

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50574769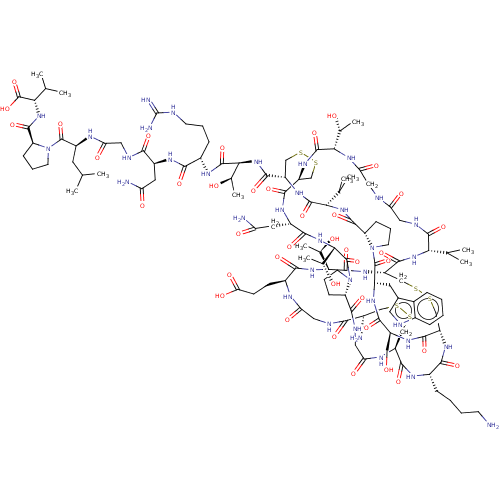 (CHEMBL4870603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Positive allosteric modulator activity in mouse kappa opioid receptor stably expressed in HEK293 cell membrane assessed as binding affinity of dyn A1... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00301 BindingDB Entry DOI: 10.7270/Q2WD44CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM214798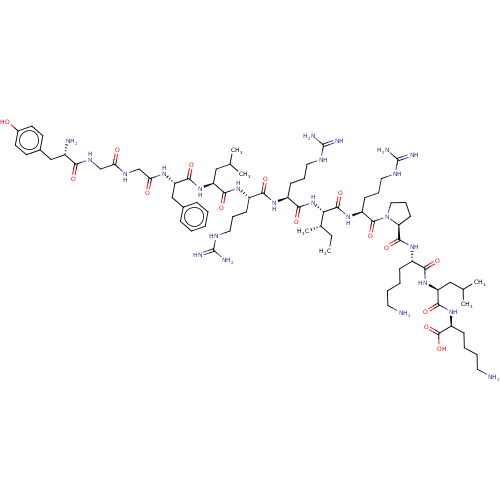 (Dynorphin A (1-17) | YGGFLRRIRPKLK) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Positive allosteric modulator activity in mouse kappa opioid receptor stably expressed in HEK293 cell membrane assessed as binding affinity of dyn A1... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00301 BindingDB Entry DOI: 10.7270/Q2WD44CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50574769 (CHEMBL4870603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Positive allosteric modulator activity in mouse kappa opioid receptor stably expressed in HEK293 cell membrane assessed as binding affinity of dyn A1... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00301 BindingDB Entry DOI: 10.7270/Q2WD44CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113787 (CHEMBL81056 | S-(2-{2-[2-(4-Carbamimidoyl-phenoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506124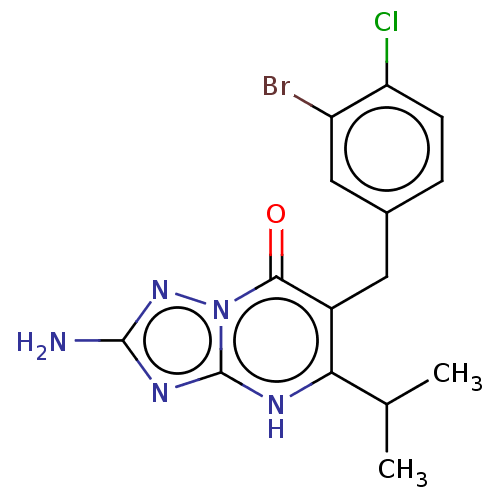 (CHEMBL4544504) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50574769 (CHEMBL4870603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Positive allosteric modulator activity in mouse kappa opioid receptor stably expressed in HEK293 cell membrane assessed as binding affinity of dyn A1... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00301 BindingDB Entry DOI: 10.7270/Q2WD44CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50574769 (CHEMBL4870603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Positive allosteric modulator activity in mouse kappa opioid receptor stably expressed in HEK293 cell membrane assessed as binding affinity of dyn A1... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00301 BindingDB Entry DOI: 10.7270/Q2WD44CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50574769 (CHEMBL4870603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Positive allosteric modulator activity in mouse kappa opioid receptor stably expressed in HEK293 cell membrane assessed as binding affinity of dyn A1... | Citation and Details Article DOI: 10.1021/acs.jnatprod.1c00301 BindingDB Entry DOI: 10.7270/Q2WD44CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506124 (CHEMBL4544504) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50201438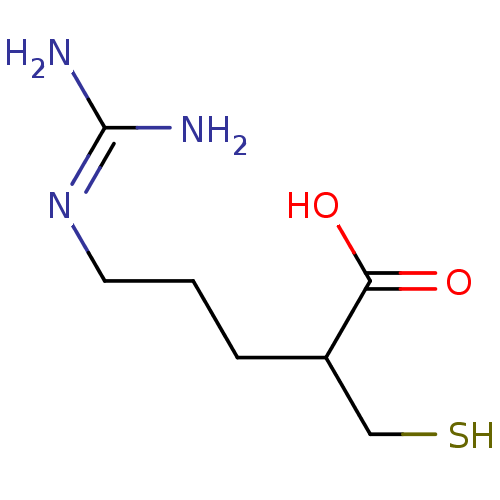 ((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic carboxypeptidase B | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506145 (CHEMBL4483373) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506145 (CHEMBL4483373) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113790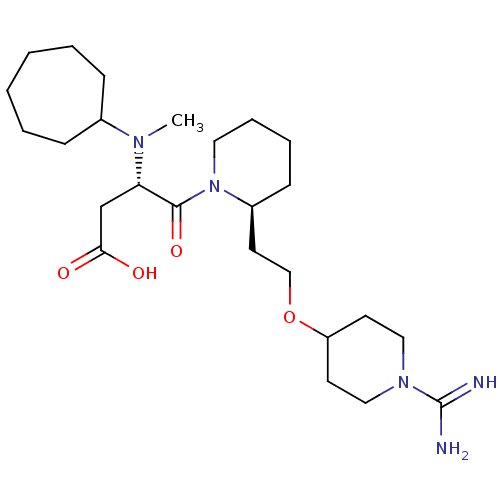 (CHEMBL84389 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506146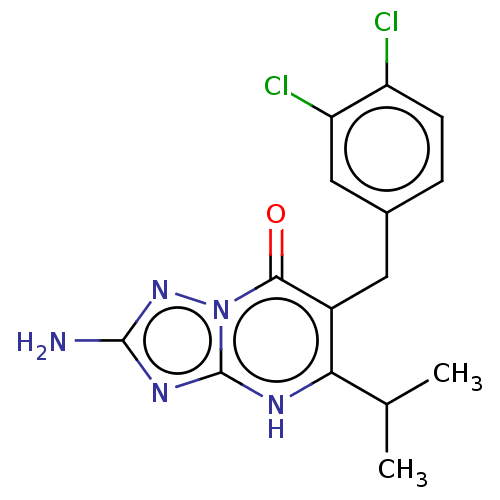 (CHEMBL4548396) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506146 (CHEMBL4548396) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113791 (CHEMBL84229 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506119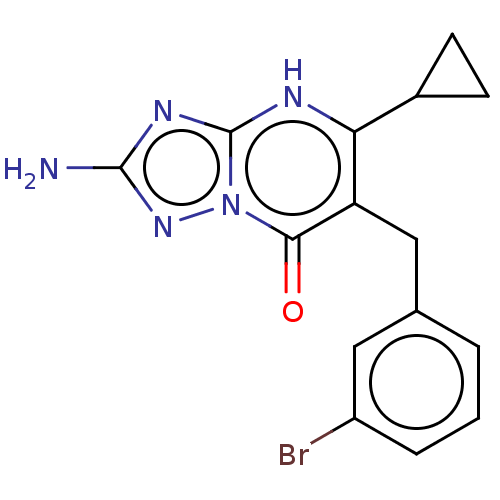 (CHEMBL4577171) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506132 (CHEMBL4571719) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506132 (CHEMBL4571719) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506119 (CHEMBL4577171) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113785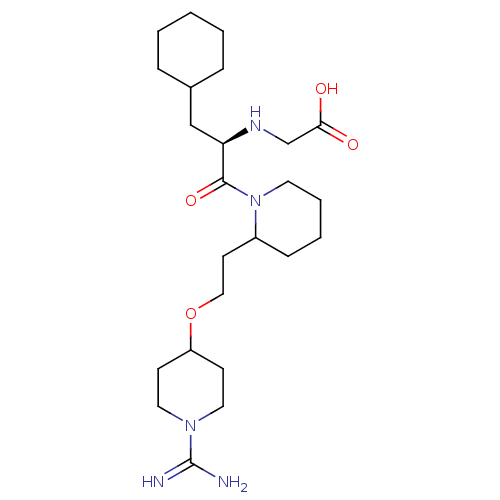 (CHEMBL309670 | RS-(2-{2-[2-(1-Carbamimidoyl-piperi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113799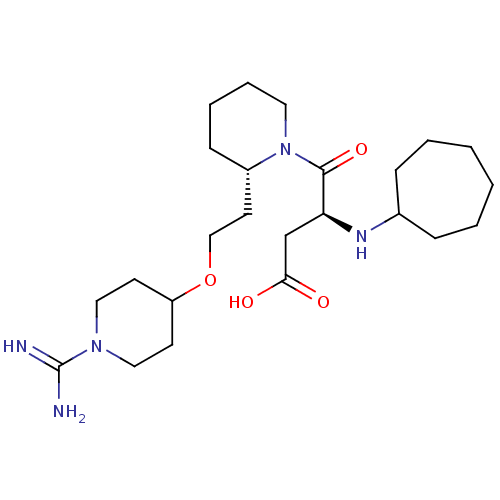 (CHEMBL309403 | S-4-{2-[2-(1-Carbamimidoyl-piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506125 (CHEMBL4517518) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506125 (CHEMBL4517518) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113794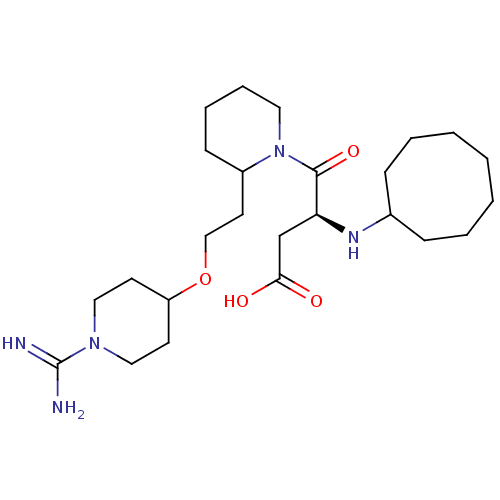 (CHEMBL82658 | RS-4-{2-[2-(1-Carbamimidoyl-piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506135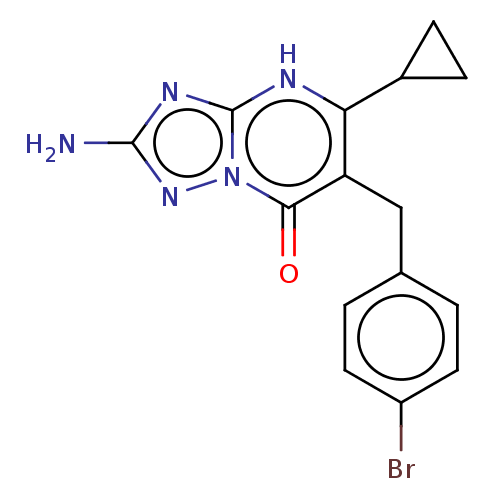 (CHEMBL4572833) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506122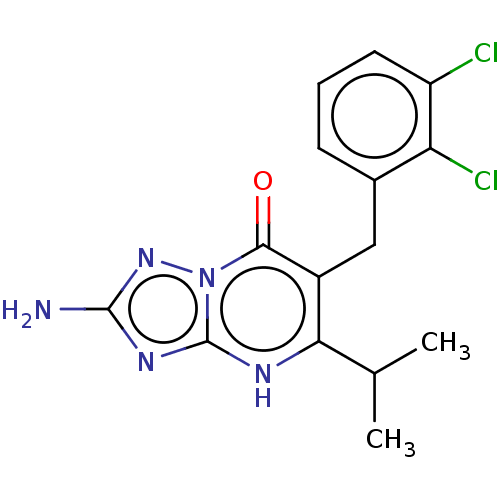 (CHEMBL4531005) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506120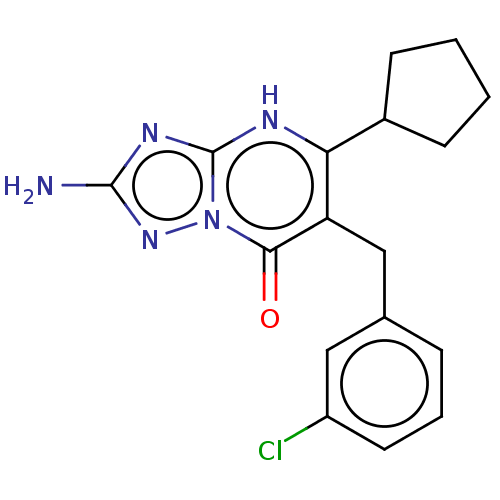 (CHEMBL4438407) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506135 (CHEMBL4572833) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506122 (CHEMBL4531005) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506120 (CHEMBL4438407) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506115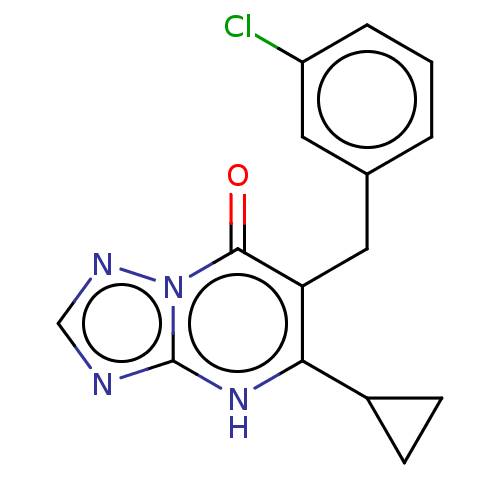 (CHEMBL4463626) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506115 (CHEMBL4463626) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506116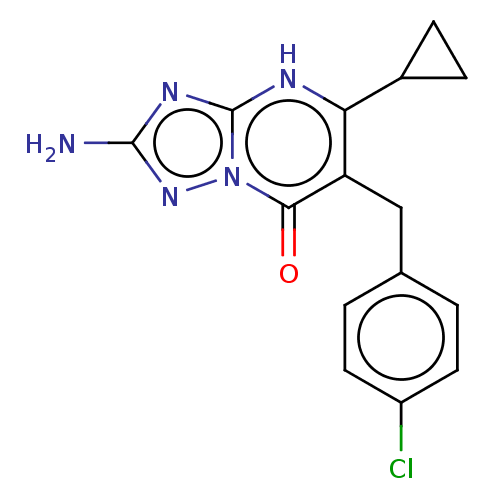 (CHEMBL4548107) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506116 (CHEMBL4548107) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113802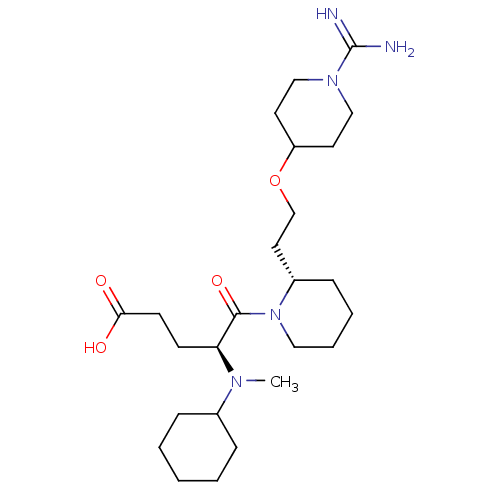 (5-{2-[2-(1-Carbamimidoyl-piperidin-4-yloxy)-ethyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506117 (CHEMBL4450273) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506117 (CHEMBL4450273) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase N catalytic chain (Homo sapiens (Human)) | BDBM50201438 ((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human plasma carboxypeptidase N | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506147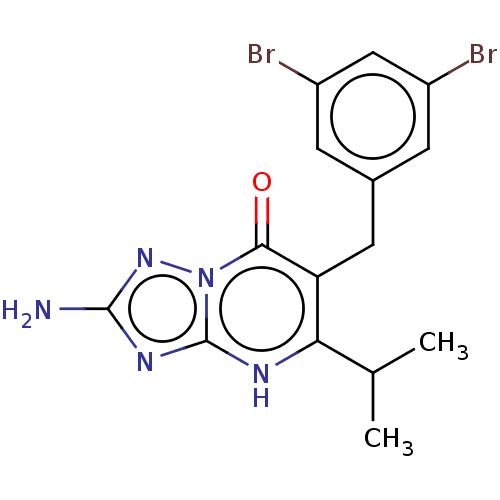 (CHEMBL4569807) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506147 (CHEMBL4569807) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113792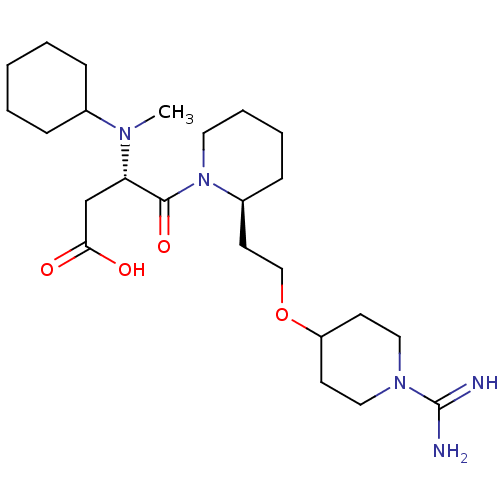 (CHEMBL81521 | S-4-{2-[2-(1-Carbamimidoyl-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human thrombin. | J Med Chem 45: 2432-53 (2002) BindingDB Entry DOI: 10.7270/Q2S181VR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506123 (CHEMBL4516788) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506123 (CHEMBL4516788) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506138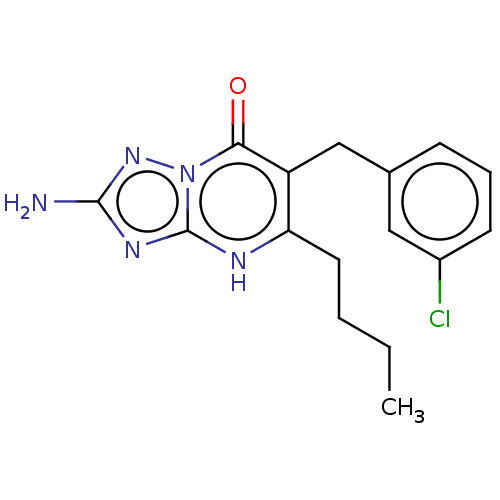 (CHEMBL4474757) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506138 (CHEMBL4474757) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506130 (CHEMBL4567816) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506130 (CHEMBL4567816) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506131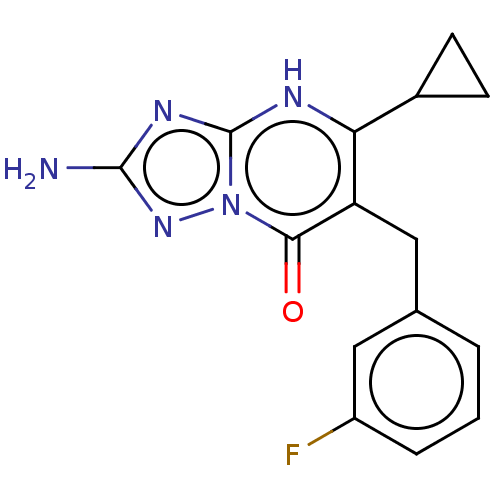 (CHEMBL4519409) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50506131 (CHEMBL4519409) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]-CCR2-RA-[R] from human CCR2 expressed in human U2OS cells incubated for 2 hrs by scintillation spectrometric method | J Med Chem 62: 11035-11053 (2019) Article DOI: 10.1021/acs.jmedchem.9b00742 BindingDB Entry DOI: 10.7270/Q2QR51DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1420 total ) | Next | Last >> |