 Found 7296 hits Enz. Inhib. hit(s) with Target = 'Muscarinic acetylcholine receptor M2'
Found 7296 hits Enz. Inhib. hit(s) with Target = 'Muscarinic acetylcholine receptor M2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M4 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095105
(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O8S2/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095105

(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O8S2/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 11: 2311-4 (2001)
BindingDB Entry DOI: 10.7270/Q28S4P7Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095105

(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O8S2/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50066861

(BA-679-BR | Spiriva | Tiotropium Bromide | Tiotrop...)Show SMILES O.[Br-].[H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:4:3:15:10.12.9,4:5:15:10.12.9,18:10:15:3.5| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG
Curated by ChEMBL
| Assay Description
Binding affinity to human M2 receptor |
J Med Chem 56: 8746-56 (2013)
Article DOI: 10.1021/jm401219y
BindingDB Entry DOI: 10.7270/Q28055J7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M2 receptor expressed in CHO cells after 24 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 576-80 (1992)
BindingDB Entry DOI: 10.7270/Q28P5Z0G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021919
(CHEMBL3298595)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ccsc1-c1ccccc1 Show InChI InChI=1S/C19H22N2O2S.HI/c1-21-11-8-19(9-12-21,10-13-21)23-18(22)20-16-7-14-24-17(16)15-5-3-2-4-6-15;/h2-7,14H,8-13H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation
Curated by PDSP Ki Database
| |
Biochem Pharmacol 45: 2352-4 (1993)
Article DOI: 10.1016/0006-2952(93)90211-e
BindingDB Entry DOI: 10.7270/Q2F76B2C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592
(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory
Curated by ChEMBL
| Assay Description
Ability to displace [3H](-)-quinuclidinyl bezilate(QNB) from M2 receptor in rat heart homogenate |
J Med Chem 36: 848-54 (1993)
BindingDB Entry DOI: 10.7270/Q2S46SM0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50451113
(CHEMBL2114068)Show SMILES COc1ccc(cc1)[S@+]([O-])c1ccc(cc1)C(=C)C1CCN(CC1)C1CCCCC1 |r| Show InChI InChI=1S/C26H33NO2S/c1-20(22-16-18-27(19-17-22)23-6-4-3-5-7-23)21-8-12-25(13-9-21)30(28)26-14-10-24(29-2)11-15-26/h8-15,22-23H,1,3-7,16-19H2,2H3/t30-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 10: 2209-12 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HMP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592

(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]QNB (quinuclidinyl benzylate) from muscarinic M2 receptor of rat heart homogenates. |
Bioorg Med Chem Lett 7: 979-984 (1997)
Article DOI: 10.1016/S0960-894X(97)00143-1
BindingDB Entry DOI: 10.7270/Q2N29XFM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50450592

(CHEMBL558910)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |r,wD:5.4,(8.38,-12.97,;7.3,-14.07,;8.63,-14.83,;8.64,-16.37,;9.97,-14.06,;11.3,-14.82,;11.3,-16.36,;12.63,-17.13,;13.96,-16.36,;13.96,-14.82,;12.63,-14.05,;13.05,-15.29,;12,-15.64,;5.97,-14.84,;4.63,-14.07,;3.3,-14.84,;3.31,-16.38,;4.65,-17.15,;5.98,-16.37,;7.29,-12.53,;8.62,-11.76,;8.62,-10.22,;7.28,-9.45,;5.95,-10.23,;5.96,-11.77,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)-quinuclidinyl benzilate(QNB) from muscarinic (M2) receptor in rat heart homogenates |
J Med Chem 34: 2984-9 (1991)
BindingDB Entry DOI: 10.7270/Q27H1K5M |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50110539
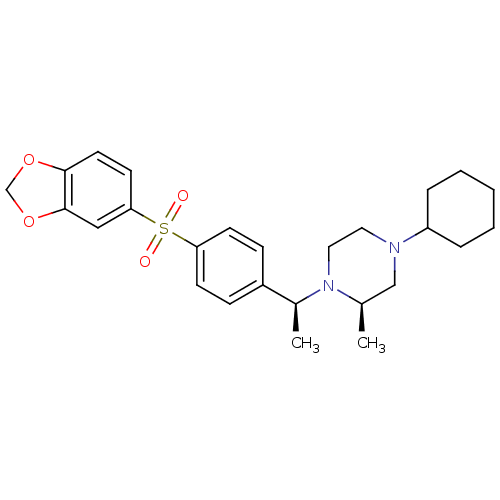
(1-{1-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-eth...)Show SMILES C[C@H](N1CCN(C[C@H]1C)C1CCCCC1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C26H34N2O4S/c1-19-17-27(22-6-4-3-5-7-22)14-15-28(19)20(2)21-8-10-23(11-9-21)33(29,30)24-12-13-25-26(16-24)32-18-31-25/h8-13,16,19-20,22H,3-7,14-15,17-18H2,1-2H3/t19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against human cloned Muscarinic acetylcholine receptor M2. |
Bioorg Med Chem Lett 12: 791-4 (2002)
BindingDB Entry DOI: 10.7270/Q2VH5N5J |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50110523
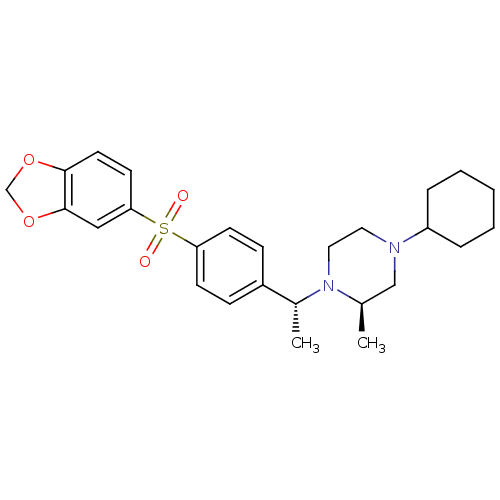
(1-{1-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-eth...)Show SMILES C[C@@H](N1CCN(C[C@H]1C)C1CCCCC1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C26H34N2O4S/c1-19-17-27(22-6-4-3-5-7-22)14-15-28(19)20(2)21-8-10-23(11-9-21)33(29,30)24-12-13-25-26(16-24)32-18-31-25/h8-13,16,19-20,22H,3-7,14-15,17-18H2,1-2H3/t19-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against human cloned Muscarinic acetylcholine receptor M2. |
Bioorg Med Chem Lett 12: 791-4 (2002)
BindingDB Entry DOI: 10.7270/Q2VH5N5J |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095111
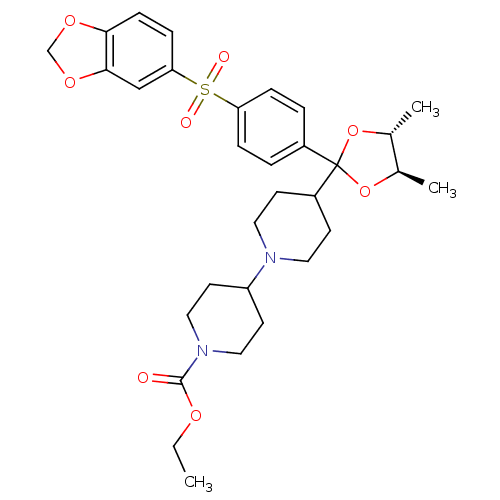
(4-{(4R,5R)-2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phe...)Show SMILES CCOC(=O)N1CCC(CC1)N1CCC(CC1)C1(O[C@H](C)[C@@H](C)O1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C31H40N2O8S/c1-4-37-30(34)33-17-13-25(14-18-33)32-15-11-24(12-16-32)31(40-21(2)22(3)41-31)23-5-7-26(8-6-23)42(35,36)27-9-10-28-29(19-27)39-20-38-28/h5-10,19,21-22,24-25H,4,11-18,20H2,1-3H3/t21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095097
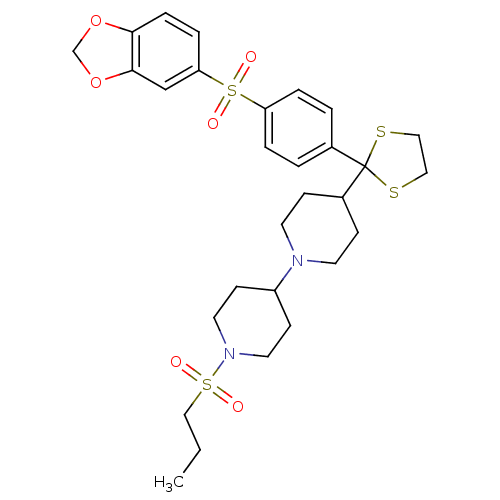
(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(SCCS1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O6S4/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 576-80 (1992)
BindingDB Entry DOI: 10.7270/Q28P5Z0G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation
Curated by PDSP Ki Database
| |
Biochem Pharmacol 45: 2352-4 (1993)
Article DOI: 10.1016/0006-2952(93)90211-e
BindingDB Entry DOI: 10.7270/Q2F76B2C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50110534
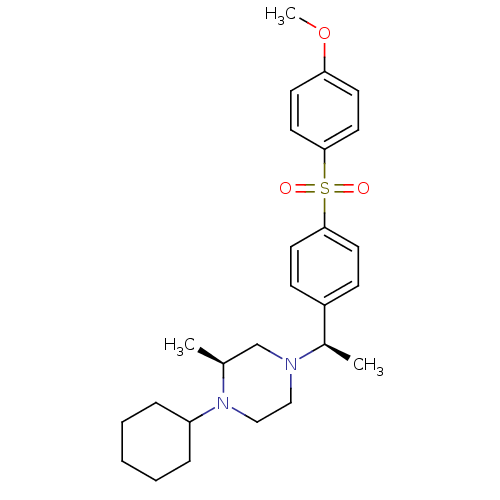
(1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfonyl)-p...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)[C@@H](C)N1CCN([C@@H](C)C1)C1CCCCC1 Show InChI InChI=1S/C26H36N2O3S/c1-20-19-27(17-18-28(20)23-7-5-4-6-8-23)21(2)22-9-13-25(14-10-22)32(29,30)26-15-11-24(31-3)12-16-26/h9-16,20-21,23H,4-8,17-19H2,1-3H3/t20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against human cloned Muscarinic acetylcholine receptor M2. |
Bioorg Med Chem Lett 12: 791-4 (2002)
BindingDB Entry DOI: 10.7270/Q2VH5N5J |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM81768

(BENZYLIC ACID | CAS_6581-06-2 | NSC_5311391 | Quin...)Show InChI InChI=1S/C14H12O3/c15-13(16)14(17,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,17H,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by PDSP Ki Database
| |
Eur J Pharmacol 103: 197-204 (1984)
Article DOI: 10.1016/0014-2999(84)90478-3
BindingDB Entry DOI: 10.7270/Q2WW7G5B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50010096
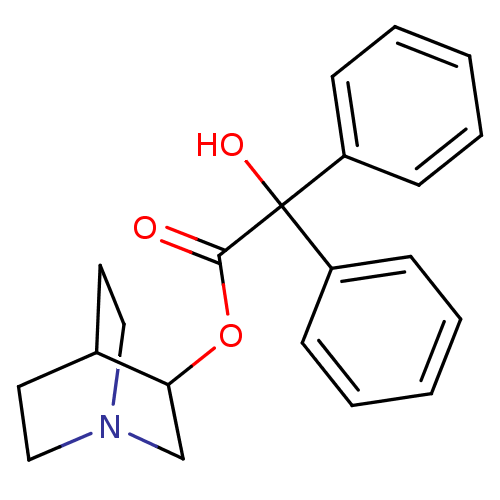
(CHEMBL12980 | Hydroxy-diphenyl-acetic acid 1-aza-b...)Show SMILES OC(C(=O)OC1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(5.41,-4.43,;4.65,-5.78,;6.2,-5.78,;6.97,-7.12,;6.97,-4.45,;8.52,-4.1,;8.52,-2.56,;9.86,-1.78,;11.19,-2.56,;11.19,-4.1,;9.86,-4.88,;9.09,-3.78,;9.09,-2.9,;3.1,-5.81,;2.36,-7.19,;.82,-7.2,;.01,-5.88,;.78,-4.52,;2.32,-4.49,;4.65,-7.23,;3.39,-7.96,;3.38,-9.41,;4.64,-10.15,;5.91,-9.42,;5.91,-7.97,)| Show InChI InChI=1S/C21H23NO3/c23-20(25-19-15-22-13-11-16(19)12-14-22)21(24,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,16,19,24H,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity for muscarinic acetylcholine receptor M2 by measuring displacement of [3H]-QNB from guinea pig heart |
J Med Chem 38: 473-87 (1995)
BindingDB Entry DOI: 10.7270/Q25Q4V42 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50021928
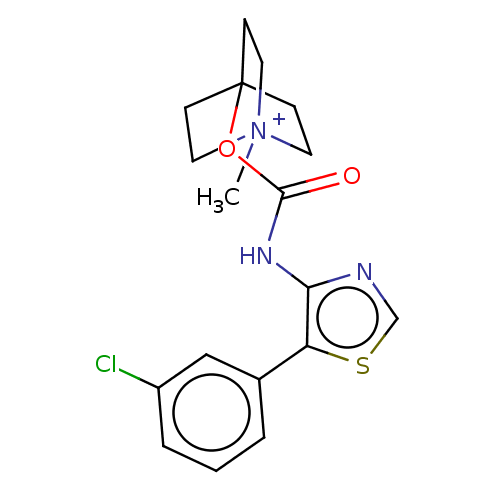
(CHEMBL3298599)Show SMILES [I-].C[N+]12CCC(CC1)(CC2)OC(=O)Nc1ncsc1-c1cccc(Cl)c1 Show InChI InChI=1S/C18H20ClN3O2S.HI/c1-22-8-5-18(6-9-22,7-10-22)24-17(23)21-16-15(25-12-20-16)13-3-2-4-14(19)11-13;/h2-4,11-12H,5-10H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart |
Bioorg Med Chem 22: 3478-87 (2014)
Article DOI: 10.1016/j.bmc.2014.04.031
BindingDB Entry DOI: 10.7270/Q2XS5X00 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50355612
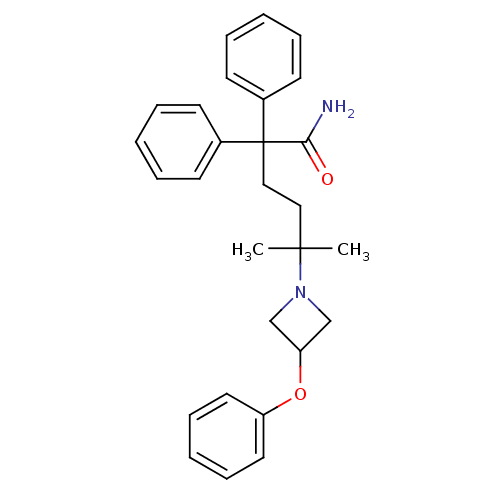
(CHEMBL1910848)Show SMILES CC(C)(CCC(C(N)=O)(c1ccccc1)c1ccccc1)N1CC(C1)Oc1ccccc1 Show InChI InChI=1S/C28H32N2O2/c1-27(2,30-20-25(21-30)32-24-16-10-5-11-17-24)18-19-28(26(29)31,22-12-6-3-7-13-22)23-14-8-4-9-15-23/h3-17,25H,18-21H2,1-2H3,(H2,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M2 receptor expressed in CHO cells after 2 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50451114
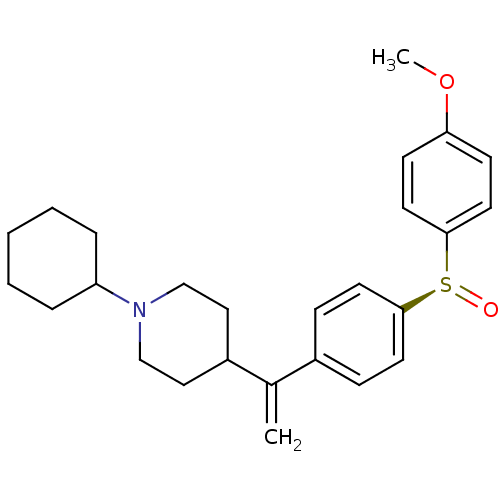
(CHEMBL2115128)Show SMILES COc1ccc(cc1)[S@@+]([O-])c1ccc(cc1)C(=C)C1CCN(CC1)C1CCCCC1 |r| Show InChI InChI=1S/C26H33NO2S/c1-20(22-16-18-27(19-17-22)23-6-4-3-5-7-23)21-8-12-25(13-9-21)30(28)26-14-10-24(29-2)11-15-26/h8-15,22-23H,1,3-7,16-19H2,2H3/t30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 10: 2209-12 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HMP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296336
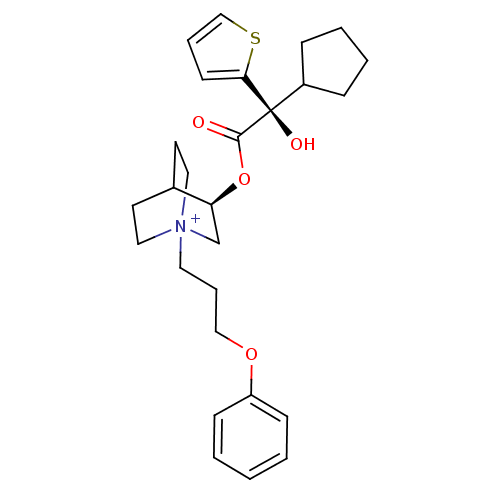
((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...)Show SMILES O[C@@](C1CCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.31,wD:10.10,1.0,(16.18,-14.66,;15.09,-15.75,;13.76,-16.53,;12.35,-15.91,;11.32,-17.05,;12.1,-18.39,;13.6,-18.06,;16.43,-16.52,;16.43,-18.06,;17.76,-15.75,;19.09,-16.51,;19.09,-18.05,;20.42,-18.81,;20.41,-20.35,;21.74,-21.14,;23.08,-20.38,;24.4,-21.16,;25.74,-20.41,;27.07,-21.19,;28.41,-20.43,;28.42,-18.89,;27.09,-18.11,;25.75,-18.87,;21.75,-18.05,;21.75,-16.51,;20.42,-15.73,;20.84,-16.98,;19.79,-17.33,;15.09,-14.21,;13.83,-13.31,;14.3,-11.85,;15.84,-11.84,;16.32,-13.31,)| Show InChI InChI=1S/C27H36NO4S/c29-26(27(30,22-8-4-5-9-22)25-12-6-19-33-25)32-24-20-28(16-13-21(24)14-17-28)15-7-18-31-23-10-2-1-3-11-23/h1-3,6,10-12,19,21-22,24,30H,4-5,7-9,13-18,20H2/q+1/t21?,24-,27+,28?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human recombinant M2 receptor expressed in CHO cells after 2 hrs by filter binding assay |
J Med Chem 54: 6888-904 (2011)
Article DOI: 10.1021/jm200884j
BindingDB Entry DOI: 10.7270/Q2CZ37JW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50241132
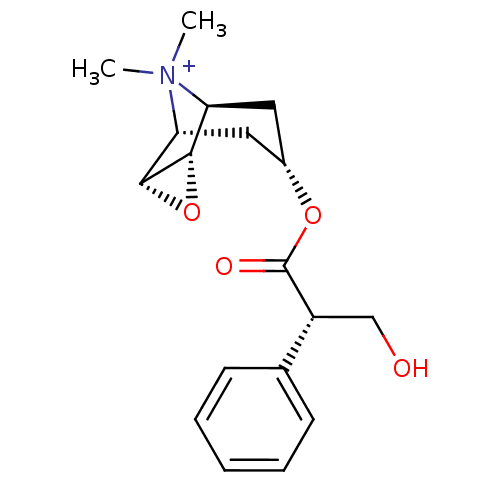
(3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...)Show SMILES C[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:9:8:4.5.6:1,9:10:4.5.6:1,THB:11:5:1:8.10| Show InChI InChI=1S/C18H24NO4/c1-19(2)14-8-12(9-15(19)17-16(14)23-17)22-18(21)13(10-20)11-6-4-3-5-7-11/h3-7,12-17,20H,8-10H2,1-2H3/q+1/t12-,13-,14-,15+,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]UNSW-MK259 from human muscarinic acetylcholine receptor M2 expressed in CHOK9 cells after 3 hrs by liquid scintillation counting ... |
J Med Chem 60: 3314-3334 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01892
BindingDB Entry DOI: 10.7270/Q29S1THC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50525130

(CHEMBL4540949)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1 |r| Show InChI InChI=1S/C44H68N12O5.5C2HF3O2/c1-32(57)50-37(14-6-8-20-45)43(61)52-36(15-10-21-49-44(46)47)42(60)48-22-26-54-29-27-53(28-30-54)23-9-7-11-33-18-24-55(25-19-33)31-40(58)56-38-16-4-2-12-34(38)41(59)51-35-13-3-5-17-39(35)56;5*3-2(4,5)1(6)7/h2-5,12-13,16-17,33,36-37H,6-11,14-15,18-31,45H2,1H3,(H,48,60)(H,50,57)(H,51,59)(H,52,61)(H4,46,47,49);5*(H,6,7)/t36-,37-;;;;;/m0...../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method |
J Med Chem 62: 5358-5369 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01967
BindingDB Entry DOI: 10.7270/Q25H7KQF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50525115

(CHEMBL4582879)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@H](NC(=O)[C@H](CCCCN)NC(C)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1 |r| Show InChI InChI=1S/C47H73N13O6.5C2HF3O2/c1-33(53-46(66)39(54-34(2)61)15-7-9-21-48)43(63)56-38(16-11-22-52-47(49)50)45(65)51-23-27-58-30-28-57(29-31-58)24-10-8-12-35-19-25-59(26-20-35)32-42(62)60-40-17-5-3-13-36(40)44(64)55-37-14-4-6-18-41(37)60;5*3-2(4,5)1(6)7/h3-6,13-14,17-18,33,35,38-39H,7-12,15-16,19-32,48H2,1-2H3,(H,51,65)(H,53,66)(H,54,61)(H,55,64)(H,56,63)(H4,49,50,52);5*(H,6,7)/t33-,38-,39-;;;;;/m0...../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells using 0.2 nM [3H]-NMS after 3 hrs by microbeta2 scin... |
J Med Chem 62: 5358-5369 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01967
BindingDB Entry DOI: 10.7270/Q25H7KQF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50525115

(CHEMBL4582879)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@H](NC(=O)[C@H](CCCCN)NC(C)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1 |r| Show InChI InChI=1S/C47H73N13O6.5C2HF3O2/c1-33(53-46(66)39(54-34(2)61)15-7-9-21-48)43(63)56-38(16-11-22-52-47(49)50)45(65)51-23-27-58-30-28-57(29-31-58)24-10-8-12-35-19-25-59(26-20-35)32-42(62)60-40-17-5-3-13-36(40)44(64)55-37-14-4-6-18-41(37)60;5*3-2(4,5)1(6)7/h3-6,13-14,17-18,33,35,38-39H,7-12,15-16,19-32,48H2,1-2H3,(H,51,65)(H,53,66)(H,54,61)(H,55,64)(H,56,63)(H4,49,50,52);5*(H,6,7)/t33-,38-,39-;;;;;/m0...../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method |
J Med Chem 62: 5358-5369 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01967
BindingDB Entry DOI: 10.7270/Q25H7KQF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50121132
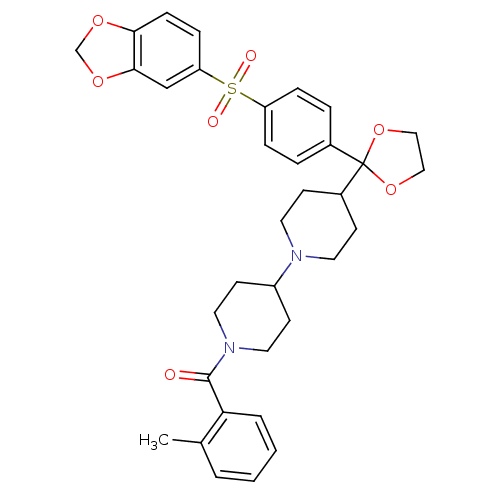
((4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1...)Show SMILES Cc1ccccc1C(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C34H38N2O7S/c1-24-4-2-3-5-30(24)33(37)36-18-14-27(15-19-36)35-16-12-26(13-17-35)34(42-20-21-43-34)25-6-8-28(9-7-25)44(38,39)29-10-11-31-32(22-29)41-23-40-31/h2-11,22,26-27H,12-21,23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 12: 3479-82 (2002)
BindingDB Entry DOI: 10.7270/Q2VT1RGD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50092959
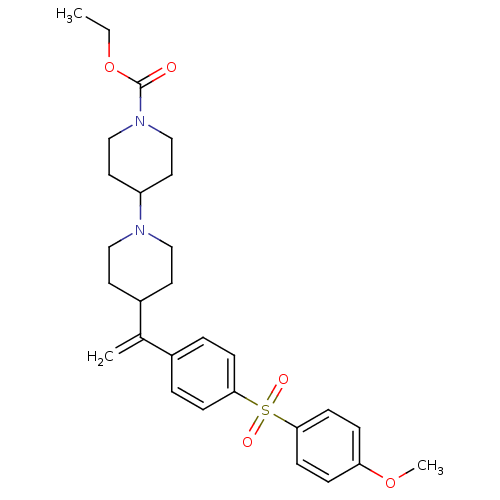
(4-{1-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-vinyl}...)Show SMILES CCOC(=O)N1CCC(CC1)N1CCC(CC1)C(=C)c1ccc(cc1)S(=O)(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C28H36N2O5S/c1-4-35-28(31)30-19-15-24(16-20-30)29-17-13-23(14-18-29)21(2)22-5-9-26(10-6-22)36(32,33)27-11-7-25(34-3)8-12-27/h5-12,23-24H,2,4,13-20H2,1,3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50092313
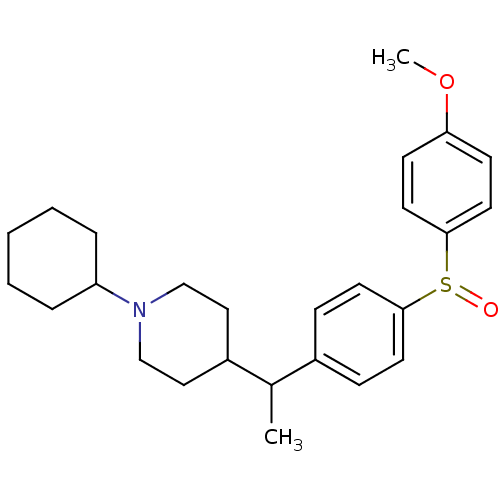
(1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfinyl)-p...)Show SMILES COc1ccc(cc1)S(=O)c1ccc(cc1)C(C)C1CCN(CC1)C1CCCCC1 Show InChI InChI=1S/C26H35NO2S/c1-20(22-16-18-27(19-17-22)23-6-4-3-5-7-23)21-8-12-25(13-9-21)30(28)26-14-10-24(29-2)11-15-26/h8-15,20,22-23H,3-7,16-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M4 |
Bioorg Med Chem Lett 10: 2209-12 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HMP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095112

(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCOC(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H36N2O8S/c1-2-35-28(32)31-15-11-23(12-16-31)30-13-9-22(10-14-30)29(38-17-18-39-29)21-3-5-24(6-4-21)40(33,34)25-7-8-26-27(19-25)37-20-36-26/h3-8,19,22-23H,2,9-18,20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50083651
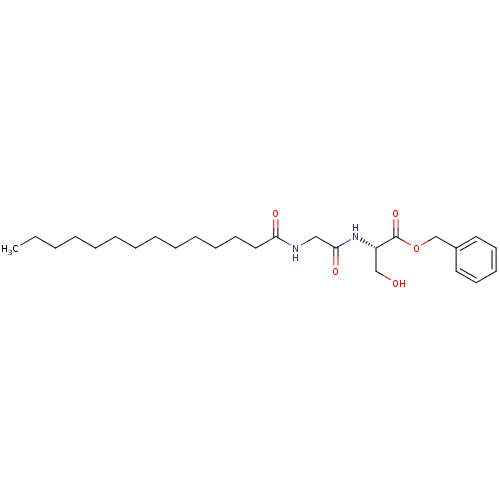
((S)-3-Hydroxy-2-(2-tetradecanoylamino-acetylamino)...)Show SMILES CCCCCCCCCCCCCC(=O)NCC(=O)N[C@@H](CO)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H42N2O5/c1-2-3-4-5-6-7-8-9-10-11-15-18-24(30)27-19-25(31)28-23(20-29)26(32)33-21-22-16-13-12-14-17-22/h12-14,16-17,23,29H,2-11,15,18-21H2,1H3,(H,27,30)(H,28,31)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM1 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50083651

((S)-3-Hydroxy-2-(2-tetradecanoylamino-acetylamino)...)Show SMILES CCCCCCCCCCCCCC(=O)NCC(=O)N[C@@H](CO)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H42N2O5/c1-2-3-4-5-6-7-8-9-10-11-15-18-24(30)27-19-25(31)28-23(20-29)26(32)33-21-22-16-13-12-14-17-22/h12-14,16-17,23,29H,2-11,15,18-21H2,1H3,(H,27,30)(H,28,31)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM2 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM85817

(NNC 11-1585)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccc(cc1)C#CCOc1nsnc1C1CN2CCC1CC2 |(2.45,.06,;3.22,1.4,;4.76,1.4,;5.39,2.81,;6.92,2.64,;7.24,1.14,;5.91,.37,;5.51,-1.12,;6.28,-2.45,;5.51,-3.79,;3.97,-3.79,;3.2,-2.45,;3.97,-1.12,;5.3,-1.89,;4.21,-2.98,;.91,.06,;-.63,.06,;-2.17,.06,;-2.94,-1.27,;-4.48,-1.27,;-5.25,.06,;-4.48,1.4,;-2.94,1.4,;-6.79,.06,;-8.33,.07,;-9.87,.06,;-10.64,1.4,;-12.18,1.4,;-12.8,2.81,;-14.33,2.64,;-14.66,1.14,;-13.32,.37,;-12.92,-1.12,;-13.69,-2.45,;-12.92,-3.79,;-11.38,-3.79,;-10.61,-2.45,;-11.38,-1.12,;-12.72,-1.89,;-11.63,-2.98,)| Show InChI InChI=1S/C30H32N6O2S2/c1(17-37-29-27(31-39-33-29)25-19-35-13-9-23(25)10-14-35)3-21-5-7-22(8-6-21)4-2-18-38-30-28(32-40-34-30)26-20-36-15-11-24(26)12-16-36/h5-8,23-26H,9-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 1260-8 (2001)
BindingDB Entry DOI: 10.7270/Q26M35D8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50568858
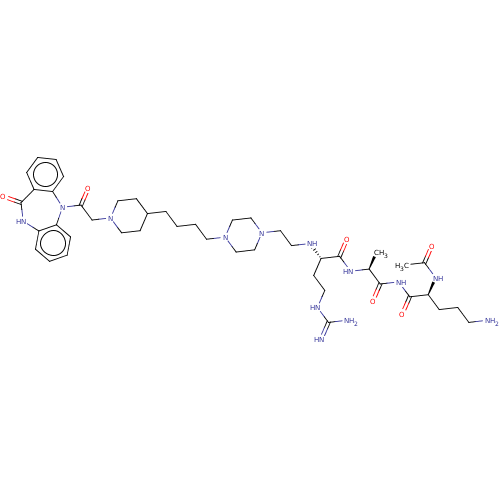
(CHEMBL4856113)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@H](NC(=O)[C@H](CCNC(N)=N)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1)C(=O)NC(=O)[C@H](CCCN)NC(C)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO cell membranes assessed as inhibition constant by radioligand competition... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113159
BindingDB Entry DOI: 10.7270/Q2F47SWV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50525110

(CHEMBL4455570)Show SMILES CN1CCC(=CC1)c1nsnc1OCCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1 |c:4| Show InChI InChI=1S/C39H52N8O3S/c1-43-20-16-31(17-21-43)37-39(42-51-41-37)50-28-8-19-45-26-24-44(25-27-45)18-7-6-9-30-14-22-46(23-15-30)29-36(48)47-34-12-4-2-10-32(34)38(49)40-33-11-3-5-13-35(33)47/h2-5,10-13,16,30H,6-9,14-15,17-29H2,1H3,(H,40,49) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method |
J Med Chem 62: 5358-5369 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01967
BindingDB Entry DOI: 10.7270/Q25H7KQF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50525124

(CHEMBL4569639)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1 |r| Show InChI InChI=1S/C47H65N9O6.4C2HF3O2/c1-34(57)50-40(13-6-8-22-48)47(62)52-41(32-36-16-18-37(58)19-17-36)46(61)49-23-27-54-30-28-53(29-31-54)24-9-7-10-35-20-25-55(26-21-35)33-44(59)56-42-14-4-2-11-38(42)45(60)51-39-12-3-5-15-43(39)56;4*3-2(4,5)1(6)7/h2-5,11-12,14-19,35,40-41,58H,6-10,13,20-33,48H2,1H3,(H,49,61)(H,50,57)(H,51,60)(H,52,62);4*(H,6,7)/t40-,41-;;;;/m0..../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method |
J Med Chem 62: 5358-5369 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01967
BindingDB Entry DOI: 10.7270/Q25H7KQF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50525132

(CHEMBL4451383)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1 |r| Show InChI InChI=1S/C47H65N9O6.4C2HF3O2/c1-34(57)50-41(32-36-16-18-37(58)19-17-36)47(62)52-40(13-6-8-22-48)46(61)49-23-27-54-30-28-53(29-31-54)24-9-7-10-35-20-25-55(26-21-35)33-44(59)56-42-14-4-2-11-38(42)45(60)51-39-12-3-5-15-43(39)56;4*3-2(4,5)1(6)7/h2-5,11-12,14-19,35,40-41,58H,6-10,13,20-33,48H2,1H3,(H,49,61)(H,50,57)(H,51,60)(H,52,62);4*(H,6,7)/t40-,41-;;;;/m0..../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method |
J Med Chem 62: 5358-5369 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01967
BindingDB Entry DOI: 10.7270/Q25H7KQF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50525115

(CHEMBL4582879)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@H](NC(=O)[C@H](CCCCN)NC(C)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1 |r| Show InChI InChI=1S/C47H73N13O6.5C2HF3O2/c1-33(53-46(66)39(54-34(2)61)15-7-9-21-48)43(63)56-38(16-11-22-52-47(49)50)45(65)51-23-27-58-30-28-57(29-31-58)24-10-8-12-35-19-25-59(26-20-35)32-42(62)60-40-17-5-3-13-36(40)44(64)55-37-14-4-6-18-41(37)60;5*3-2(4,5)1(6)7/h3-6,13-14,17-18,33,35,38-39H,7-12,15-16,19-32,48H2,1-2H3,(H,51,65)(H,53,66)(H,54,61)(H,55,64)(H,56,63)(H4,49,50,52);5*(H,6,7)/t33-,38-,39-;;;;;/m0...../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Competitive displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells using 2 nM [3H]-NMS after 3 hrs by microbeta2 scinti... |
J Med Chem 62: 5358-5369 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01967
BindingDB Entry DOI: 10.7270/Q25H7KQF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50525122

(CHEMBL4589047)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCCN1CCN(CCCCC2CCN(CC(=O)N3c4ccccc4NC(=O)c4ccccc34)CC2)CC1 |r| Show InChI InChI=1S/C55H68N10O7.3C2HF3O2/c1-37(58-55(72)48(59-38(2)66)34-41-35-57-45-14-5-3-12-43(41)45)52(69)61-47(33-40-18-20-42(67)21-19-40)54(71)56-24-28-63-31-29-62(30-32-63)25-10-9-11-39-22-26-64(27-23-39)36-51(68)65-49-16-7-4-13-44(49)53(70)60-46-15-6-8-17-50(46)65;3*3-2(4,5)1(6)7/h3-8,12-21,35,37,39,47-48,57,67H,9-11,22-34,36H2,1-2H3,(H,56,71)(H,58,72)(H,59,66)(H,60,70)(H,61,69);3*(H,6,7)/t37-,47-,48-;;;/m0.../s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg
Curated by ChEMBL
| Assay Description
Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method |
J Med Chem 62: 5358-5369 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01967
BindingDB Entry DOI: 10.7270/Q25H7KQF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296329
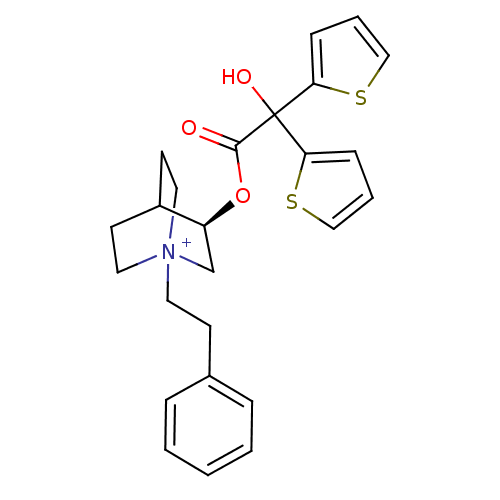
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(12.26,-17.08,;11.17,-18.17,;12.51,-18.94,;12.51,-20.48,;13.84,-18.17,;15.17,-18.93,;15.17,-20.47,;16.5,-21.23,;16.49,-22.77,;17.82,-23.56,;19.16,-22.8,;20.48,-23.59,;21.82,-22.83,;21.84,-21.29,;20.5,-20.51,;19.17,-21.27,;17.83,-20.47,;17.83,-18.93,;16.5,-18.15,;16.92,-19.4,;15.87,-19.75,;11.17,-16.63,;9.92,-15.73,;10.39,-14.26,;11.93,-14.26,;12.41,-15.72,;9.84,-18.95,;8.42,-18.32,;7.4,-19.47,;8.17,-20.8,;9.68,-20.48,)| Show InChI InChI=1S/C25H28NO3S2/c27-24(25(28,22-8-4-16-30-22)23-9-5-17-31-23)29-21-18-26(14-11-20(21)12-15-26)13-10-19-6-2-1-3-7-19/h1-9,16-17,20-21,28H,10-15,18H2/q+1/t20?,21-,26?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296331
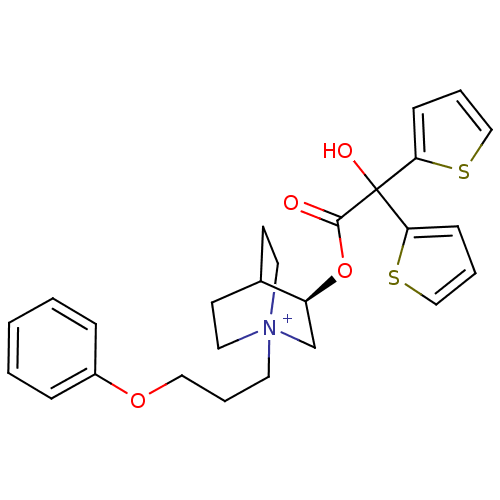
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-4.87,-33.87,;-5.96,-34.96,;-4.62,-35.72,;-4.61,-37.26,;-3.29,-34.95,;-1.95,-35.72,;-1.95,-37.26,;-.62,-38.02,;-.64,-39.56,;.69,-40.34,;2.03,-39.58,;3.36,-40.37,;4.7,-39.61,;6.02,-40.39,;7.36,-39.64,;7.37,-38.1,;6.04,-37.31,;4.7,-38.07,;.71,-37.26,;.71,-35.72,;-.62,-34.94,;-.2,-36.18,;-1.25,-36.53,;-5.96,-33.42,;-7.21,-32.51,;-6.74,-31.04,;-5.2,-31.04,;-4.72,-32.5,;-7.29,-35.73,;-8.7,-35.11,;-9.73,-36.25,;-8.96,-37.59,;-7.45,-37.26,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2/q+1/t20?,22-,27?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50451113

(CHEMBL2114068)Show SMILES COc1ccc(cc1)[S@+]([O-])c1ccc(cc1)C(=C)C1CCN(CC1)C1CCCCC1 |r| Show InChI InChI=1S/C26H33NO2S/c1-20(22-16-18-27(19-17-22)23-6-4-3-5-7-23)21-8-12-25(13-9-21)30(28)26-14-10-24(29-2)11-15-26/h8-15,22-23H,1,3-7,16-19H2,2H3/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor M4 |
Bioorg Med Chem Lett 10: 2209-12 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HMP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50110555
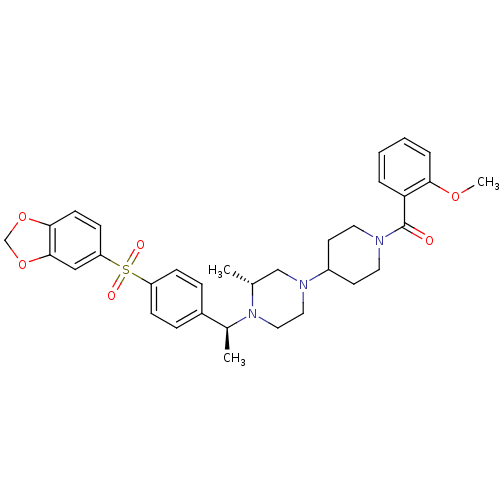
(CHEMBL164935 | [4-((R)-4-{(S)-1-[4-(Benzo[1,3]diox...)Show SMILES COc1ccccc1C(=O)N1CCC(CC1)N1CCN([C@@H](C)c2ccc(cc2)S(=O)(=O)c2ccc3OCOc3c2)[C@H](C)C1 Show InChI InChI=1S/C33H39N3O6S/c1-23-21-35(26-14-16-34(17-15-26)33(37)29-6-4-5-7-30(29)40-3)18-19-36(23)24(2)25-8-10-27(11-9-25)43(38,39)28-12-13-31-32(20-28)42-22-41-31/h4-13,20,23-24,26H,14-19,21-22H2,1-3H3/t23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against human muscarinic acetylcholine receptor M2 using [3H]-N-methylscopolamine as radioligand |
Bioorg Med Chem Lett 12: 795-8 (2002)
BindingDB Entry DOI: 10.7270/Q2QR4WG8 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50381654
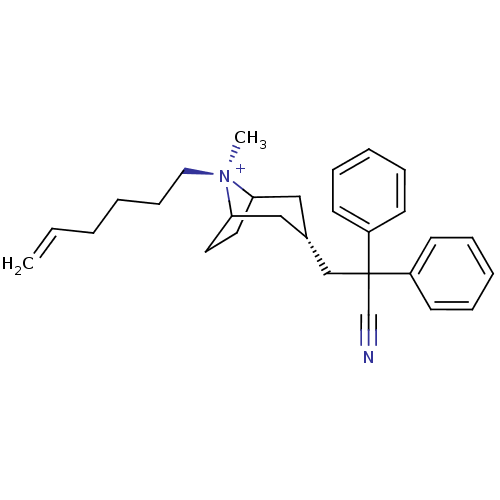
(CHEMBL2023764)Show SMILES C[N@+]1(CCCCC=C)C2CCC1C[C@@H](CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,wU:1.1,wD:13.14,1.0,TLB:0:1:13.12.30:10.9,THB:2:1:13.12.30:10.9,14:13:1:10.9,(20.22,-1.4,;21.72,-1.81,;22.48,-.47,;21.7,.86,;22.46,2.2,;21.68,3.53,;22.44,4.87,;21.66,6.2,;22.77,-2.64,;22.17,-3.98,;20.99,-4.69,;21.98,-3.34,;23.82,-3.34,;24.8,-4.15,;25.57,-5.48,;27.11,-5.48,;27.5,-3.98,;27.89,-2.48,;27.88,-6.81,;27.11,-8.14,;27.89,-9.47,;29.43,-9.47,;30.19,-8.12,;29.42,-6.8,;28.59,-5.07,;29.68,-6.15,;31.16,-5.75,;31.55,-4.26,;30.45,-3.18,;28.97,-3.59,;24.53,-2.61,)| Show InChI InChI=1S/C29H37N2/c1-3-4-5-12-19-31(2)27-17-18-28(31)21-24(20-27)22-29(23-30,25-13-8-6-9-14-25)26-15-10-7-11-16-26/h3,6-11,13-16,24,27-28H,1,4-5,12,17-22H2,2H3/q+1/t24-,27?,28?,31- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M2 receptor expressed in CHO cell membrane |
Bioorg Med Chem Lett 22: 3366-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.015
BindingDB Entry DOI: 10.7270/Q2C82B9Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data