 Found 107 hits with Last Name = 'ondeyka' and Initial = 'jg'
Found 107 hits with Last Name = 'ondeyka' and Initial = 'jg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167697

((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H43NO4/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(36)19-25(21)31)29(38)35-30(39)34(4)18-6-16-32(2)26-20-24(37)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,36-37H,5-6,9-10,13-18H2,1-4H3,(H,35,38,39)/t27-,28-,31-,32-,33+,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167698
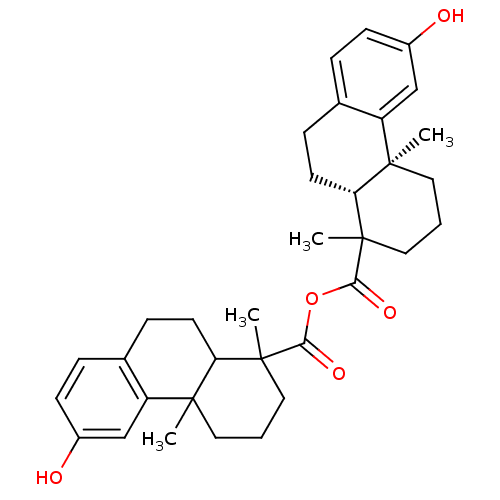
(13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...)Show SMILES CC1(CCCC2(C)C1CCc1ccc(O)cc21)C(=O)OC(=O)C1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H42O5/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(35)19-25(21)31)29(37)39-30(38)34(4)18-6-16-32(2)26-20-24(36)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,35-36H,5-6,9-10,13-18H2,1-4H3/t27-,28?,31-,32?,33?,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167694

(2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....)Show SMILES CC(=O)Oc1ccc2CC[C@H]3C(C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167698

(13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...)Show SMILES CC1(CCCC2(C)C1CCc1ccc(O)cc21)C(=O)OC(=O)C1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H42O5/c1-31-15-5-17-33(3,27(31)13-9-21-7-11-23(35)19-25(21)31)29(37)39-30(38)34(4)18-6-16-32(2)26-20-24(36)12-8-22(26)10-14-28(32)34/h7-8,11-12,19-20,27-28,35-36H,5-6,9-10,13-18H2,1-4H3/t27-,28?,31-,32?,33?,34?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167700
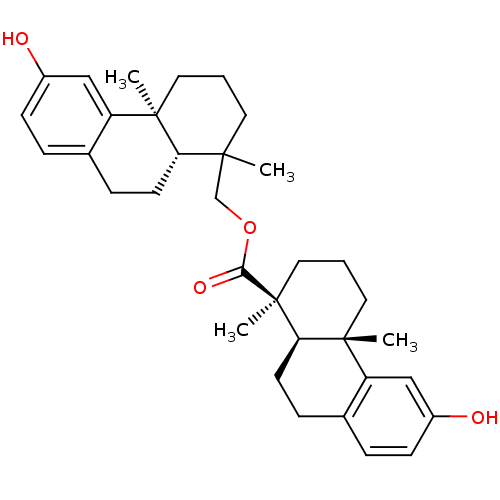
((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...)Show SMILES CC1(COC(=O)[C@@]2(C)CCC[C@@]3(C)[C@H]2CCc2ccc(O)cc32)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H44O4/c1-31(15-5-16-32(2)26-19-24(35)11-7-22(26)9-13-28(31)32)21-38-30(37)34(4)18-6-17-33(3)27-20-25(36)12-8-23(27)10-14-29(33)34/h7-8,11-12,19-20,28-29,35-36H,5-6,9-10,13-18,21H2,1-4H3/t28-,29+,31?,32+,33+,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167700

((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...)Show SMILES CC1(COC(=O)[C@@]2(C)CCC[C@@]3(C)[C@H]2CCc2ccc(O)cc32)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C34H44O4/c1-31(15-5-16-32(2)26-19-24(35)11-7-22(26)9-13-28(31)32)21-38-30(37)34(4)18-6-17-33(3)27-20-25(36)12-8-23(27)10-14-29(33)34/h7-8,11-12,19-20,28-29,35-36H,5-6,9-10,13-18,21H2,1-4H3/t28-,29+,31?,32+,33+,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50126018
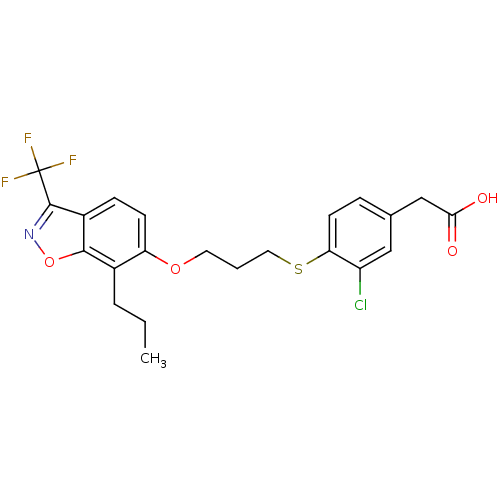
(2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...)Show SMILES CCCc1c(OCCCSc2ccc(CC(O)=O)cc2Cl)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C22H21ClF3NO4S/c1-2-4-14-17(7-6-15-20(14)31-27-21(15)22(24,25)26)30-9-3-10-32-18-8-5-13(11-16(18)23)12-19(28)29/h5-8,11H,2-4,9-10,12H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167695

(13-[13-hydroxy-2,6-dimethyl-(2S,6S,7R)-tricyclo[8....)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(OC(=O)[C@@]3(C)CCC[C@@]4(C)[C@H]3CCc3ccc(O)cc43)cc21)C(O)=O Show InChI InChI=1S/C34H42O5/c1-31-16-6-18-34(4,28(31)14-10-21-7-11-23(35)19-25(21)31)30(38)39-24-12-8-22-9-13-27-32(2,26(22)20-24)15-5-17-33(27,3)29(36)37/h7-8,11-12,19-20,27-28,35H,5-6,9-10,13-18H2,1-4H3,(H,36,37)/t27-,28-,31-,32-,33?,34+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50126018

(2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...)Show SMILES CCCc1c(OCCCSc2ccc(CC(O)=O)cc2Cl)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C22H21ClF3NO4S/c1-2-4-14-17(7-6-15-20(14)31-27-21(15)22(24,25)26)30-9-3-10-32-18-8-5-13(11-16(18)23)12-19(28)29/h5-8,11H,2-4,9-10,12H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-alpha in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167699
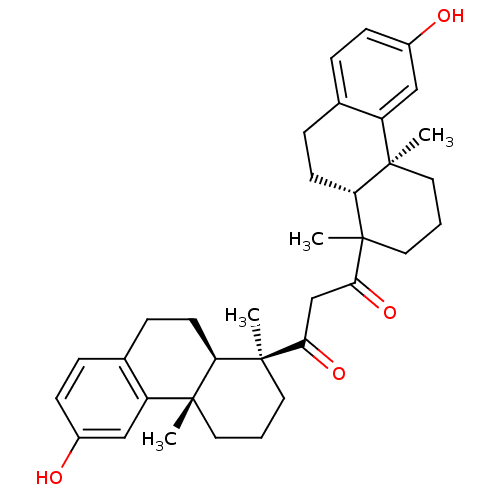
(1-((9R,10S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)CC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C35H44O4/c1-32-15-5-17-34(3,28(32)13-9-22-7-11-24(36)19-26(22)32)30(38)21-31(39)35(4)18-6-16-33(2)27-20-25(37)12-8-23(27)10-14-29(33)35/h7-8,11-12,19-20,28-29,36-37H,5-6,9-10,13-18,21H2,1-4H3/t28-,29-,32-,33-,34+,35?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167695

(13-[13-hydroxy-2,6-dimethyl-(2S,6S,7R)-tricyclo[8....)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(OC(=O)[C@@]3(C)CCC[C@@]4(C)[C@H]3CCc3ccc(O)cc43)cc21)C(O)=O Show InChI InChI=1S/C34H42O5/c1-31-16-6-18-34(4,28(31)14-10-21-7-11-23(35)19-25(21)31)30(38)39-24-12-8-22-9-13-27-32(2,26(22)20-24)15-5-17-33(27,3)29(36)37/h7-8,11-12,19-20,27-28,35H,5-6,9-10,13-18H2,1-4H3,(H,36,37)/t27-,28-,31-,32-,33?,34+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Effective concentration against liver X receptor-alpha in HEK293 cell transactivation assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167699

(1-((9R,10S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,1...)Show SMILES CC1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)CC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21 Show InChI InChI=1S/C35H44O4/c1-32-15-5-17-34(3,28(32)13-9-22-7-11-24(36)19-26(22)32)30(38)21-31(39)35(4)18-6-16-33(2)27-20-25(37)12-8-23(27)10-14-29(33)35/h7-8,11-12,19-20,28-29,36-37H,5-6,9-10,13-18,21H2,1-4H3/t28-,29-,32-,33-,34+,35?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50242053

(6,8-didec-(1Z)-enyl-5,7-dimethyl-2,3-dihydro-1H-in...)Show SMILES CCCCCCCC\C=C/c1c2CCC[n+]2c(C)c(\C=C/CCCCCCCC)c1C Show InChI InChI=1S/C30H50N/c1-5-7-9-11-13-15-17-19-22-28-26(3)29(30-24-21-25-31(30)27(28)4)23-20-18-16-14-12-10-8-6-2/h19-20,22-23H,5-18,21,24-25H2,1-4H3/q+1/b22-19-,23-20- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]gp-120 from human CCR5 receptor expressed in CHO cells |
J Nat Prod 67: 1036-8 (2004)
Article DOI: 10.1021/np049974l
BindingDB Entry DOI: 10.7270/Q29Z94P3 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50269497

(CHEMBL445689 | Guttiferone I)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6@H]1-[#6][C@@]2([#6]\[#6]=[#6](\[#6])-[#6])[#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6](=O)[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]2=O)[C@]1([#6])[#6]-[#6]\[#6]=[#6](\[#6])-[#6] |r,THB:40:39:31.20.18:10.41.11| Show InChI InChI=1S/C43H58O6/c1-27(2)13-11-15-31(9)16-18-33-26-42(23-20-29(5)6)38(47)36(37(46)32-17-19-34(44)35(45)25-32)39(48)43(40(42)49,24-21-30(7)8)41(33,10)22-12-14-28(3)4/h13-14,16-17,19-21,25,33,36,44-45H,11-12,15,18,22-24,26H2,1-10H3/b31-16+/t33-,36?,41+,42-,43+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]F3-methylAA from human LXRalpha expressed in Escherichia coli BL21 cells by scintillation proximity assay |
J Nat Prod 68: 617-9 (2005)
Article DOI: 10.1021/np050045j
BindingDB Entry DOI: 10.7270/Q24749MK |
More data for this
Ligand-Target Pair | |
cGMP-dependent protein kinase
(Eimeria tenella) | BDBM50269479
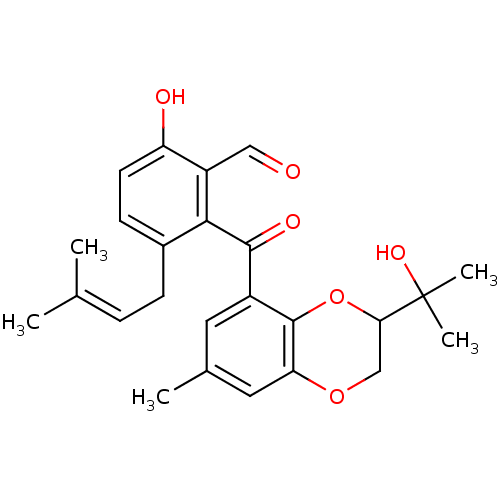
(CHEMBL515063 | Tenellone B)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1ccc(-[#8])c(-[#6]=O)c1-[#6](=O)-c1cc(-[#6])cc2-[#8]-[#6]-[#6](-[#8]-c12)C([#6])([#6])[#8] Show InChI InChI=1S/C25H28O6/c1-14(2)6-7-16-8-9-19(27)18(12-26)22(16)23(28)17-10-15(3)11-20-24(17)31-21(13-30-20)25(4,5)29/h6,8-12,21,27,29H,7,13H2,1-5H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Eimera tenella CGMP-dependent protein kinase by radiometric assay |
J Nat Prod 68: 611-3 (2005)
Article DOI: 10.1021/np049591n
BindingDB Entry DOI: 10.7270/Q28052CR |
More data for this
Ligand-Target Pair | |
cGMP-dependent protein kinase
(Eimeria tenella) | BDBM50269478
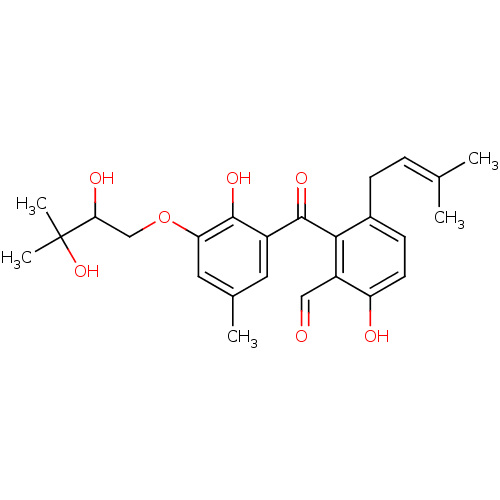
(CHEMBL457810 | Tenellone A)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1ccc(-[#8])c(-[#6]=O)c1-[#6](=O)-c1cc(-[#6])cc(-[#8]-[#6]-[#6](-[#8])C([#6])([#6])[#8])c1-[#8] Show InChI InChI=1S/C25H30O7/c1-14(2)6-7-16-8-9-19(27)18(12-26)22(16)24(30)17-10-15(3)11-20(23(17)29)32-13-21(28)25(4,5)31/h6,8-12,21,27-29,31H,7,13H2,1-5H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Eimera tenella CGMP-dependent protein kinase by radiometric assay |
J Nat Prod 68: 611-3 (2005)
Article DOI: 10.1021/np049591n
BindingDB Entry DOI: 10.7270/Q28052CR |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50269497

(CHEMBL445689 | Guttiferone I)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6@H]1-[#6][C@@]2([#6]\[#6]=[#6](\[#6])-[#6])[#6](=O)-[#6](-[#6](=O)-c3ccc(-[#8])c(-[#8])c3)-[#6](=O)[C@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]2=O)[C@]1([#6])[#6]-[#6]\[#6]=[#6](\[#6])-[#6] |r,THB:40:39:31.20.18:10.41.11| Show InChI InChI=1S/C43H58O6/c1-27(2)13-11-15-31(9)16-18-33-26-42(23-20-29(5)6)38(47)36(37(46)32-17-19-34(44)35(45)25-32)39(48)43(40(42)49,24-21-30(7)8)41(33,10)22-12-14-28(3)4/h13-14,16-17,19-21,25,33,36,44-45H,11-12,15,18,22-24,26H2,1-10H3/b31-16+/t33-,36?,41+,42-,43+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]F3-methylAA from human LXRbeta expressed in Escherichia coli BL21 cells by scintillation proximity assay |
J Nat Prod 68: 617-9 (2005)
Article DOI: 10.1021/np050045j
BindingDB Entry DOI: 10.7270/Q24749MK |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50242056
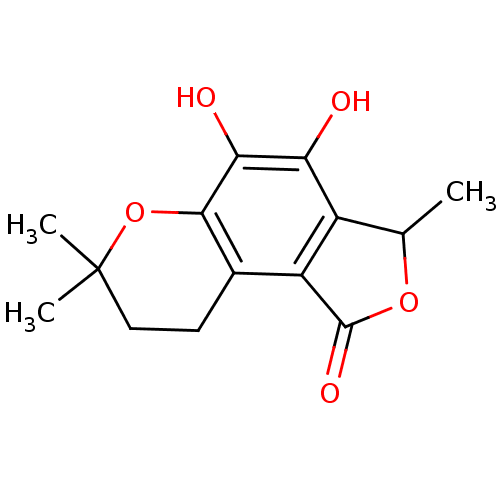
(CHEMBL469856 | fuscinarin)Show InChI InChI=1S/C14H16O5/c1-6-8-9(13(17)18-6)7-4-5-14(2,3)19-12(7)11(16)10(8)15/h6,15-16H,4-5H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MIP1alpha binding to human CCR5 receptor |
J Nat Prod 67: 1036-8 (2004)
Article DOI: 10.1021/np049974l
BindingDB Entry DOI: 10.7270/Q29Z94P3 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50242054

(CHEMBL469855 | ophiobolin C)Show SMILES [#6]-[#6@@H](-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6@H]-1-[#6]-[#6][C@]2([#6])[#6]-[#6@H]3-[#6@H](-[#6](=O)-[#6][C@@]3([#6])[#8])\[#6](-[#6]=O)=[#6]/[#6]-[#6@@H]-12 |r,c:25| Show InChI InChI=1S/C25H38O3/c1-16(2)7-6-8-17(3)19-11-12-24(4)13-21-23(22(27)14-25(21,5)28)18(15-26)9-10-20(19)24/h7,9,15,17,19-21,23,28H,6,8,10-14H2,1-5H3/b18-9-/t17-,19+,20-,21-,23+,24+,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]gp-120 from human CCR5 receptor expressed in CHO cells |
J Nat Prod 67: 1036-8 (2004)
Article DOI: 10.1021/np049974l
BindingDB Entry DOI: 10.7270/Q29Z94P3 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50167696
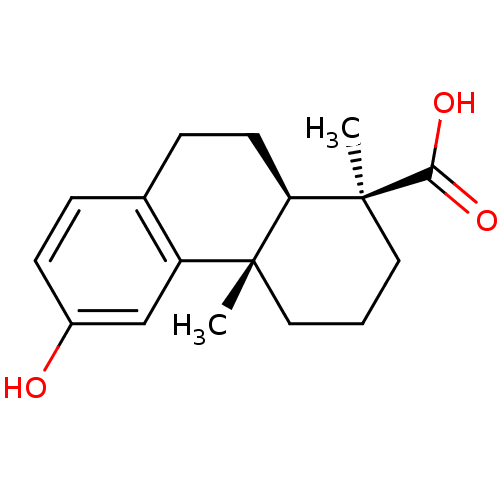
((1S,4aS,10aR)-1,2,3,4,4a,9,10,10a-octahydro-6-hydr...)Show SMILES C[C@@]1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(O)=O |r| Show InChI InChI=1S/C17H22O3/c1-16-8-3-9-17(2,15(19)20)14(16)7-5-11-4-6-12(18)10-13(11)16/h4,6,10,14,18H,3,5,7-9H2,1-2H3,(H,19,20)/t14-,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]-F3-methyl AA (1) from liver X receptor-beta in SPA assay |
Bioorg Med Chem Lett 15: 2824-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.100
BindingDB Entry DOI: 10.7270/Q29G5NM2 |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250712
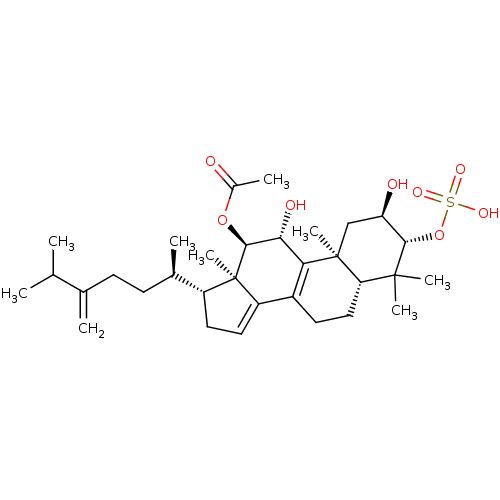
(CHEMBL446297 | Integracide A)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](OS(O)(=O)=O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C32H50O8S/c1-17(2)18(3)10-11-19(4)22-13-14-23-21-12-15-25-30(6,7)28(40-41(36,37)38)24(34)16-31(25,8)26(21)27(35)29(32(22,23)9)39-20(5)33/h14,17,19,22,24-25,27-29,34-35H,3,10-13,15-16H2,1-2,4-9H3,(H,36,37,38)/t19-,22-,24-,25+,27-,28+,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250713
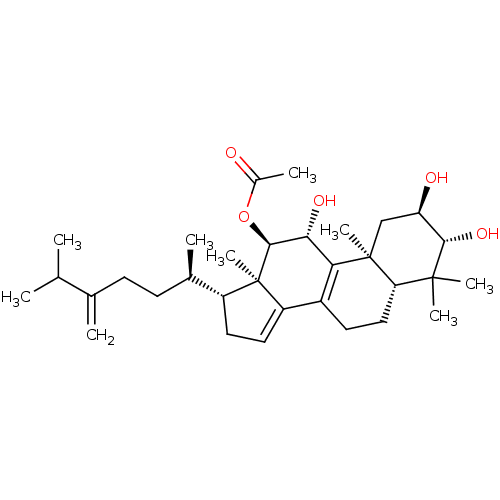
(CHEMBL464128 | Integracide B)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C32H50O5/c1-17(2)18(3)10-11-19(4)22-13-14-23-21-12-15-25-30(6,7)28(36)24(34)16-31(25,8)26(21)27(35)29(32(22,23)9)37-20(5)33/h14,17,19,22,24-25,27-29,34-36H,3,10-13,15-16H2,1-2,4-9H3/t19-,22-,24-,25+,27-,28+,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250714

((2R,3R,5R,10S,11R,12R,13R,14S,17R)-2,11-dihydroxy-...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC[C@H]2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](OS(O)(=O)=O)C(C)(C)[C@@H]1CC3 |r,c:13| Show InChI InChI=1S/C32H52O8S/c1-17(2)18(3)10-11-19(4)22-13-14-23-21-12-15-25-30(6,7)28(40-41(36,37)38)24(34)16-31(25,8)26(21)27(35)29(32(22,23)9)39-20(5)33/h17,19,22-25,27-29,34-35H,3,10-16H2,1-2,4-9H3,(H,36,37,38)/t19-,22-,23+,24-,25+,27-,28+,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250715
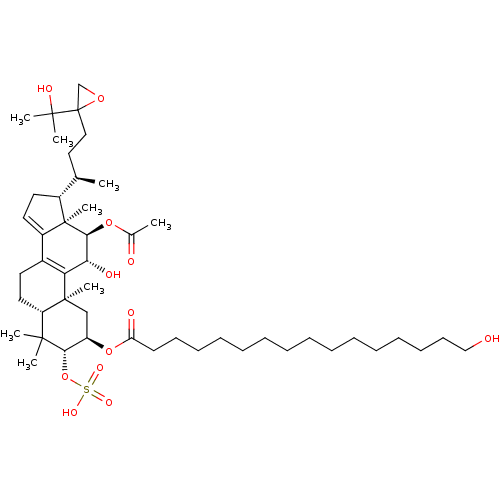
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-11-hydro...)Show SMILES C[C@H](CCC1(CO1)C(C)(C)O)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CCCCCCCCCCCCCCCO)[C@H](OS(O)(=O)=O)C(C)(C)[C@@H]1CC3 |r,c:16,t:14| Show InChI InChI=1S/C48H80O12S/c1-32(27-28-48(31-57-48)45(5,6)53)35-24-25-36-34-23-26-38-44(3,4)42(60-61(54,55)56)37(30-46(38,7)40(34)41(52)43(47(35,36)8)58-33(2)50)59-39(51)22-20-18-16-14-12-10-9-11-13-15-17-19-21-29-49/h25,32,35,37-38,41-43,49,52-53H,9-24,26-31H2,1-8H3,(H,54,55,56)/t32-,35-,37-,38+,41-,42+,43+,46+,47-,48?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250716

((2R,3R,5R,10S,11R,12R,13R,14S,17R)-12-acetoxy-11-h...)Show SMILES C[C@H](CCC1(CO1)C(C)(C)O)[C@H]1CC[C@H]2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CCCCCCCCCCCCCCCO)[C@H](OS(O)(=O)=O)C(C)(C)[C@@H]1CC3 |r,c:16| Show InChI InChI=1S/C48H82O12S/c1-32(27-28-48(31-57-48)45(5,6)53)35-24-25-36-34-23-26-38-44(3,4)42(60-61(54,55)56)37(30-46(38,7)40(34)41(52)43(47(35,36)8)58-33(2)50)59-39(51)22-20-18-16-14-12-10-9-11-13-15-17-19-21-29-49/h32,35-38,41-43,49,52-53H,9-31H2,1-8H3,(H,54,55,56)/t32-,35-,36+,37-,38+,41-,42+,43+,46+,47-,48?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250717
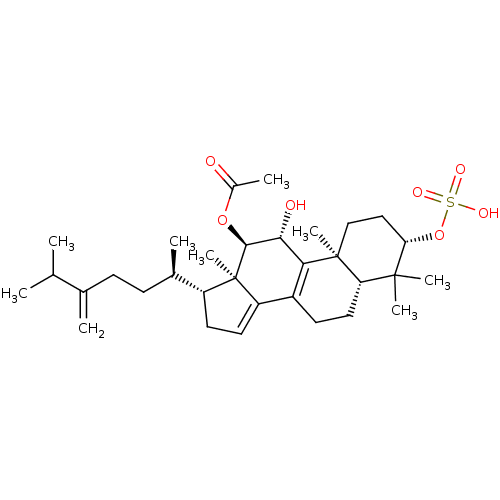
((3S,5R,10S,11R,12R,13R,17R)-11-hydroxy-4,4,10,13-t...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)CC[C@H](OS(O)(=O)=O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C32H50O7S/c1-18(2)19(3)10-11-20(4)23-13-14-24-22-12-15-25-30(6,7)26(39-40(35,36)37)16-17-31(25,8)27(22)28(34)29(32(23,24)9)38-21(5)33/h14,18,20,23,25-26,28-29,34H,3,10-13,15-17H2,1-2,4-9H3,(H,35,36,37)/t20-,23-,25+,26+,28-,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250718

(2-alpha-(3'-hydroxy-3'-methylglutaroyl)-24,25-dihy...)Show SMILES C[C@H](CCC(O)C(C)(C)O)[C@H]1CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CC(C)(O)CC(O)=O)C(=O)C(C)(C)[C@@H]1CC3 |r,c:15| Show InChI InChI=1S/C36H58O8/c1-21(10-13-27(37)32(4,5)42)22-14-16-36(9)24-11-12-26-31(2,3)30(41)25(44-29(40)20-33(6,43)19-28(38)39)18-34(26,7)23(24)15-17-35(22,36)8/h21-22,25-27,37,42-43H,10-20H2,1-9H3,(H,38,39)/t21-,22-,25-,26+,27?,33?,34-,35-,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250719
((3R)-1-((2R,5R,10S,13R,14R,17R)-17-((2R)-5,6-dihyd...)Show SMILES COC(=O)CC(C)(O)CC(=O)O[C@@H]1C[C@@]2(C)[C@@H](CCC3=C2CC[C@]2(C)[C@H](CC[C@@]32C)[C@H](C)CCC(O)C(C)(C)O)C(C)(C)C1=O |r,c:19| Show InChI InChI=1S/C37H60O8/c1-22(11-14-28(38)33(4,5)42)23-15-17-37(9)25-12-13-27-32(2,3)31(41)26(19-35(27,7)24(25)16-18-36(23,37)8)45-30(40)21-34(6,43)20-29(39)44-10/h22-23,26-28,38,42-43H,11-21H2,1-10H3/t22-,23-,26-,27+,28?,34?,35-,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250727
((2R,5R,10S,13R,14R,17R)-17-((2R)-5,6-dihydroxy-6-m...)Show SMILES C[C@H](CCC(O)C(C)(C)O)[C@H]1CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]1(C)C[C@@H](O)C(=O)C(C)(C)[C@@H]1CC3 |r,c:15| Show InChI InChI=1S/C30H50O4/c1-18(9-12-24(32)27(4,5)34)19-13-15-30(8)21-10-11-23-26(2,3)25(33)22(31)17-28(23,6)20(21)14-16-29(19,30)7/h18-19,22-24,31-32,34H,9-17H2,1-8H3/t18-,19-,22-,23+,24?,28-,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250728
((2R,3R,5R,10S,15S,17R)-15-methoxy-4,4,10,12-tetram...)Show SMILES CO[C@H]1C[C@H]([C@H](C)CCC(=C)C(C)C)c2c1c1CC[C@H]3C(C)(C)[C@@H](O)[C@H](O)C[C@]3(C)c1cc2C |r| Show InChI InChI=1S/C31H48O3/c1-17(2)18(3)10-11-19(4)22-15-25(34-9)28-21-12-13-26-30(6,7)29(33)24(32)16-31(26,8)23(21)14-20(5)27(22)28/h14,17,19,22,24-26,29,32-33H,3,10-13,15-16H2,1-2,4-9H3/t19-,22-,24-,25+,26+,29+,31-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250729
((2R,3R,5R,10S,15R,17R)-15-methoxy-4,4,10,12-tetram...)Show SMILES CO[C@@H]1C[C@H]([C@H](C)CCC(=C)C(C)C)c2c1c1CC[C@H]3C(C)(C)[C@@H](O)[C@H](O)C[C@]3(C)c1cc2C |r| Show InChI InChI=1S/C31H48O3/c1-17(2)18(3)10-11-19(4)22-15-25(34-9)28-21-12-13-26-30(6,7)29(33)24(32)16-31(26,8)23(21)14-20(5)27(22)28/h14,17,19,22,24-26,29,32-33H,3,10-13,15-16H2,1-2,4-9H3/t19-,22-,24-,25-,26+,29+,31-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250730
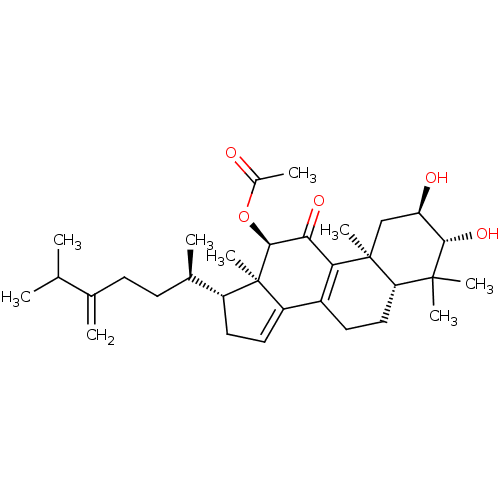
((2R,3R,5R,10S,12R,13R,17R)-2,3-dihydroxy-4,4,10,13...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C(C(=O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C32H48O5/c1-17(2)18(3)10-11-19(4)22-13-14-23-21-12-15-25-30(6,7)28(36)24(34)16-31(25,8)26(21)27(35)29(32(22,23)9)37-20(5)33/h14,17,19,22,24-25,28-29,34,36H,3,10-13,15-16H2,1-2,4-9H3/t19-,22-,24-,25+,28+,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250735
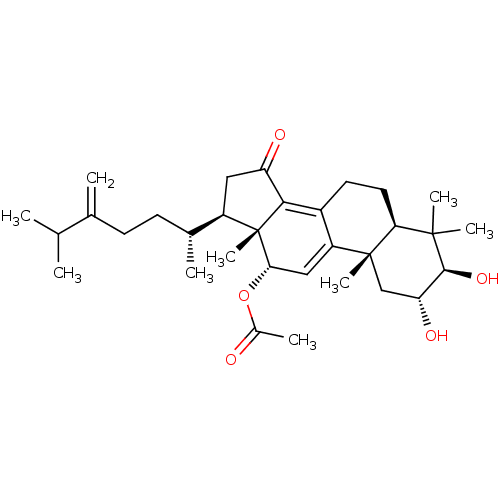
((2R,3R,5R,10S,12S,13R,17R)-2,3-dihydroxy-4,4,10,13...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC(=O)C2=C3CC[C@H]4C(C)(C)[C@@H](O)[C@H](O)C[C@]4(C)C3=C[C@H](OC(C)=O)[C@]12C |r,c:13,30| Show InChI InChI=1S/C32H48O5/c1-17(2)18(3)10-11-19(4)22-14-24(34)28-21-12-13-26-30(6,7)29(36)25(35)16-31(26,8)23(21)15-27(32(22,28)9)37-20(5)33/h15,17,19,22,25-27,29,35-36H,3,10-14,16H2,1-2,4-9H3/t19-,22-,25-,26+,27+,29+,31-,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250736
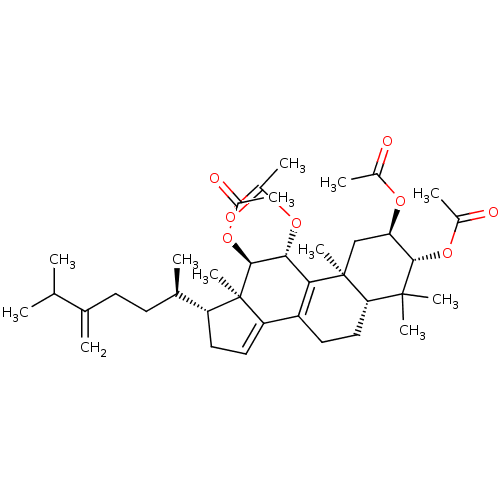
((2R,3R,5R,10S,11R,12R,13R,17R)-4,4,10,13-tetrameth...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](OC(C)=O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(C)=O)[C@H](OC(C)=O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C38H56O8/c1-20(2)21(3)13-14-22(4)28-16-17-29-27-15-18-31-36(9,10)34(45-25(7)41)30(43-23(5)39)19-37(31,11)32(27)33(44-24(6)40)35(38(28,29)12)46-26(8)42/h17,20,22,28,30-31,33-35H,3,13-16,18-19H2,1-2,4-12H3/t22-,28-,30-,31+,33-,34+,35+,37+,38-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250737
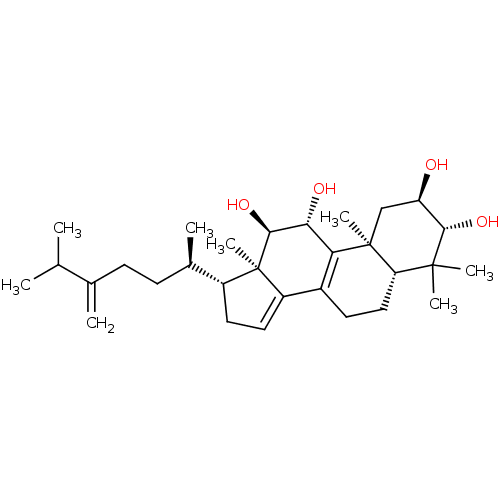
((2R,3R,5R,10S,11R,12R,13R,17R)-4,4,10,13-tetrameth...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C30H48O4/c1-16(2)17(3)9-10-18(4)20-12-13-21-19-11-14-23-28(5,6)26(33)22(31)15-29(23,7)24(19)25(32)27(34)30(20,21)8/h13,16,18,20,22-23,25-27,31-34H,3,9-12,14-15H2,1-2,4-8H3/t18-,20-,22-,23+,25-,26+,27+,29+,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250738

(Acetic acid (1R,5aR,8R,9R,10aS,11R,12R,12aR)-1-((R...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@H]2OC(C)(C)O[C@@H]2C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C35H54O5/c1-19(2)20(3)12-13-21(4)24-15-16-25-23-14-17-27-32(6,7)30-26(39-33(8,9)40-30)18-34(27,10)28(23)29(37)31(35(24,25)11)38-22(5)36/h16,19,21,24,26-27,29-31,37H,3,12-15,17-18H2,1-2,4-11H3/t21-,24-,26-,27+,29-,30+,31+,34+,35-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250739

(Acetic acid (5R,10S,11R,12R,13R,17R)-17-((R)-1,5-d...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H]2O[C@@H]2C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C32H48O4/c1-17(2)18(3)10-11-19(4)22-13-14-23-21-12-15-25-30(6,7)28-24(36-28)16-31(25,8)26(21)27(34)29(32(22,23)9)35-20(5)33/h14,17,19,22,24-25,27-29,34H,3,10-13,15-16H2,1-2,4-9H3/t19-,22-,24+,25+,27-,28+,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250740

((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-11-hydro...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)c2ccccc2)[C@H](OC(=O)c2ccccc2)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C46H58O7/c1-27(2)28(3)20-21-29(4)34-23-24-35-33-22-25-37-44(6,7)40(53-43(50)32-18-14-11-15-19-32)36(52-42(49)31-16-12-10-13-17-31)26-45(37,8)38(33)39(48)41(46(34,35)9)51-30(5)47/h10-19,24,27,29,34,36-37,39-41,48H,3,20-23,25-26H2,1-2,4-9H3/t29-,34-,36-,37+,39-,40+,41+,45+,46-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250741
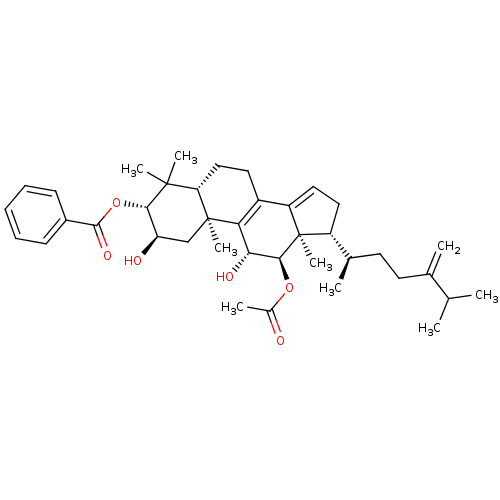
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-2,11-dih...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](OC(=O)c2ccccc2)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C39H54O6/c1-22(2)23(3)15-16-24(4)28-18-19-29-27-17-20-31-37(6,7)34(45-36(43)26-13-11-10-12-14-26)30(41)21-38(31,8)32(27)33(42)35(39(28,29)9)44-25(5)40/h10-14,19,22,24,28,30-31,33-35,41-42H,3,15-18,20-21H2,1-2,4-9H3/t24-,28-,30-,31+,33-,34+,35+,38+,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250742
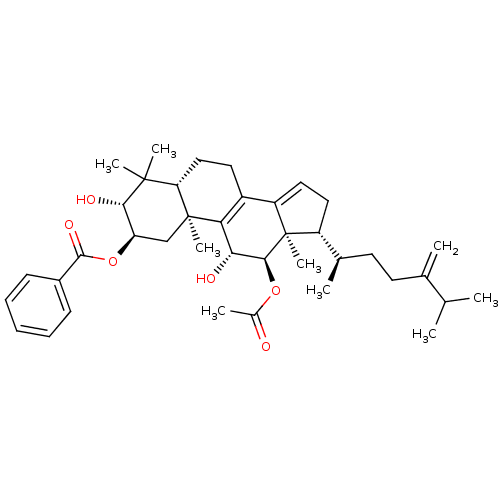
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-dih...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)c2ccccc2)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C39H54O6/c1-22(2)23(3)15-16-24(4)28-18-19-29-27-17-20-31-37(6,7)34(42)30(45-36(43)26-13-11-10-12-14-26)21-38(31,8)32(27)33(41)35(39(28,29)9)44-25(5)40/h10-14,19,22,24,28,30-31,33-35,41-42H,3,15-18,20-21H2,1-2,4-9H3/t24-,28-,30-,31+,33-,34+,35+,38+,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250743

((2R,3R,5R,10S,11R,12R,13R,17R)-11-hydroxy-2,3-bis(...)Show SMILES COCO[C@@H]1C[C@@]2(C)[C@@H](CCC3=C2[C@@H](O)[C@H](OC(C)=O)[C@]2(C)[C@H](CC=C32)[C@H](C)CCC(=C)C(C)C)C(C)(C)[C@H]1OCOC |r,c:11,t:25| Show InChI InChI=1S/C36H58O7/c1-21(2)22(3)12-13-23(4)26-15-16-27-25-14-17-29-34(6,7)32(42-20-40-11)28(41-19-39-10)18-35(29,8)30(25)31(38)33(36(26,27)9)43-24(5)37/h16,21,23,26,28-29,31-33,38H,3,12-15,17-20H2,1-2,4-11H3/t23-,26-,28-,29+,31-,32+,33+,35+,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250744
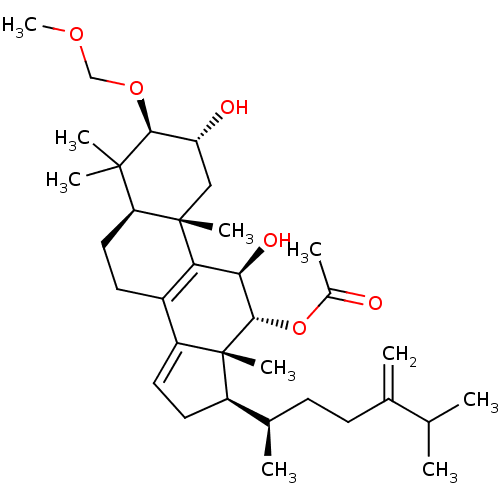
((2R,3R,5R,10S,11R,12R,13R,17R)-2,11-dihydroxy-3-(m...)Show SMILES COCO[C@H]1[C@H](O)C[C@@]2(C)[C@@H](CCC3=C2[C@@H](O)[C@H](OC(C)=O)[C@]2(C)[C@H](CC=C32)[C@H](C)CCC(=C)C(C)C)C1(C)C |r,c:13,t:27| Show InChI InChI=1S/C34H54O6/c1-19(2)20(3)11-12-21(4)24-14-15-25-23-13-16-27-32(6,7)30(39-18-38-10)26(36)17-33(27,8)28(23)29(37)31(34(24,25)9)40-22(5)35/h15,19,21,24,26-27,29-31,36-37H,3,11-14,16-18H2,1-2,4-10H3/t21-,24-,26-,27+,29-,30+,31+,33+,34-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250745
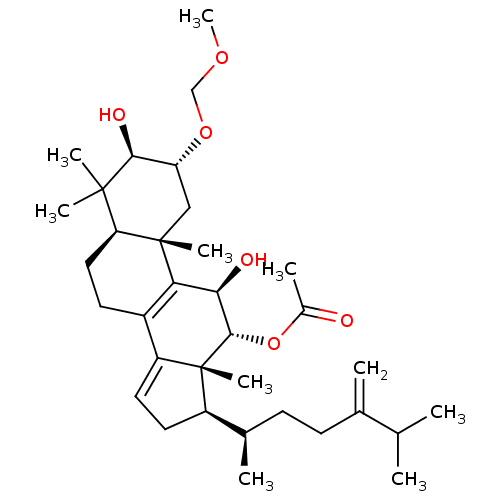
((2R,3R,5R,10S,11R,12R,13R,17R)-3,11-dihydroxy-2-(m...)Show SMILES COCO[C@@H]1C[C@@]2(C)[C@@H](CCC3=C2[C@@H](O)[C@H](OC(C)=O)[C@]2(C)[C@H](CC=C32)[C@H](C)CCC(=C)C(C)C)C(C)(C)[C@H]1O |r,c:11,t:25| Show InChI InChI=1S/C34H54O6/c1-19(2)20(3)11-12-21(4)24-14-15-25-23-13-16-27-32(6,7)30(37)26(39-18-38-10)17-33(27,8)28(23)29(36)31(34(24,25)9)40-22(5)35/h15,19,21,24,26-27,29-31,36-37H,3,11-14,16-18H2,1-2,4-10H3/t21-,24-,26-,27+,29-,30+,31+,33+,34-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250746

(4-((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-2,11-...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](OC(=O)CCC(O)=O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C36H54O8/c1-19(2)20(3)10-11-21(4)24-13-14-25-23-12-15-27-34(6,7)32(44-29(41)17-16-28(39)40)26(38)18-35(27,8)30(23)31(42)33(36(24,25)9)43-22(5)37/h14,19,21,24,26-27,31-33,38,42H,3,10-13,15-18H2,1-2,4-9H3,(H,39,40)/t21-,24-,26-,27+,31-,32+,33+,35+,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250747

(4-((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CCC(O)=O)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C36H54O8/c1-19(2)20(3)10-11-21(4)24-13-14-25-23-12-15-27-34(6,7)32(42)26(44-29(40)17-16-28(38)39)18-35(27,8)30(23)31(41)33(36(24,25)9)43-22(5)37/h14,19,21,24,26-27,31-33,41-42H,3,10-13,15-18H2,1-2,4-9H3,(H,38,39)/t21-,24-,26-,27+,31-,32+,33+,35+,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250748

((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-2,11-dih...)Show SMILES COC(=O)CCC(=O)O[C@H]1[C@H](O)C[C@@]2(C)[C@@H](CCC3=C2[C@@H](O)[C@H](OC(C)=O)[C@]2(C)[C@H](CC=C32)[C@H](C)CCC(=C)C(C)C)C1(C)C |r,c:18,t:32| Show InChI InChI=1S/C37H56O8/c1-20(2)21(3)11-12-22(4)25-14-15-26-24-13-16-28-35(6,7)33(45-30(41)18-17-29(40)43-10)27(39)19-36(28,8)31(24)32(42)34(37(25,26)9)44-23(5)38/h15,20,22,25,27-28,32-34,39,42H,3,11-14,16-19H2,1-2,4-10H3/t22-,25-,27-,28+,32-,33+,34+,36+,37-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250749

((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-dih...)Show SMILES COC(=O)CCC(=O)O[C@@H]1C[C@@]2(C)[C@@H](CCC3=C2[C@@H](O)[C@H](OC(C)=O)[C@]2(C)[C@H](CC=C32)[C@H](C)CCC(=C)C(C)C)C(C)(C)[C@H]1O |r,c:16,t:30| Show InChI InChI=1S/C37H56O8/c1-20(2)21(3)11-12-22(4)25-14-15-26-24-13-16-28-35(6,7)33(42)27(45-30(40)18-17-29(39)43-10)19-36(28,8)31(24)32(41)34(37(25,26)9)44-23(5)38/h15,20,22,25,27-28,32-34,41-42H,3,11-14,16-19H2,1-2,4-10H3/t22-,25-,27-,28+,32-,33+,34+,36+,37-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250750
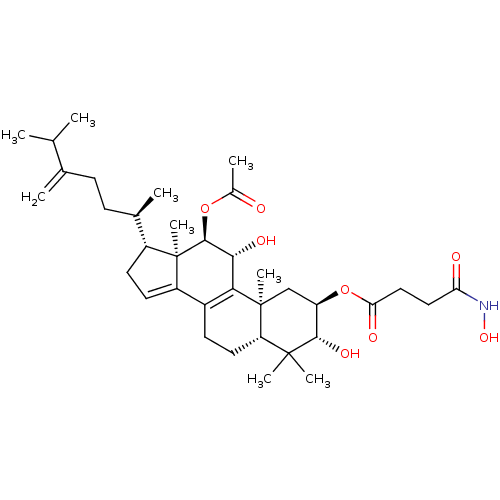
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-dih...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CCC(=O)NO)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C36H55NO8/c1-19(2)20(3)10-11-21(4)24-13-14-25-23-12-15-27-34(6,7)32(42)26(45-29(40)17-16-28(39)37-43)18-35(27,8)30(23)31(41)33(36(24,25)9)44-22(5)38/h14,19,21,24,26-27,31-33,41-43H,3,10-13,15-18H2,1-2,4-9H3,(H,37,39)/t21-,24-,26-,27+,31-,32+,33+,35+,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data