-14,21H,8H2,1-5H3,(H,19,23)/t10-,13?,14?,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM86679

(CP8863)Show SMILES CNC(=O)OC1C[C@@]2(C)[C@H](C)C3=C(C)C(=O)N(C)C3=CC2=CC1O |r,c:11,19,22| Show InChI InChI=1S/C18H24N2O4/c1-9-15-10(2)18(3)8-14(24-17(23)19-4)13(21)7-11(18)6-12(15)20(5)16(9)22/h6-7,10,13-14,21H,8H2,1-5H3,(H,19,23)/t10-,13?,14?,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM86680

(CAS_4042 | Mpa | NSC_4042)Show SMILES CC1CC2C3CCC(OC(C)=O)(C(C)=O)C3(C)CCC2C2(C)CCC(=O)C=C12 |t:28| Show InChI InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM86679

(CP8863)Show SMILES CNC(=O)OC1C[C@@]2(C)[C@H](C)C3=C(C)C(=O)N(C)C3=CC2=CC1O |r,c:11,19,22| Show InChI InChI=1S/C18H24N2O4/c1-9-15-10(2)18(3)8-14(24-17(23)19-4)13(21)7-11(18)6-12(15)20(5)16(9)22/h6-7,10,13-14,21H,8H2,1-5H3,(H,19,23)/t10-,13?,14?,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM86678

(CP8816)Show SMILES COC1C=C2C=C3N(C)C(=O)C(C)=C3[C@@H](C)[C@]2(C)CC1OC(=O)N(C)C |r,c:12,t:3,5| Show InChI InChI=1S/C20H28N2O4/c1-11-17-12(2)20(3)10-16(26-19(24)21(4)5)15(25-7)9-13(20)8-14(17)22(6)18(11)23/h8-9,12,15-16H,10H2,1-7H3/t12-,15?,16?,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 916-20 (2005)
Article DOI: 10.1124/jpet.104.074146
BindingDB Entry DOI: 10.7270/Q24T6GZR |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50291167
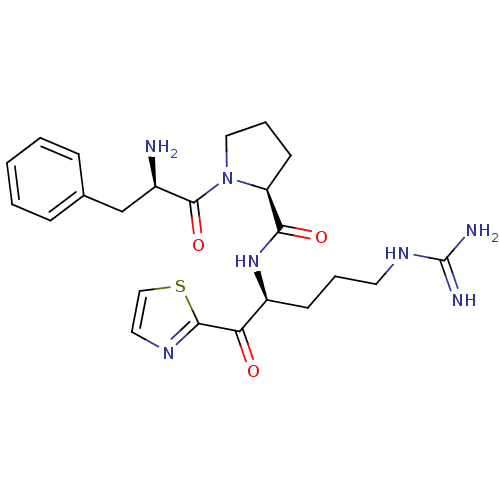
(1-((S)-Oxo-3-(R)-2-amino-1-phenyl-propyl)-pyrrolid...)Show SMILES N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C23H31N7O3S/c24-16(14-15-6-2-1-3-7-15)22(33)30-12-5-9-18(30)20(32)29-17(8-4-10-28-23(25)26)19(31)21-27-11-13-34-21/h1-3,6-7,11,13,16-18H,4-5,8-10,12,14,24H2,(H,29,32)(H4,25,26,28)/t16-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50291175
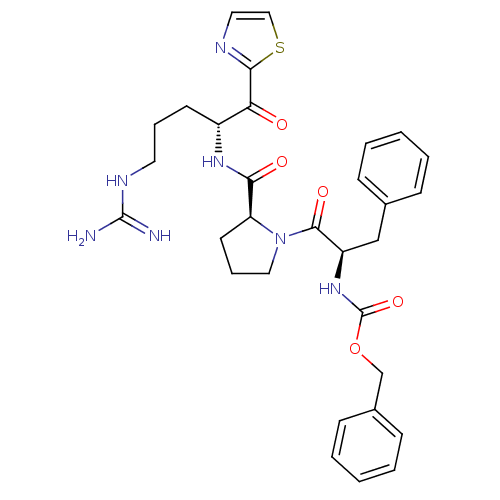
(((R)-1-Benzyl-2-{(S)-2-[(R)-4-guanidino-1-(thiazol...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C31H37N7O5S/c32-30(33)35-15-7-13-23(26(39)28-34-16-18-44-28)36-27(40)25-14-8-17-38(25)29(41)24(19-21-9-3-1-4-10-21)37-31(42)43-20-22-11-5-2-6-12-22/h1-6,9-12,16,18,23-25H,7-8,13-15,17,19-20H2,(H,36,40)(H,37,42)(H4,32,33,35)/t23-,24-,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50291175

(((R)-1-Benzyl-2-{(S)-2-[(R)-4-guanidino-1-(thiazol...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C31H37N7O5S/c32-30(33)35-15-7-13-23(26(39)28-34-16-18-44-28)36-27(40)25-14-8-17-38(25)29(41)24(19-21-9-3-1-4-10-21)37-31(42)43-20-22-11-5-2-6-12-22/h1-6,9-12,16,18,23-25H,7-8,13-15,17,19-20H2,(H,36,40)(H,37,42)(H4,32,33,35)/t23-,24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50291173
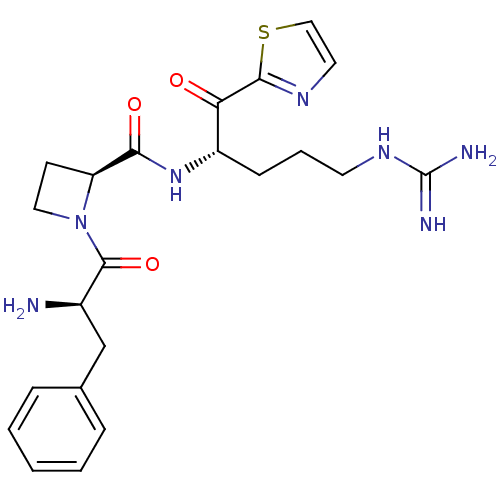
(1-((S)-2-Amino-3-(R)-phenyl-propionyl)-azetidine-2...)Show SMILES N[C@H](Cc1ccccc1)C(=O)N1CC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C22H29N7O3S/c23-15(13-14-5-2-1-3-6-14)21(32)29-11-8-17(29)19(31)28-16(7-4-9-27-22(24)25)18(30)20-26-10-12-33-20/h1-3,5-6,10,12,15-17H,4,7-9,11,13,23H2,(H,28,31)(H4,24,25,27)/t15-,16+,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50284127
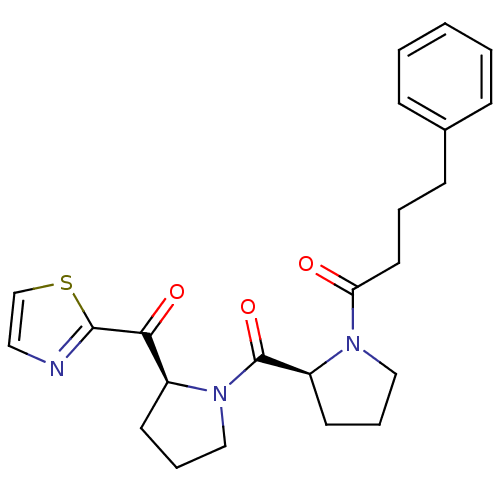
(4-Phenyl-1-{(S)-2-[(S)-2-(thiazole-2-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nccs1 Show InChI InChI=1S/C23H27N3O3S/c27-20(12-4-9-17-7-2-1-3-8-17)25-14-6-11-19(25)23(29)26-15-5-10-18(26)21(28)22-24-13-16-30-22/h1-3,7-8,13,16,18-19H,4-6,9-12,14-15H2/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Prolyl endopeptidase (PEP) from pig kidney using Z-Gly-Pro-p-nitroanilide as substrate |
Bioorg Med Chem Lett 4: 831-834 (1994)
Article DOI: 10.1016/S0960-894X(01)80857-X
BindingDB Entry DOI: 10.7270/Q28W3D8R |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037610
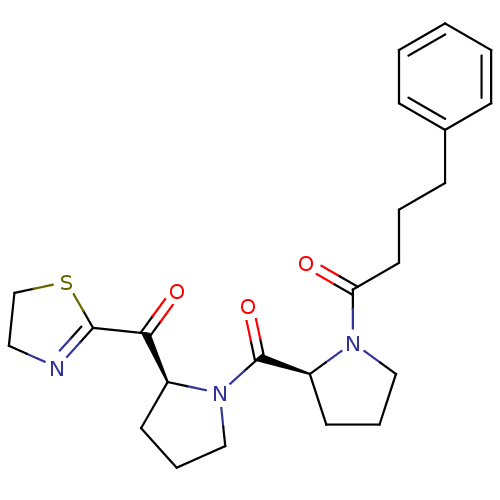
(1-{(S)-2-[(S)-2-(4,5-Dihydro-thiazole-2-carbonyl)-...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)C1=NCCS1 |t:28| Show InChI InChI=1S/C23H29N3O3S/c27-20(12-4-9-17-7-2-1-3-8-17)25-14-6-11-19(25)23(29)26-15-5-10-18(26)21(28)22-24-13-16-30-22/h1-3,7-8,18-19H,4-6,9-16H2/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037612
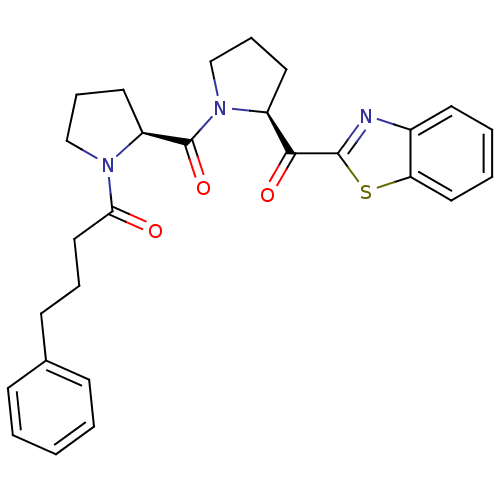
(1-{(S)-2-[(S)-2-(Benzothiazole-2-carbonyl)-pyrroli...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C27H29N3O3S/c31-24(16-6-11-19-9-2-1-3-10-19)29-17-8-14-22(29)27(33)30-18-7-13-21(30)25(32)26-28-20-12-4-5-15-23(20)34-26/h1-5,9-10,12,15,21-22H,6-8,11,13-14,16-18H2/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50291167

(1-((S)-Oxo-3-(R)-2-amino-1-phenyl-propyl)-pyrrolid...)Show SMILES N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C23H31N7O3S/c24-16(14-15-6-2-1-3-7-15)22(33)30-12-5-9-18(30)20(32)29-17(8-4-10-28-23(25)26)19(31)21-27-11-13-34-21/h1-3,6-7,11,13,16-18H,4-5,8-10,12,14,24H2,(H,29,32)(H4,25,26,28)/t16-,17+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50291163
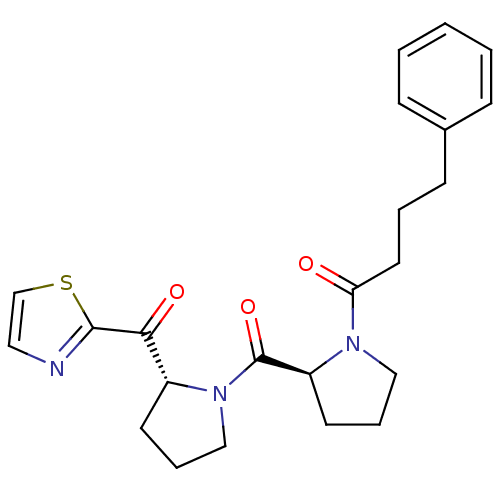
(4-Phenyl-1-{(S)-2-[(R)-2-(thiazole-2-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@@H]1C(=O)c1nccs1 Show InChI InChI=1S/C23H27N3O3S/c27-20(12-4-9-17-7-2-1-3-8-17)25-14-6-11-19(25)23(29)26-15-5-10-18(26)21(28)22-24-13-16-30-22/h1-3,7-8,13,16,18-19H,4-6,9-12,14-15H2/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibitory activity against prolyl endo peptidase(PEP) enzyme |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037605
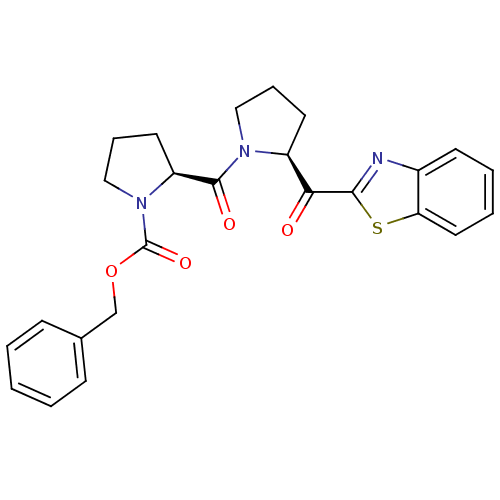
((S)-2-[(S)-2-(Benzothiazole-2-carbonyl)-pyrrolidin...)Show SMILES O=C(OCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C25H25N3O4S/c29-22(23-26-18-10-4-5-13-21(18)33-23)19-11-6-14-27(19)24(30)20-12-7-15-28(20)25(31)32-16-17-8-2-1-3-9-17/h1-5,8-10,13,19-20H,6-7,11-12,14-16H2/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50284127

(4-Phenyl-1-{(S)-2-[(S)-2-(thiazole-2-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nccs1 Show InChI InChI=1S/C23H27N3O3S/c27-20(12-4-9-17-7-2-1-3-8-17)25-14-6-11-19(25)23(29)26-15-5-10-18(26)21(28)22-24-13-16-30-22/h1-3,7-8,13,16,18-19H,4-6,9-12,14-15H2/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50291168
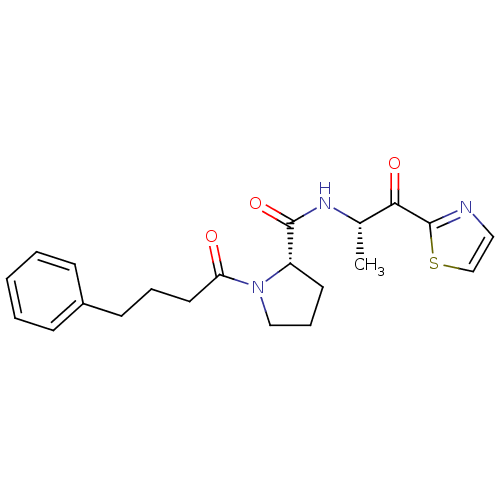
((S)-1-(4-Phenyl-butyryl)-pyrrolidine-2-carboxylic ...)Show SMILES C[C@H](NC(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C21H25N3O3S/c1-15(19(26)21-22-12-14-28-21)23-20(27)17-10-6-13-24(17)18(25)11-5-9-16-7-3-2-4-8-16/h2-4,7-8,12,14-15,17H,5-6,9-11,13H2,1H3,(H,23,27)/t15-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibitory activity against prolyl endo peptidase(PEP) enzyme |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037611
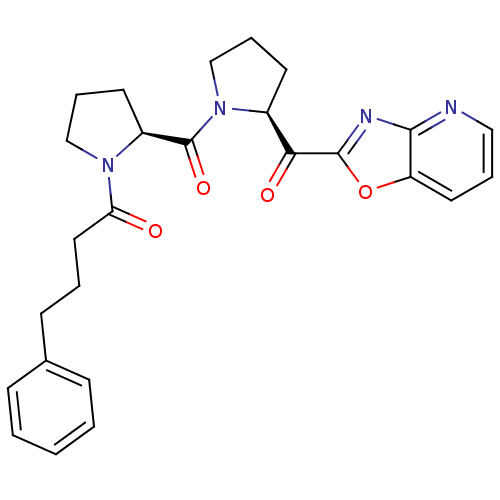
(1-{(S)-2-[(S)-2-(Oxazolo[4,5-b]pyridine-2-carbonyl...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nc2ncccc2o1 Show InChI InChI=1S/C26H28N4O4/c31-22(14-4-10-18-8-2-1-3-9-18)29-16-7-12-20(29)26(33)30-17-6-11-19(30)23(32)25-28-24-21(34-25)13-5-15-27-24/h1-3,5,8-9,13,15,19-20H,4,6-7,10-12,14,16-17H2/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037606
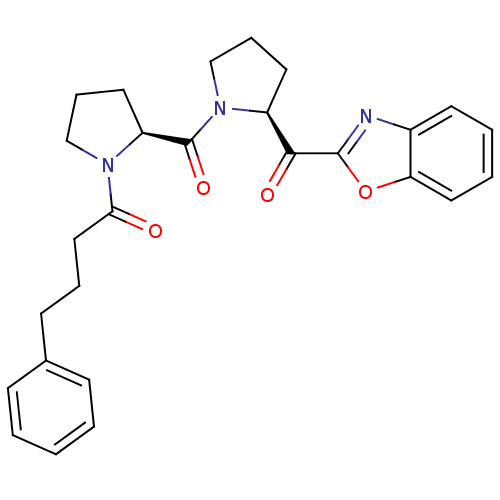
(1-{(S)-2-[(S)-2-(Benzooxazole-2-carbonyl)-pyrrolid...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1nc2ccccc2o1 Show InChI InChI=1S/C27H29N3O4/c31-24(16-6-11-19-9-2-1-3-10-19)29-17-8-14-22(29)27(33)30-18-7-13-21(30)25(32)26-28-20-12-4-5-15-23(20)34-26/h1-5,9-10,12,15,21-22H,6-8,11,13-14,16-18H2/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037597
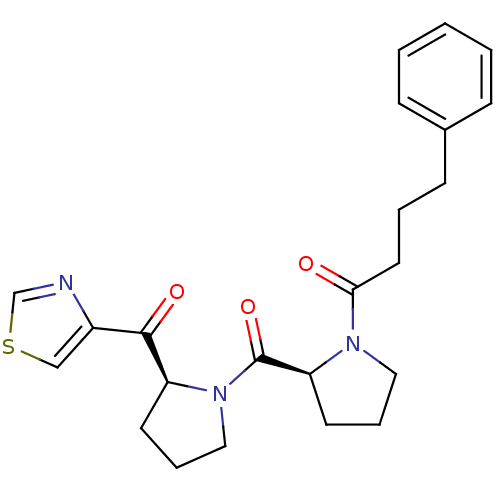
(4-Phenyl-1-{(S)-2-[(S)-2-(thiazole-4-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cscn1 Show InChI InChI=1S/C23H27N3O3S/c27-21(12-4-9-17-7-2-1-3-8-17)25-13-6-11-20(25)23(29)26-14-5-10-19(26)22(28)18-15-30-16-24-18/h1-3,7-8,15-16,19-20H,4-6,9-14H2/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Prolyl endopeptidase (PEP) from pig kidney using Z-Gly-Pro-p-nitroanilide as substrate |
Bioorg Med Chem Lett 4: 831-834 (1994)
Article DOI: 10.1016/S0960-894X(01)80857-X
BindingDB Entry DOI: 10.7270/Q28W3D8R |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037597

(4-Phenyl-1-{(S)-2-[(S)-2-(thiazole-4-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cscn1 Show InChI InChI=1S/C23H27N3O3S/c27-21(12-4-9-17-7-2-1-3-8-17)25-13-6-11-20(25)23(29)26-14-5-10-19(26)22(28)18-15-30-16-24-18/h1-3,7-8,15-16,19-20H,4-6,9-14H2/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037600
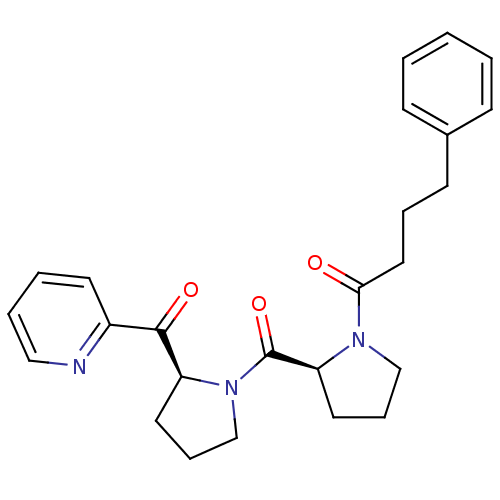
(4-Phenyl-1-{(S)-2-[(S)-2-(pyridine-2-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1ccccn1 Show InChI InChI=1S/C25H29N3O3/c29-23(15-6-11-19-9-2-1-3-10-19)27-17-8-14-22(27)25(31)28-18-7-13-21(28)24(30)20-12-4-5-16-26-20/h1-5,9-10,12,16,21-22H,6-8,11,13-15,17-18H2/t21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037609
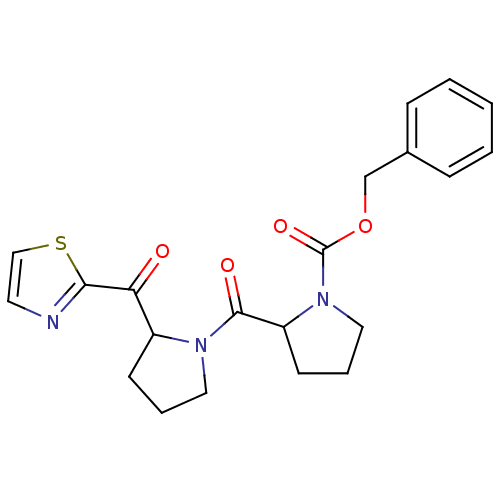
(2-[2-(Thiazole-2-carbonyl)-pyrrolidine-1-carbonyl]...)Show SMILES O=C(OCc1ccccc1)N1CCCC1C(=O)N1CCCC1C(=O)c1nccs1 Show InChI InChI=1S/C21H23N3O4S/c25-18(19-22-10-13-29-19)16-8-4-11-23(16)20(26)17-9-5-12-24(17)21(27)28-14-15-6-2-1-3-7-15/h1-3,6-7,10,13,16-17H,4-5,8-9,11-12,14H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50291166
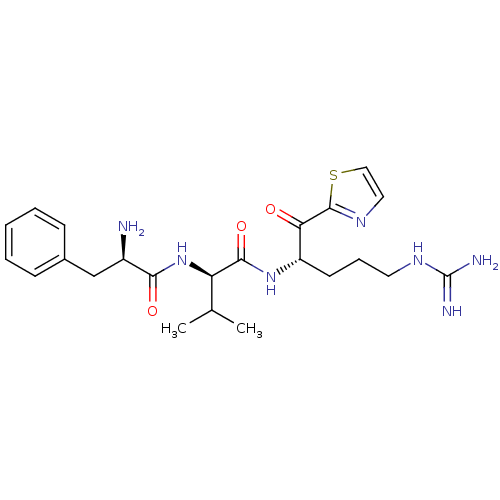
((R)-2-((R)-2-Amino-3-phenyl-propionylamino)-N-[(S)...)Show SMILES CC(C)[C@@H](NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C23H33N7O3S/c1-14(2)18(30-20(32)16(24)13-15-7-4-3-5-8-15)21(33)29-17(9-6-10-28-23(25)26)19(31)22-27-11-12-34-22/h3-5,7-8,11-12,14,16-18H,6,9-10,13,24H2,1-2H3,(H,29,33)(H,30,32)(H4,25,26,28)/t16-,17+,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50284126
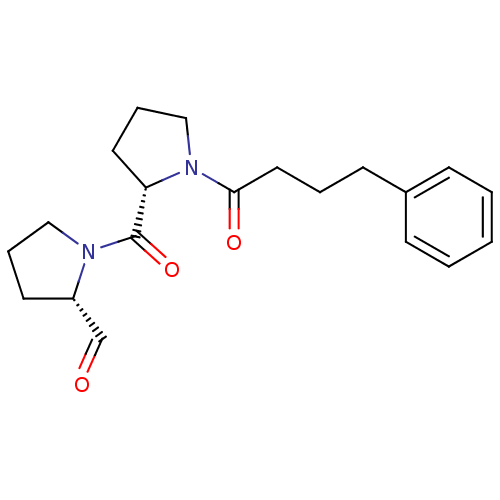
((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1 Show InChI InChI=1S/C20H26N2O3/c23-15-17-10-5-13-21(17)20(25)18-11-6-14-22(18)19(24)12-4-9-16-7-2-1-3-8-16/h1-3,7-8,15,17-18H,4-6,9-14H2/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Prolyl endopeptidase (PEP) from pig kidney using Z-Gly-Pro-p-nitroanilide as substrate |
Bioorg Med Chem Lett 4: 831-834 (1994)
Article DOI: 10.1016/S0960-894X(01)80857-X
BindingDB Entry DOI: 10.7270/Q28W3D8R |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037602
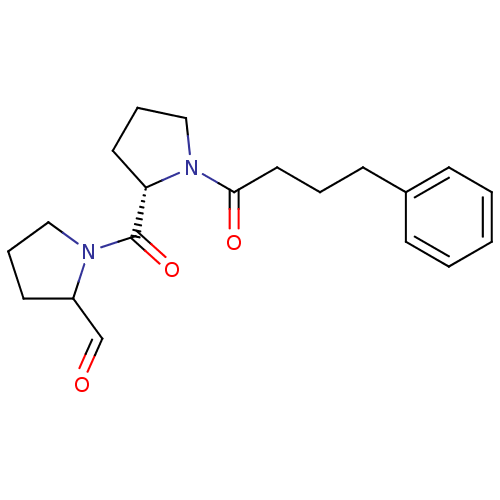
(1-[(S)-1-(4-Phenyl-butyryl)-pyrrolidine-2-carbonyl...)Show SMILES O=CC1CCCN1C(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1 Show InChI InChI=1S/C20H26N2O3/c23-15-17-10-5-13-21(17)20(25)18-11-6-14-22(18)19(24)12-4-9-16-7-2-1-3-8-16/h1-3,7-8,15,17-18H,4-6,9-14H2/t17?,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037604
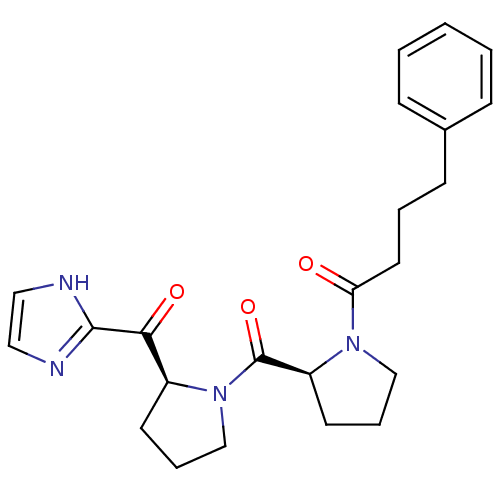
(1-{(S)-2-[(S)-2-(1H-Imidazole-2-carbonyl)-pyrrolid...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1ncc[nH]1 Show InChI InChI=1S/C23H28N4O3/c28-20(12-4-9-17-7-2-1-3-8-17)26-15-6-11-19(26)23(30)27-16-5-10-18(27)21(29)22-24-13-14-25-22/h1-3,7-8,13-14,18-19H,4-6,9-12,15-16H2,(H,24,25)/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50291173

(1-((S)-2-Amino-3-(R)-phenyl-propionyl)-azetidine-2...)Show SMILES N[C@H](Cc1ccccc1)C(=O)N1CC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C22H29N7O3S/c23-15(13-14-5-2-1-3-6-14)21(32)29-11-8-17(29)19(31)28-16(7-4-9-27-22(24)25)18(30)20-26-10-12-33-20/h1-3,5-6,10,12,15-17H,4,7-9,11,13,23H2,(H,28,31)(H4,24,25,27)/t15-,16+,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50088247

(CHEMBL303966 | Oxo-{(S)-1-[(S)-1-(4-phenyl-butyryl...)Show SMILES CCOC(=O)C(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1 Show InChI InChI=1S/C23H30N2O5/c1-2-30-23(29)21(27)18-12-7-16-25(18)22(28)19-13-8-15-24(19)20(26)14-6-11-17-9-4-3-5-10-17/h3-5,9-10,18-19H,2,6-8,11-16H2,1H3/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Prolyl endopeptidase (PEP) from pig kidney using Z-Gly-Pro-p-nitroanilide as substrate |
Bioorg Med Chem Lett 4: 831-834 (1994)
Article DOI: 10.1016/S0960-894X(01)80857-X
BindingDB Entry DOI: 10.7270/Q28W3D8R |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037607
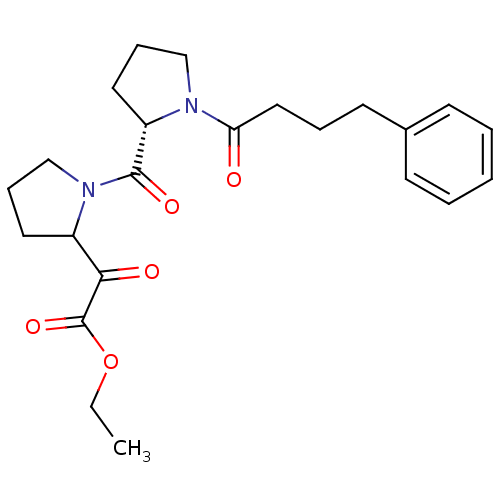
(CHEMBL333638 | Oxo-{1-[(S)-1-(4-phenyl-butyryl)-py...)Show SMILES CCOC(=O)C(=O)C1CCCN1C(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1 Show InChI InChI=1S/C23H30N2O5/c1-2-30-23(29)21(27)18-12-7-16-25(18)22(28)19-13-8-15-24(19)20(26)14-6-11-17-9-4-3-5-10-17/h3-5,9-10,18-19H,2,6-8,11-16H2,1H3/t18?,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037603
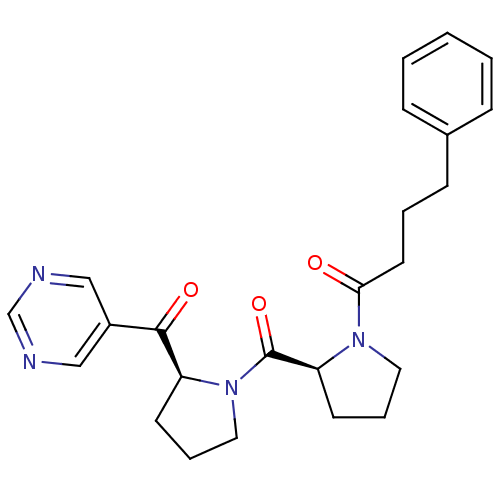
(4-Phenyl-1-{(S)-2-[(S)-2-(pyrimidine-5-carbonyl)-p...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cncnc1 Show InChI InChI=1S/C24H28N4O3/c29-22(12-4-9-18-7-2-1-3-8-18)27-13-6-11-21(27)24(31)28-14-5-10-20(28)23(30)19-15-25-17-26-16-19/h1-3,7-8,15-17,20-21H,4-6,9-14H2/t20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50291166

((R)-2-((R)-2-Amino-3-phenyl-propionylamino)-N-[(S)...)Show SMILES CC(C)[C@@H](NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C23H33N7O3S/c1-14(2)18(30-20(32)16(24)13-15-7-4-3-5-8-15)21(33)29-17(9-6-10-28-23(25)26)19(31)22-27-11-12-34-22/h3-5,7-8,11-12,14,16-18H,6,9-10,13,24H2,1-2H3,(H,29,33)(H,30,32)(H4,25,26,28)/t16-,17+,18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50291172
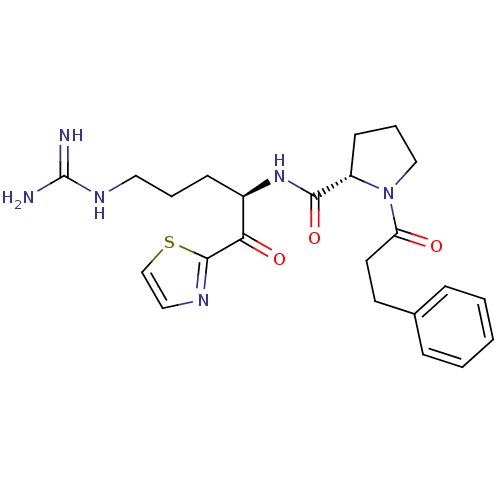
((S)-1-(3-Phenyl-propionyl)-pyrrolidine-2-carboxyli...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)CCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C23H30N6O3S/c24-23(25)27-12-4-8-17(20(31)22-26-13-15-33-22)28-21(32)18-9-5-14-29(18)19(30)11-10-16-6-2-1-3-7-16/h1-3,6-7,13,15,17-18H,4-5,8-12,14H2,(H,28,32)(H4,24,25,27)/t17-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50291171
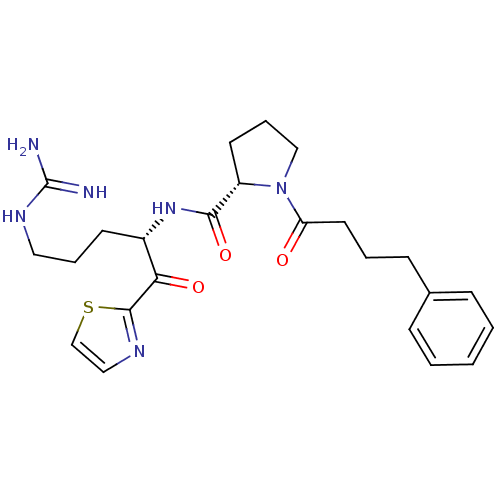
((S)-1-(4-Phenyl-butyryl)-pyrrolidine-2-carboxylic ...)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)CCCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C24H32N6O3S/c25-24(26)28-13-5-10-18(21(32)23-27-14-16-34-23)29-22(33)19-11-6-15-30(19)20(31)12-4-9-17-7-2-1-3-8-17/h1-3,7-8,14,16,18-19H,4-6,9-13,15H2,(H,29,33)(H4,25,26,28)/t18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Bos taurus (bovine)) | BDBM50291170
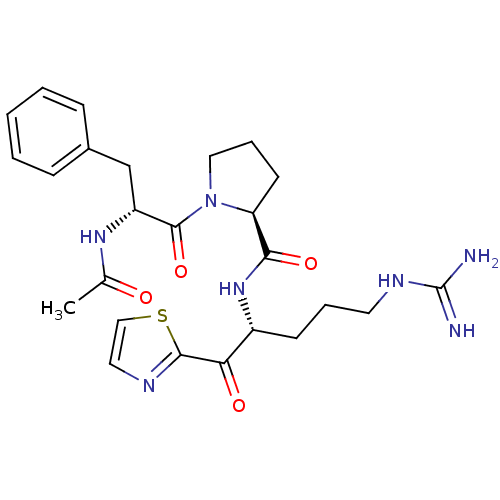
((S)-1-((R)-2-Acetylamino-3-phenyl-propionyl)-pyrro...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C25H33N7O4S/c1-16(33)30-19(15-17-7-3-2-4-8-17)24(36)32-13-6-10-20(32)22(35)31-18(9-5-11-29-25(26)27)21(34)23-28-12-14-37-23/h2-4,7-8,12,14,18-20H,5-6,9-11,13,15H2,1H3,(H,30,33)(H,31,35)(H4,26,27,29)/t18-,19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118724
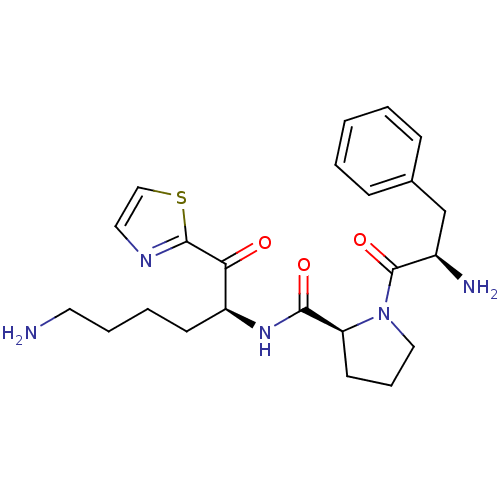
((S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C23H31N5O3S/c24-11-5-4-9-18(20(29)22-26-12-14-32-22)27-21(30)19-10-6-13-28(19)23(31)17(25)15-16-7-2-1-3-8-16/h1-3,7-8,12,14,17-19H,4-6,9-11,13,15,24-25H2,(H,27,30)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50038001
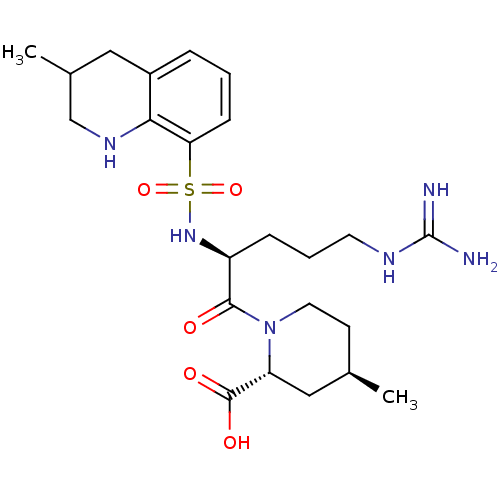
((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...)Show SMILES C[C@@H]1CCN([C@H](C1)C(O)=O)C(=O)[C@H](CCCNC(N)=N)NS(=O)(=O)c1cccc2CC(C)CNc12 Show InChI InChI=1S/C23H36N6O5S/c1-14-8-10-29(18(12-14)22(31)32)21(30)17(6-4-9-26-23(24)25)28-35(33,34)19-7-3-5-16-11-15(2)13-27-20(16)19/h3,5,7,14-15,17-18,27-28H,4,6,8-13H2,1-2H3,(H,31,32)(H4,24,25,26)/t14-,15?,17+,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037599
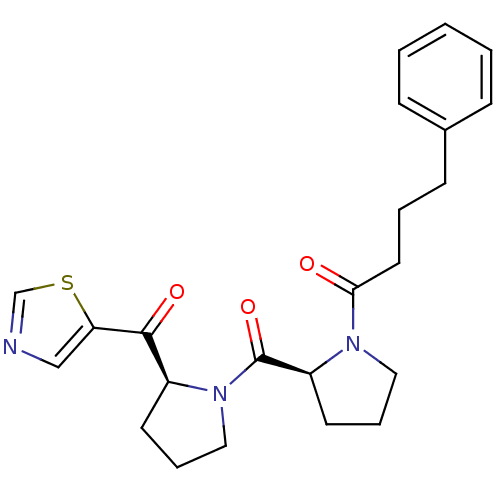
(4-Phenyl-1-{(S)-2-[(S)-2-(thiazole-5-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cncs1 Show InChI InChI=1S/C23H27N3O3S/c27-21(12-4-9-17-7-2-1-3-8-17)25-13-6-11-19(25)23(29)26-14-5-10-18(26)22(28)20-15-24-16-30-20/h1-3,7-8,15-16,18-19H,4-6,9-14H2/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 646 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Prolyl endopeptidase (PEP) from pig kidney using Z-Gly-Pro-p-nitroanilide as substrate |
Bioorg Med Chem Lett 4: 831-834 (1994)
Article DOI: 10.1016/S0960-894X(01)80857-X
BindingDB Entry DOI: 10.7270/Q28W3D8R |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50291174
((S)-1-((R)-2-Amino-3-phenyl-propionyl)-piperidine-...)Show SMILES N[C@H](Cc1ccccc1)C(=O)N1CCCC[C@H]1C(=O)N[C@H](CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C24H33N7O3S/c25-17(15-16-7-2-1-3-8-16)23(34)31-13-5-4-10-19(31)21(33)30-18(9-6-11-29-24(26)27)20(32)22-28-12-14-35-22/h1-3,7-8,12,14,17-19H,4-6,9-11,13,15,25H2,(H,30,33)(H4,26,27,29)/t17-,18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50291172

((S)-1-(3-Phenyl-propionyl)-pyrrolidine-2-carboxyli...)Show SMILES NC(=N)NCCC[C@@H](NC(=O)[C@@H]1CCCN1C(=O)CCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C23H30N6O3S/c24-23(25)27-12-4-8-17(20(31)22-26-13-15-33-22)28-21(32)18-9-5-14-29(18)19(30)11-10-16-6-2-1-3-7-16/h1-3,6-7,13,15,17-18H,4-5,8-12,14H2,(H,28,32)(H4,24,25,27)/t17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 533-538 (1997)
Article DOI: 10.1016/S0960-894X(97)00057-7
BindingDB Entry DOI: 10.7270/Q28S4QF2 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Sus scrofa) | BDBM50037599

(4-Phenyl-1-{(S)-2-[(S)-2-(thiazole-5-carbonyl)-pyr...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)c1cncs1 Show InChI InChI=1S/C23H27N3O3S/c27-21(12-4-9-17-7-2-1-3-8-17)25-13-6-11-19(25)23(29)26-14-5-10-18(26)22(28)20-15-24-16-30-20/h1-3,7-8,15-16,18-19H,4-6,9-14H2/t18-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against prolyl endopeptidase (PEP) |
J Med Chem 37: 3492-502 (1994)
BindingDB Entry DOI: 10.7270/Q2RB73N9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data