

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50070679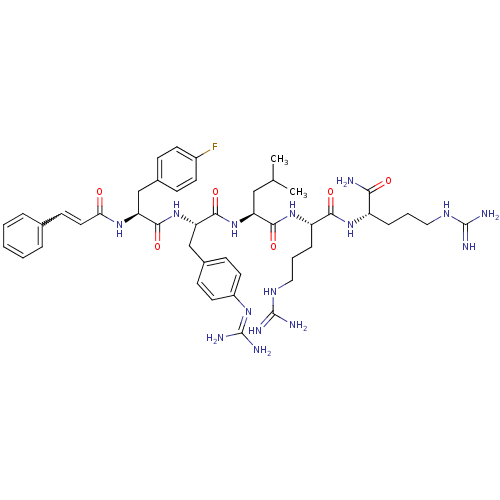 ((S)-2-[(S)-2-{(S)-3-(4-Fluoro-phenyl)-2-[(E)-(3-ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]S-(p-F-Phe)-Har-L-Har-KY-NH2 binding to a thrombin receptor (PAR-1) membrane preparation. | Bioorg Med Chem Lett 8: 1649-54 (1999) BindingDB Entry DOI: 10.7270/Q26T0N4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 using in human liver microsomes using testosterone as substrate after 5 to 15 mins | ACS Med Chem Lett 8: 321-326 (2017) Article DOI: 10.1021/acsmedchemlett.6b00491 BindingDB Entry DOI: 10.7270/Q21J9D2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM151585 (US11739089, Compound Ketoconazole | US8987315, Ket...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 using in human liver microsomes using midazolam as substrate after 5 to 15 mins | ACS Med Chem Lett 8: 321-326 (2017) Article DOI: 10.1021/acsmedchemlett.6b00491 BindingDB Entry DOI: 10.7270/Q21J9D2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129484 (CHEMBL308050 | N-(2-Amino-ethyl)-2-[(S)-2-{3-[1-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129486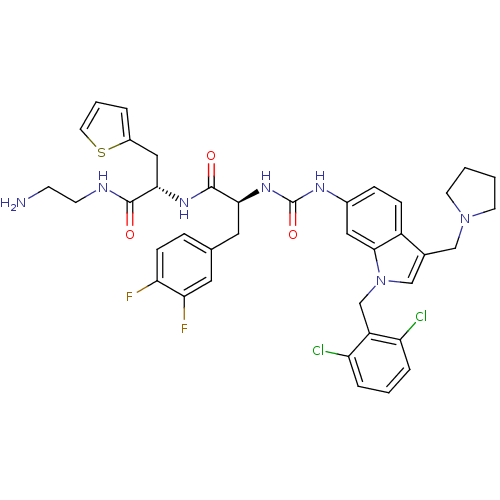 ((S)-N-[1-((S)-2-Amino-ethylcarbamoyl)-2-thiophen-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 5 to 15 mins | ACS Med Chem Lett 8: 321-326 (2017) Article DOI: 10.1021/acsmedchemlett.6b00491 BindingDB Entry DOI: 10.7270/Q21J9D2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129487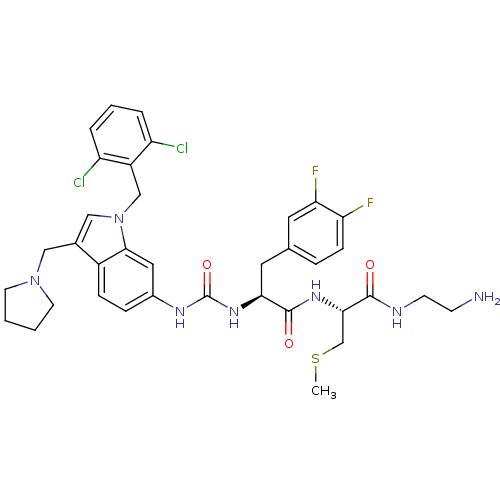 ((S)-N-[1-((R)-2-Amino-ethylcarbamoyl)-2-methylsulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103034 ((S)-2-((S)-3-(4-Cyano-phenyl)-2-{3-[1-(2,6-dichlor...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129488 ((S)-N-[1-((S)-2-Amino-ethylcarbamoyl)-2-pyridin-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129478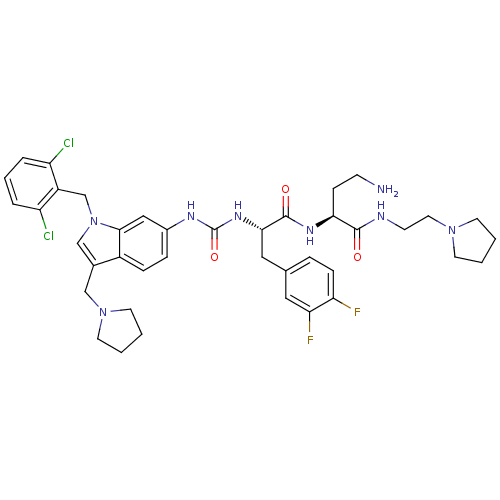 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129495 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103015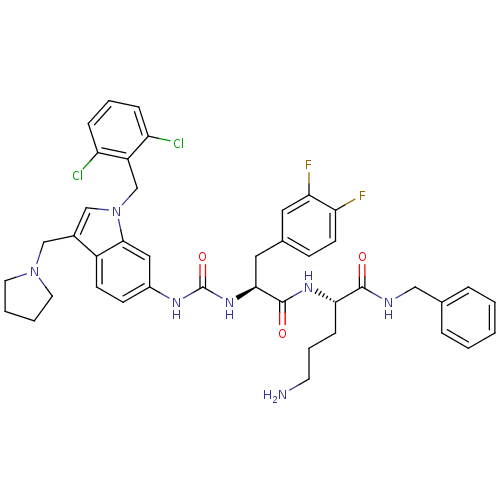 ((S)-5-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103029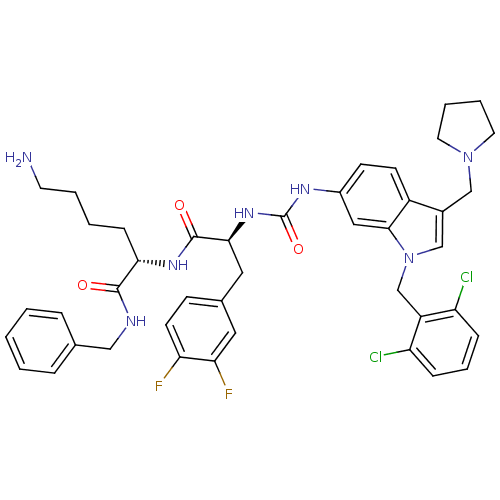 ((S)-6-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103015 ((S)-5-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129493 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103014 ((S)-2-[(S)-2-{3-[1-(4-Fluoro-benzyl)-3-pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129491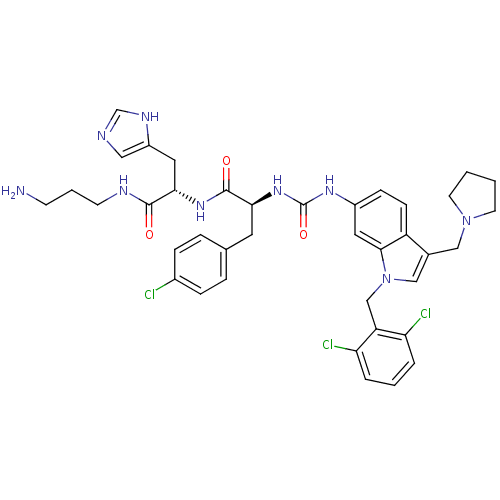 ((S)-N-[1-((S)-3-Amino-propylcarbamoyl)-2-(1H-imida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103038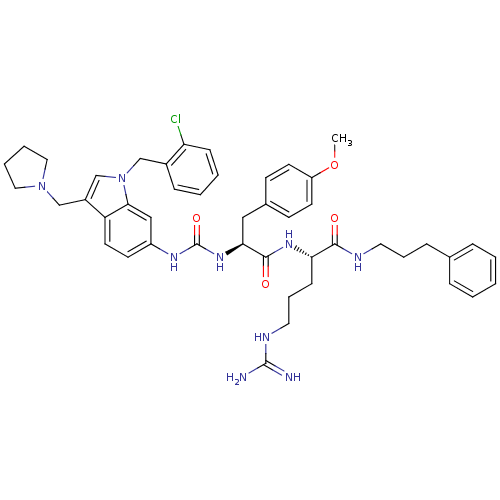 ((S)-2-[(S)-2-{3-[1-(2-Chloro-benzyl)-3-pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103025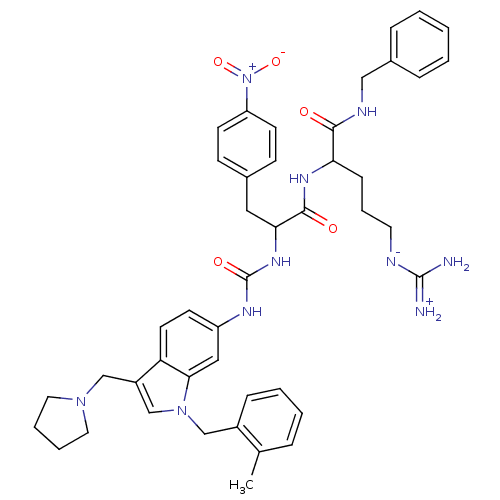 ((S)-5-Guanidino-2-[(S)-2-{3-[1-(2-methyl-benzyl)-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro displacement of [3H]-S-(p-F-Phe)-homoarginine-K Y-NH2 (at a concentration of 10 microM) from thrombin receptor (PAR-1) on the membranes of C... | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103016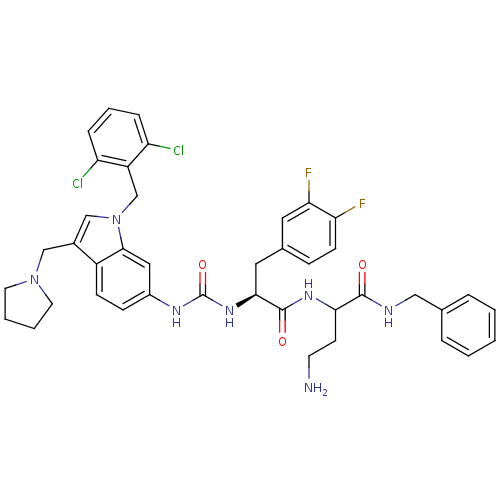 (4-Amino-N-benzyl-2-[(S)-2-{3-[1-(2,6-dichloro-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro displacement of [3H]-S-(p-F-Phe)-homoarginine-K Y-NH2 (at a concentration of 10 microM) from thrombin receptor (PAR-1) on the membranes of C... | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129489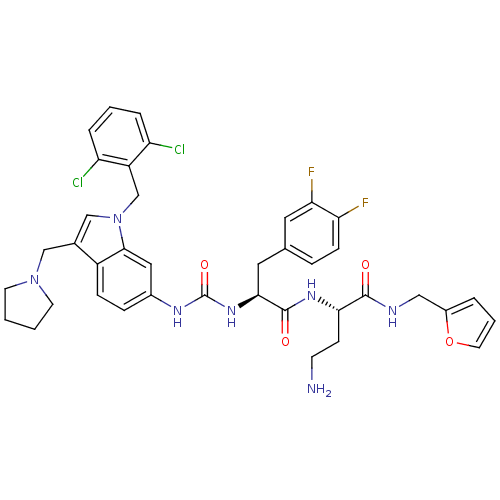 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103016 (4-Amino-N-benzyl-2-[(S)-2-{3-[1-(2,6-dichloro-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103024 ((S)-5-Guanidino-2-((S)-3-(4-methoxy-phenyl)-2-{3-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103017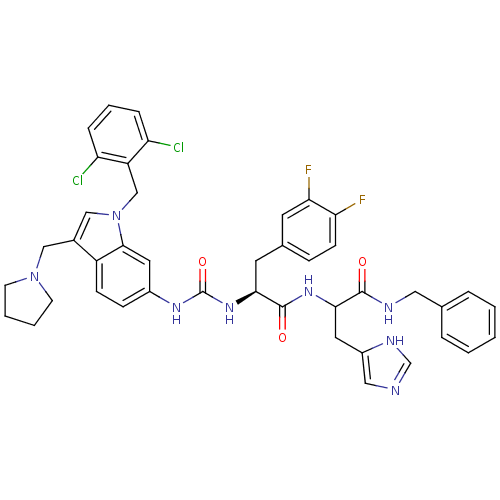 ((S)-N-[1-Benzylcarbamoyl-2-(3H-imidazol-4-yl)-ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50090677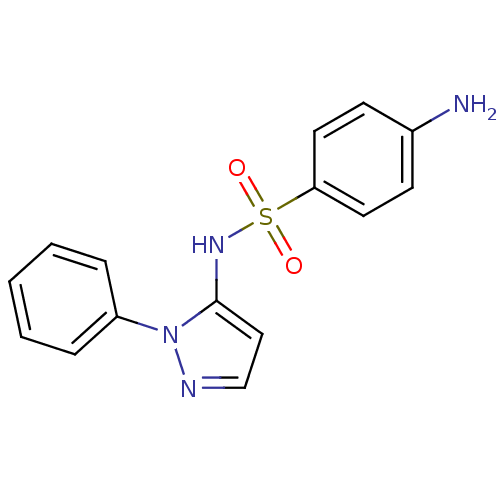 (4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 5 to 15 mins | ACS Med Chem Lett 8: 321-326 (2017) Article DOI: 10.1021/acsmedchemlett.6b00491 BindingDB Entry DOI: 10.7270/Q21J9D2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103020 ((S)-2-[(S)-2-{3-[3-Diethylaminomethyl-1-(3-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129490 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103018 ((S)-2-[(S)-2-{3-[3-Diethylaminomethyl-1-(2-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103036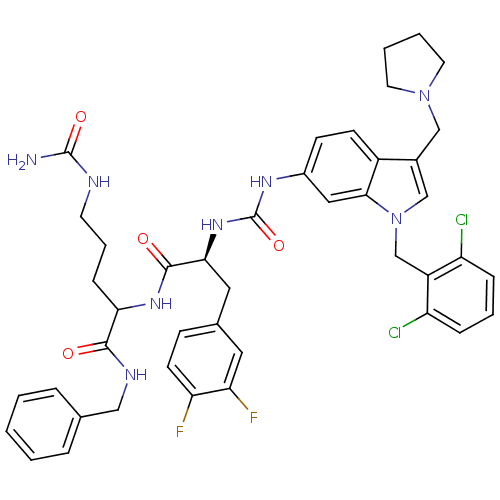 (2-[(S)-2-{3-[1-(2,6-Dichloro-benzyl)-3-pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro displacement of [3H]-S-(p-F-Phe)-homoarginine-K Y-NH2 (at a concentration of 10 microM) from thrombin receptor (PAR-1) on the membranes of C... | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103036 (2-[(S)-2-{3-[1-(2,6-Dichloro-benzyl)-3-pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro displacement of [3H]-S-(p-F-Phe)-homoarginine-K Y-NH2 (at a concentration of 10 microM) from thrombin receptor (PAR-1) on the membranes of C... | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098218 ((S)-2-[(S)-2-{3-[1-(4-Fluoro-benzyl)-3-pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103036 (2-[(S)-2-{3-[1-(2,6-Dichloro-benzyl)-3-pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129480 (5-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103026 ((S)-5-Guanidino-2-((S)-3-(4-methoxy-phenyl)-2-{3-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129485 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098216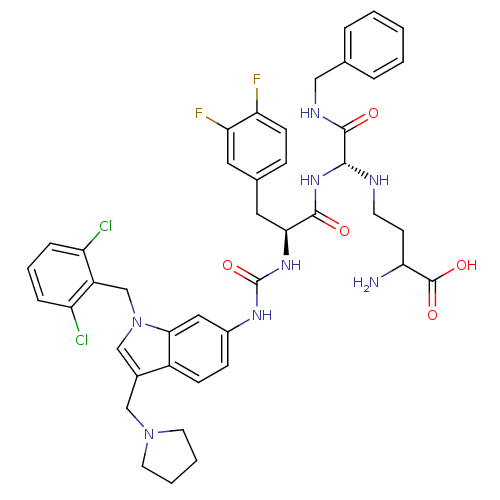 (2-Amino-4-({benzylcarbamoyl-[2-{3-[1-(2,6-dichloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103016 (4-Amino-N-benzyl-2-[(S)-2-{3-[1-(2,6-dichloro-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098215 (4-Amino-N-benzyl-2-[2-{3-[1-(2,6-dichloro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103038 ((S)-2-[(S)-2-{3-[1-(2-Chloro-benzyl)-3-pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro displacement of [3H]-S-(p-F-Phe)-homoarginine-K Y-NH2 (at a concentration of 10 microM) from thrombin receptor (PAR-1) on the membranes of C... | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103037 ((S)-2-[(S)-2-{3-[3-Azetidin-1-ylmethyl-1-(4-fluoro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103028 (CHEMBL305188 | N-Benzyl-2-[(S)-2-{3-[1-(2,6-dichlo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103028 (CHEMBL305188 | N-Benzyl-2-[(S)-2-{3-[1-(2,6-dichlo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129492 (4-Amino-N-(3-amino-propyl)-2-((S)-3-(4-chloro-phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129481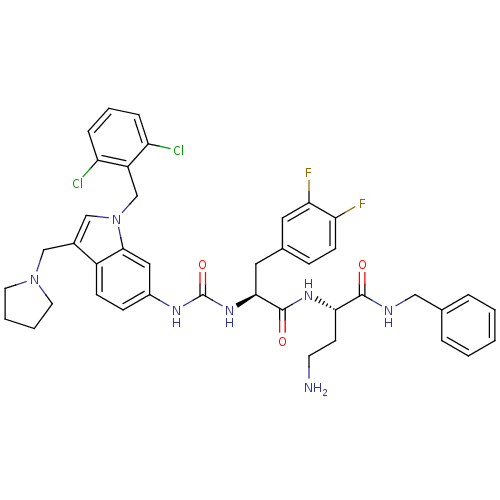 (4-Amino-N-benzyl-2-[(S)-2-{3-[1-(2,6-dichloro-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103017 ((S)-N-[1-Benzylcarbamoyl-2-(3H-imidazol-4-yl)-ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by alpha-thrombin (at a concentration of 0.15 nM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103017 ((S)-N-[1-Benzylcarbamoyl-2-(3H-imidazol-4-yl)-ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human platelet aggregation induced by SFLLRN-NH2 (at a concentration of 2 uM) | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129482 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103021 ((S)-N-(2-Amino-1-benzylcarbamoyl-ethyl)-2-{3-[1-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro displacement of [3H]-S-(p-F-Phe)-homoarginine-K Y-NH2 (at a concentration of 10 microM) from thrombin receptor (PAR-1) on the membranes of C... | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129479 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50103015 ((S)-5-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro displacement of [3H]-S-(p-F-Phe)-homoarginine-K Y-NH2 (at a concentration of 10 microM) from thrombin receptor (PAR-1) on the membranes of C... | Bioorg Med Chem Lett 11: 2105-9 (2001) BindingDB Entry DOI: 10.7270/Q27W6BGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 260 total ) | Next | Last >> |