 Found 262 hits with Last Name = 'olsen' and Initial = 'ge'
Found 262 hits with Last Name = 'olsen' and Initial = 'ge' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM10972
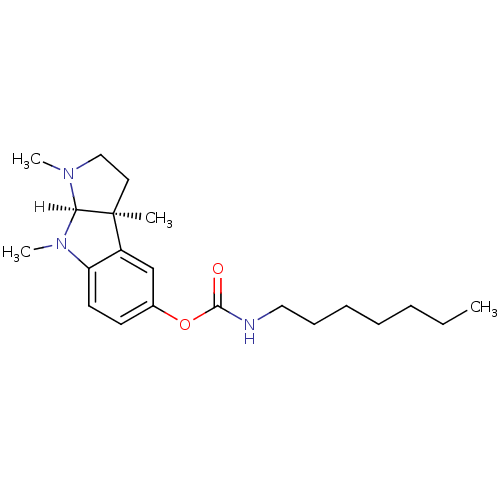
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.00029-0.001 |
Bioorg Med Chem Lett 7: 157-162 (1997)
Article DOI: 10.1016/S0960-894X(96)00592-6
BindingDB Entry DOI: 10.7270/Q2CC10P5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50452855

(Isoptpo Hyoscine | Scopolamine)Show SMILES [H][C@@]12O[C@@]1([H])[C@]1([H])C[C@@H](C[C@@]2([H])N1C)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:14:8:12:1.3,2:1:12:8.7.9,2:3:12:8.7.9| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3/t11-,12-,13-,14+,15+,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the absence of Zn |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50452855

(Isoptpo Hyoscine | Scopolamine)Show SMILES [H][C@@]12O[C@@]1([H])[C@]1([H])C[C@@H](C[C@@]2([H])N1C)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:14:8:12:1.3,2:1:12:8.7.9,2:3:12:8.7.9| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3/t11-,12-,13-,14+,15+,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048608
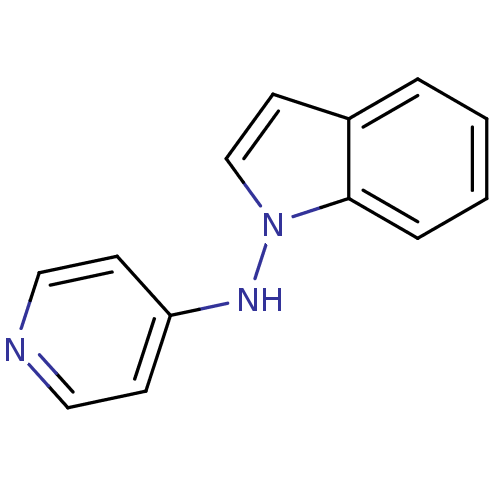
(CHEMBL154488 | Indol-1-yl-pyridin-4-yl-amine)Show InChI InChI=1S/C13H11N3/c1-2-4-13-11(3-1)7-10-16(13)15-12-5-8-14-9-6-12/h1-10H,(H,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10972

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase. |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM10972

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... |
Bioorg Med Chem Lett 7: 157-162 (1997)
Article DOI: 10.1016/S0960-894X(96)00592-6
BindingDB Entry DOI: 10.7270/Q2CC10P5 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048589
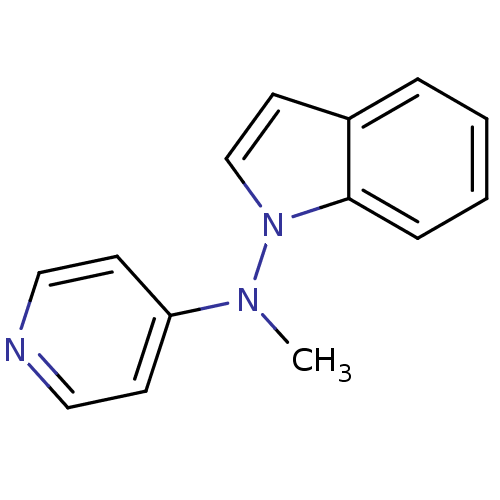
(CHEMBL152842 | Indol-1-yl-methyl-pyridin-4-yl-amin...)Show InChI InChI=1S/C14H13N3/c1-16(13-6-9-15-10-7-13)17-11-8-12-4-2-3-5-14(12)17/h2-11H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50290392
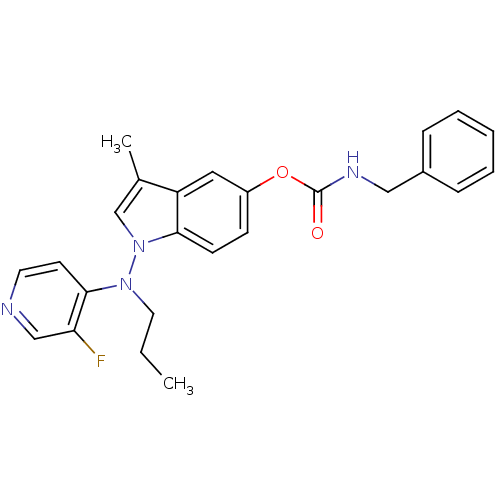
(Benzyl-carbamic acid 1-[(3-fluoro-pyridin-4-yl)-pr...)Show SMILES CCCN(c1ccncc1F)n1cc(C)c2cc(OC(=O)NCc3ccccc3)ccc12 Show InChI InChI=1S/C25H25FN4O2/c1-3-13-29(24-11-12-27-16-22(24)26)30-17-18(2)21-14-20(9-10-23(21)30)32-25(31)28-15-19-7-5-4-6-8-19/h4-12,14,16-17H,3,13,15H2,1-2H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.01-0.069 |
Bioorg Med Chem Lett 7: 157-162 (1997)
Article DOI: 10.1016/S0960-894X(96)00592-6
BindingDB Entry DOI: 10.7270/Q2CC10P5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50290391
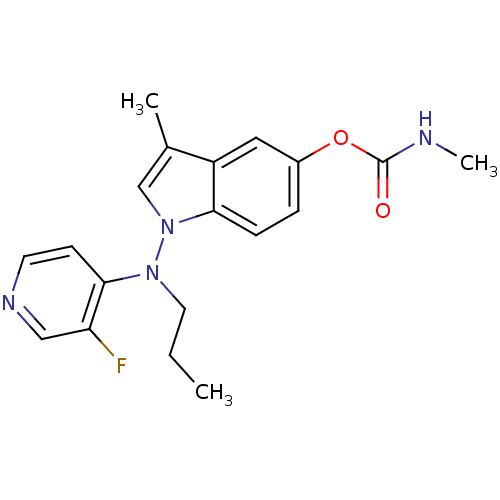
(CHEMBL42531 | Methyl-carbamic acid 1-[(3-fluoro-py...)Show InChI InChI=1S/C19H21FN4O2/c1-4-9-23(18-7-8-22-11-16(18)20)24-12-13(2)15-10-14(5-6-17(15)24)26-19(25)21-3/h5-8,10-12H,4,9H2,1-3H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.023-0.041 |
Bioorg Med Chem Lett 7: 157-162 (1997)
Article DOI: 10.1016/S0960-894X(96)00592-6
BindingDB Entry DOI: 10.7270/Q2CC10P5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50290392

(Benzyl-carbamic acid 1-[(3-fluoro-pyridin-4-yl)-pr...)Show SMILES CCCN(c1ccncc1F)n1cc(C)c2cc(OC(=O)NCc3ccccc3)ccc12 Show InChI InChI=1S/C25H25FN4O2/c1-3-13-29(24-11-12-27-16-22(24)26)30-17-18(2)21-14-20(9-10-23(21)30)32-25(31)28-15-19-7-5-4-6-8-19/h4-12,14,16-17H,3,13,15H2,1-2H3,(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... |
Bioorg Med Chem Lett 7: 157-162 (1997)
Article DOI: 10.1016/S0960-894X(96)00592-6
BindingDB Entry DOI: 10.7270/Q2CC10P5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50290391

(CHEMBL42531 | Methyl-carbamic acid 1-[(3-fluoro-py...)Show InChI InChI=1S/C19H21FN4O2/c1-4-9-23(18-7-8-22-11-16(18)20)24-12-13(2)15-10-14(5-6-17(15)24)26-19(25)21-3/h5-8,10-12H,4,9H2,1-3H3,(H,21,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... |
Bioorg Med Chem Lett 7: 157-162 (1997)
Article DOI: 10.1016/S0960-894X(96)00592-6
BindingDB Entry DOI: 10.7270/Q2CC10P5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50231956
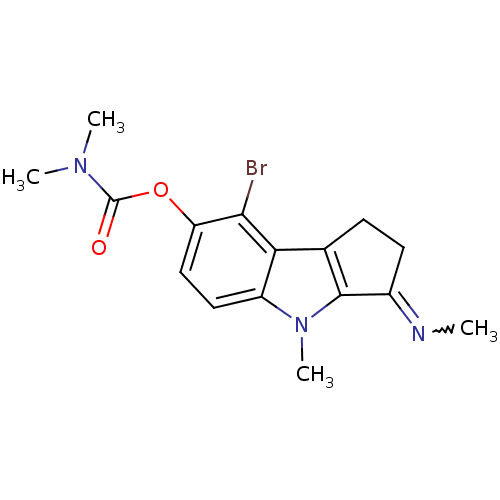
((Z)-8-bromo-4-methyl-3-(methylimino)-1,2,3,4-tetra...)Show SMILES CN=C1CCc2c1n(C)c1ccc(OC(=O)N(C)C)c(Br)c21 |w:1.0| Show InChI InChI=1S/C16H18BrN3O2/c1-18-10-6-5-9-13-11(20(4)15(9)10)7-8-12(14(13)17)22-16(21)19(2)3/h7-8H,5-6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase. |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50288976
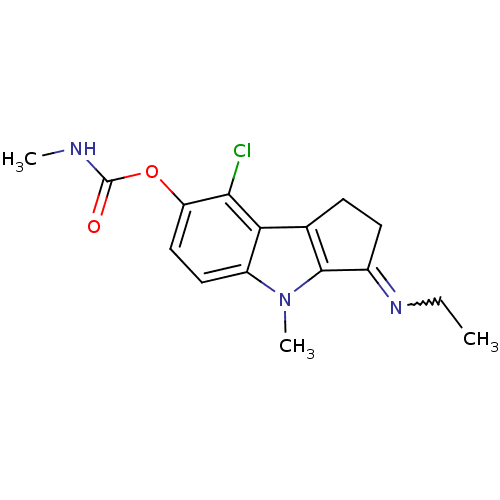
(CHEMBL155350 | Methyl-carbamic acid 8-chloro-3-[(Z...)Show SMILES CCN=C1CCc2c1n(C)c1ccc(OC(=O)NC)c(Cl)c21 |w:2.1| Show InChI InChI=1S/C16H18ClN3O2/c1-4-19-10-6-5-9-13-11(20(3)15(9)10)7-8-12(14(13)17)22-16(21)18-2/h7-8H,4-6H2,1-3H3,(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of monoamine oxidase-A(MAO-A). |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048600
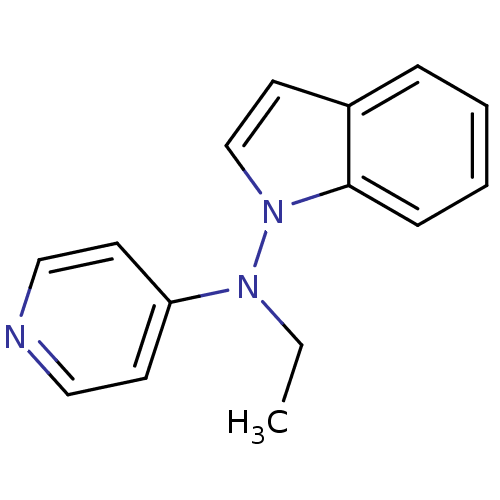
(CHEMBL154541 | Ethyl-indol-1-yl-pyridin-4-yl-amine)Show InChI InChI=1S/C15H15N3/c1-2-17(14-7-10-16-11-8-14)18-12-9-13-5-3-4-6-15(13)18/h3-12H,2H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50290389

(CHEMBL295462 | Methyl-carbamic acid 1-(3-fluoro-py...)Show InChI InChI=1S/C16H15FN4O2/c1-10-9-21(20-14-5-6-19-8-13(14)17)15-4-3-11(7-12(10)15)23-16(22)18-2/h3-9H,1-2H3,(H,18,22)(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro in rats for the inhibition of brain (striatal) acetylcholinesterase (AChEI) using acetylthiocholine as substrate; 0.0... |
Bioorg Med Chem Lett 7: 157-162 (1997)
Article DOI: 10.1016/S0960-894X(96)00592-6
BindingDB Entry DOI: 10.7270/Q2CC10P5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50288977

(CHEMBL160836 | Methyl-carbamic acid 8-bromo-3-[(Z)...)Show SMILES CCN=C1CCc2c1n(C)c1ccc(OC(=O)NC)c(Br)c21 |w:2.1| Show InChI InChI=1S/C16H18BrN3O2/c1-4-19-10-6-5-9-13-11(20(3)15(9)10)7-8-12(14(13)17)22-16(21)18-2/h7-8H,4-6H2,1-3H3,(H,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase. |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048580

(CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...)Show InChI InChI=1S/C16H17N3/c1-2-12-18(15-7-10-17-11-8-15)19-13-9-14-5-3-4-6-16(14)19/h3-11,13H,2,12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]idazoxan binding to Alpha-2 adrenergic receptor in rat cortex, in the presence of GPP |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50288976

(CHEMBL155350 | Methyl-carbamic acid 8-chloro-3-[(Z...)Show SMILES CCN=C1CCc2c1n(C)c1ccc(OC(=O)NC)c(Cl)c21 |w:2.1| Show InChI InChI=1S/C16H18ClN3O2/c1-4-19-10-6-5-9-13-11(20(3)15(9)10)7-8-12(14(13)17)22-16(21)18-2/h7-8H,4-6H2,1-3H3,(H,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase. |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50288981

(CHEMBL160219 | Methyl-carbamic acid 4-methyl-3-[(Z...)Show SMILES CNC(=O)Oc1ccc2n(C)c3C(CCc3c2c1)=NCCc1ccccc1 |w:18.21| Show InChI InChI=1S/C22H23N3O2/c1-23-22(26)27-16-8-11-20-18(14-16)17-9-10-19(21(17)25(20)2)24-13-12-15-6-4-3-5-7-15/h3-8,11,14H,9-10,12-13H2,1-2H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase. |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50288966

(CHEMBL156861 | Methyl-carbamic acid 8-bromo-4-meth...)Show SMILES CNC(=O)Oc1ccc2n(C)c3C(CCc3c2c1Br)=NC |w:19.22| Show InChI InChI=1S/C15H16BrN3O2/c1-17-9-5-4-8-12-10(19(3)14(8)9)6-7-11(13(12)16)21-15(20)18-2/h6-7H,4-5H2,1-3H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase. |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50288969

(4-(7-Bromo-5-methoxy-benzofuran-2-yl)-piperidine |...)Show InChI InChI=1S/C14H16BrNO2/c1-17-11-6-10-7-13(9-2-4-16-5-3-9)18-14(10)12(15)8-11/h6-9,16H,2-5H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of monoamine oxidase-A(MAO-A). |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50290389

(CHEMBL295462 | Methyl-carbamic acid 1-(3-fluoro-py...)Show InChI InChI=1S/C16H15FN4O2/c1-10-9-21(20-14-5-6-19-8-13(14)17)15-4-3-11(7-12(10)15)23-16(22)18-2/h3-9H,1-2H3,(H,18,22)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the inhibition of human serum Butyrylcholinesterase using butyrylthiocholine as substrate; 0.15-0.22 |
Bioorg Med Chem Lett 7: 157-162 (1997)
Article DOI: 10.1016/S0960-894X(96)00592-6
BindingDB Entry DOI: 10.7270/Q2CC10P5 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50288977

(CHEMBL160836 | Methyl-carbamic acid 8-bromo-3-[(Z)...)Show SMILES CCN=C1CCc2c1n(C)c1ccc(OC(=O)NC)c(Br)c21 |w:2.1| Show InChI InChI=1S/C16H18BrN3O2/c1-4-19-10-6-5-9-13-11(20(3)15(9)10)7-8-12(14(13)17)22-16(21)18-2/h7-8H,4-6H2,1-3H3,(H,18,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of monoamine oxidase-A(MAO-A). |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50288975
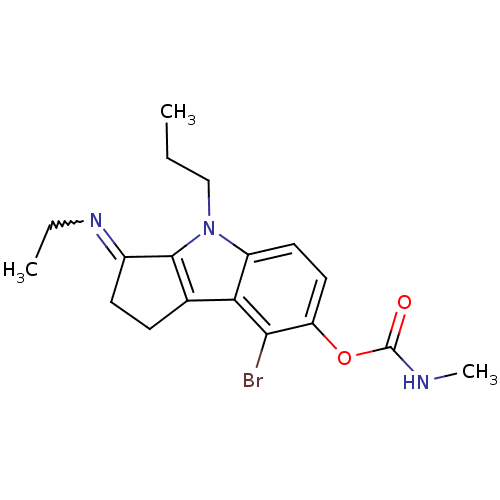
(CHEMBL445916 | Methyl-carbamic acid 8-bromo-3-[(Z)...)Show SMILES CCCn1c2C(CCc2c2c(Br)c(OC(=O)NC)ccc12)=NCC |w:21.24| Show InChI InChI=1S/C18H22BrN3O2/c1-4-10-22-13-8-9-14(24-18(23)20-3)16(19)15(13)11-6-7-12(17(11)22)21-5-2/h8-9H,4-7,10H2,1-3H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase. |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50288979

(CHEMBL155754 | Methyl-carbamic acid 8-chloro-4-met...)Show SMILES CNC(=O)Oc1ccc2n(C)c3C(CCc3c2c1Cl)=NC |w:19.22| Show InChI InChI=1S/C15H16ClN3O2/c1-17-9-5-4-8-12-10(19(3)14(8)9)6-7-11(13(12)16)21-15(20)18-2/h6-7H,4-5H2,1-3H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase. |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048580

(CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...)Show InChI InChI=1S/C16H17N3/c1-2-12-18(15-7-10-17-11-8-15)19-13-9-14-5-3-4-6-16(14)19/h3-11,13H,2,12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]yohimbine binding to Alpha-2 adrenergic receptor in rat cortex |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50048600

(CHEMBL154541 | Ethyl-indol-1-yl-pyridin-4-yl-amine)Show InChI InChI=1S/C15H15N3/c1-2-17(14-7-10-16-11-8-14)18-12-9-13-5-3-4-6-15(13)18/h3-12H,2H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat striatal preparation |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048580

(CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...)Show InChI InChI=1S/C16H17N3/c1-2-12-18(15-7-10-17-11-8-15)19-13-9-14-5-3-4-6-16(14)19/h3-11,13H,2,12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50048580

(CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...)Show InChI InChI=1S/C16H17N3/c1-2-12-18(15-7-10-17-11-8-15)19-13-9-14-5-3-4-6-16(14)19/h3-11,13H,2,12H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50048576
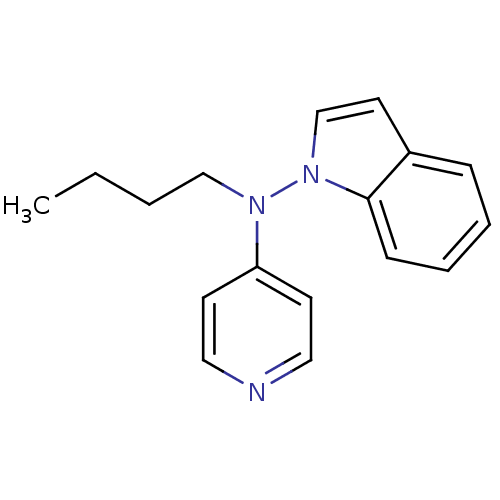
(Butyl-indol-1-yl-pyridin-4-yl-amine | CHEMBL155109)Show InChI InChI=1S/C17H19N3/c1-2-3-13-19(16-8-11-18-12-9-16)20-14-10-15-6-4-5-7-17(15)20/h4-12,14H,2-3,13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50048580

(CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...)Show InChI InChI=1S/C16H17N3/c1-2-12-18(15-7-10-17-11-8-15)19-13-9-14-5-3-4-6-16(14)19/h3-11,13H,2,12H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]oxotremorine-M binding to muscarinic receptors in rat forebrain membrane |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048580

(CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...)Show InChI InChI=1S/C16H17N3/c1-2-12-18(15-7-10-17-11-8-15)19-13-9-14-5-3-4-6-16(14)19/h3-11,13H,2,12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]-idazoxan binding to Alpha-2 adrenergic receptor in rat cortex |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50048610
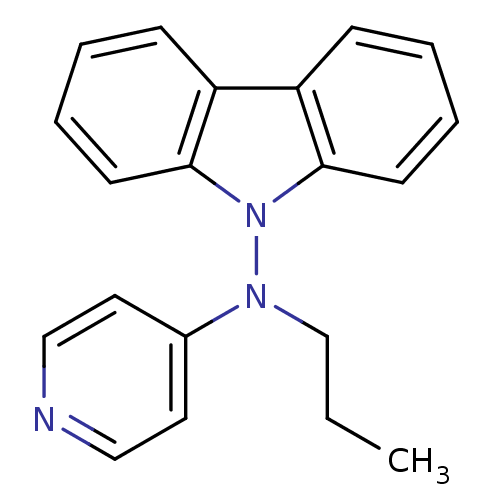
(CHEMBL435034 | Carbazol-9-yl-propyl-pyridin-4-yl-a...)Show InChI InChI=1S/C20H19N3/c1-2-15-22(16-11-13-21-14-12-16)23-19-9-5-3-7-17(19)18-8-4-6-10-20(18)23/h3-14H,2,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048602
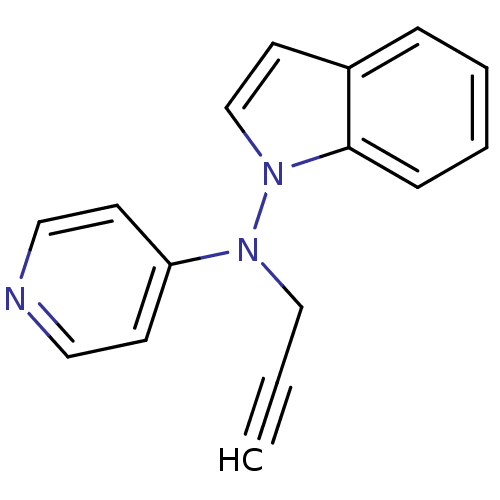
(CHEMBL348034 | Indol-1-yl-prop-2-ynyl-pyridin-4-yl...)Show InChI InChI=1S/C16H13N3/c1-2-12-18(15-7-10-17-11-8-15)19-13-9-14-5-3-4-6-16(14)19/h1,3-11,13H,12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50048608

(CHEMBL154488 | Indol-1-yl-pyridin-4-yl-amine)Show InChI InChI=1S/C13H11N3/c1-2-4-13-11(3-1)7-10-16(13)15-12-5-8-14-9-6-12/h1-10H,(H,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]WB-4101 binding to Alpha-1 adrenergic receptor in rat whole brain membrane |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50231956

((Z)-8-bromo-4-methyl-3-(methylimino)-1,2,3,4-tetra...)Show SMILES CN=C1CCc2c1n(C)c1ccc(OC(=O)N(C)C)c(Br)c21 |w:1.0| Show InChI InChI=1S/C16H18BrN3O2/c1-18-10-6-5-9-13-11(20(4)15(9)10)7-8-12(14(13)17)22-16(21)19(2)3/h7-8H,5-6H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of monoamine oxidase-A(MAO-A). |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048610

(CHEMBL435034 | Carbazol-9-yl-propyl-pyridin-4-yl-a...)Show InChI InChI=1S/C20H19N3/c1-2-15-22(16-11-13-21-14-12-16)23-19-9-5-3-7-17(19)18-8-4-6-10-20(18)23/h3-14H,2,15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50048575
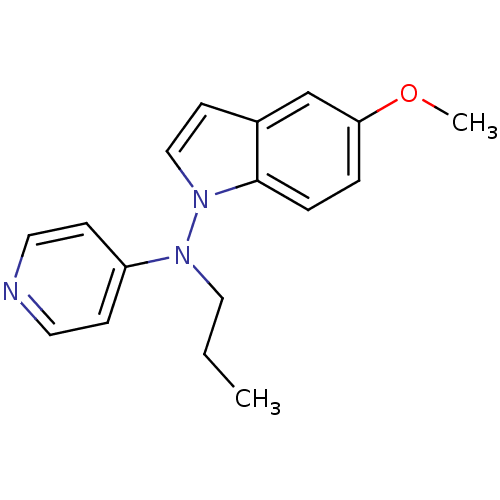
((5-Methoxy-indol-1-yl)-propyl-pyridin-4-yl-amine |...)Show InChI InChI=1S/C17H19N3O/c1-3-11-19(15-6-9-18-10-7-15)20-12-8-14-13-16(21-2)4-5-17(14)20/h4-10,12-13H,3,11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50048590

(CHEMBL154319 | Indol-1-yl-isopropyl-pyridin-4-yl-a...)Show InChI InChI=1S/C16H17N3/c1-13(2)19(15-7-10-17-11-8-15)18-12-9-14-5-3-4-6-16(14)18/h3-13H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50048576

(Butyl-indol-1-yl-pyridin-4-yl-amine | CHEMBL155109)Show InChI InChI=1S/C17H19N3/c1-2-3-13-19(16-8-11-18-12-9-16)20-14-10-15-6-4-5-7-17(15)20/h4-12,14H,2-3,13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]clonidine binding to Alpha-2 adrenergic receptor in rat cortex |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50288966

(CHEMBL156861 | Methyl-carbamic acid 8-bromo-4-meth...)Show SMILES CNC(=O)Oc1ccc2n(C)c3C(CCc3c2c1Br)=NC |w:19.22| Show InChI InChI=1S/C15H16BrN3O2/c1-17-9-5-4-8-12-10(19(3)14(8)9)6-7-11(13(12)16)21-15(20)18-2/h6-7H,4-5H2,1-3H3,(H,18,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of monoamine oxidase-A(MAO-A). |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50048580

(CHEMBL29835 | Indol-1-yl-propyl-pyridin-4-yl-amine...)Show InChI InChI=1S/C16H17N3/c1-2-12-18(15-7-10-17-11-8-15)19-13-9-14-5-3-4-6-16(14)19/h3-11,13H,2,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against [3H]N-methyl-scopolamine in rat Muscarinic acetylcholine receptor M2 cerebellum in the presence GPP(NH)P |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50288973

(CHEMBL157182 | Methyl-carbamic acid 6-chloro-4-met...)Show SMILES CNC(=O)Oc1cc2c3CCC(=NC)c3n(C)c2cc1Cl |w:12.12| Show InChI InChI=1S/C15H16ClN3O2/c1-17-11-5-4-8-9-6-13(21-15(20)18-2)10(16)7-12(9)19(3)14(8)11/h6-7H,4-5H2,1-3H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase. |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50454302
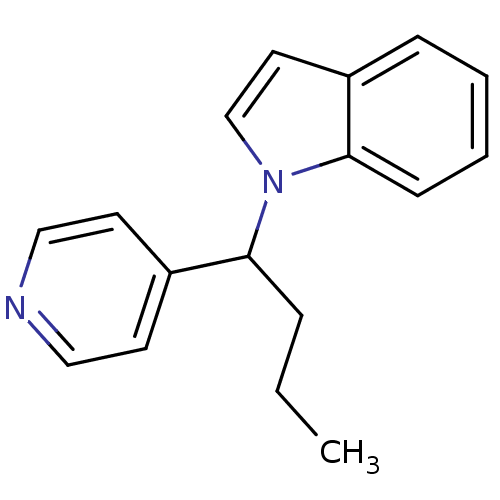
(CHEMBL347199)Show InChI InChI=1S/C17H18N2/c1-2-5-16(15-8-11-18-12-9-15)19-13-10-14-6-3-4-7-17(14)19/h3-4,6-13,16H,2,5H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50048602

(CHEMBL348034 | Indol-1-yl-prop-2-ynyl-pyridin-4-yl...)Show InChI InChI=1S/C16H13N3/c1-2-12-18(15-7-10-17-11-8-15)19-13-9-14-5-3-4-6-16(14)19/h1,3-11,13H,12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50048611
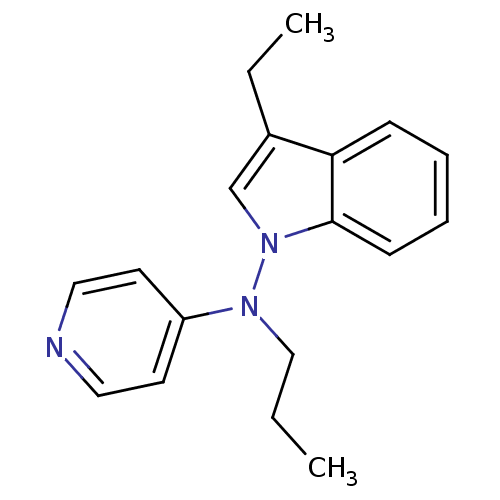
((3-Ethyl-indol-1-yl)-propyl-pyridin-4-yl-amine | C...)Show InChI InChI=1S/C18H21N3/c1-3-13-20(16-9-11-19-12-10-16)21-14-15(4-2)17-7-5-6-8-18(17)21/h5-12,14H,3-4,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50048583
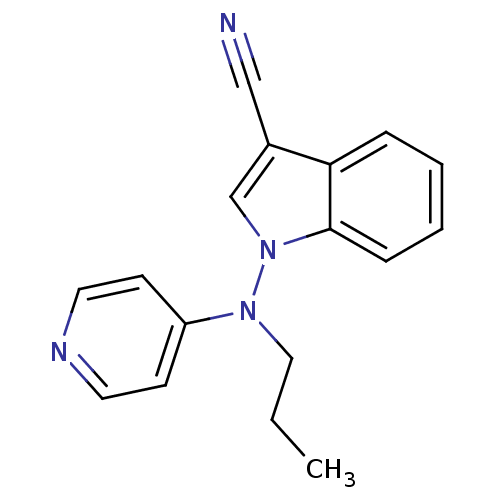
(1-(Propyl-pyridin-4-yl-amino)-1H-indole-3-carbonit...)Show InChI InChI=1S/C17H16N4/c1-2-11-20(15-7-9-19-10-8-15)21-13-14(12-18)16-5-3-4-6-17(16)21/h3-10,13H,2,11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against norepinephrine (NE) uptake in rat whole brain synaptosome preparation |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50288967

(Butyl-carbamic acid 8-bromo-4-methyl-3-[(Z)-methyl...)Show SMILES CCCCNC(=O)Oc1ccc2n(C)c3C(CCc3c2c1Br)=NC |w:22.25| Show InChI InChI=1S/C18H22BrN3O2/c1-4-5-10-21-18(23)24-14-9-8-13-15(16(14)19)11-6-7-12(20-2)17(11)22(13)3/h8-9H,4-7,10H2,1-3H3,(H,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of monoamine oxidase-A(MAO-A). |
Bioorg Med Chem Lett 6: 625-630 (1996)
Article DOI: 10.1016/0960-894X(96)00072-8
BindingDB Entry DOI: 10.7270/Q22J6BVC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data