

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135844 ((2S,8S)-2-[({[({[(1S)-1-{[(1S,2R)-2-(benzyloxy)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135845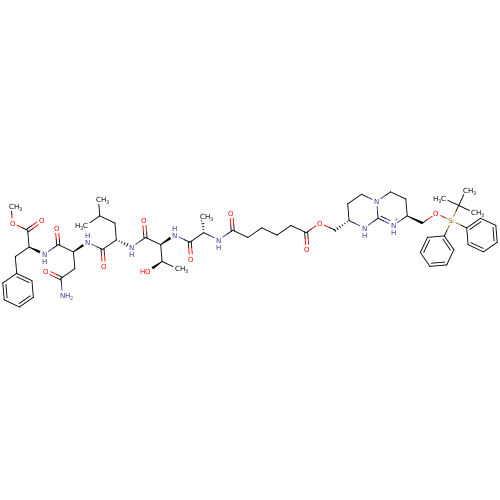 (Bicyclic Guanidinium Subunit | CHEMBL266754) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135842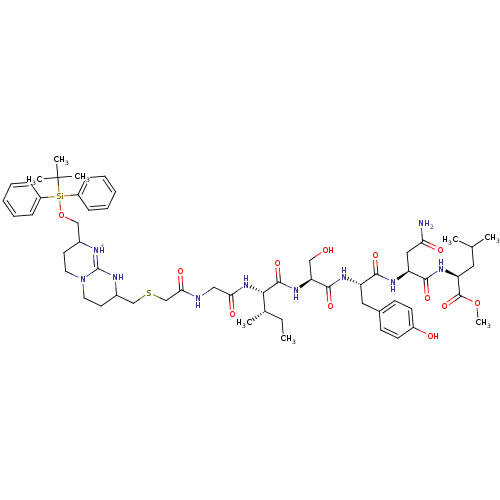 (Bicyclic Guanidinium Subunit | CHEMBL386994) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135847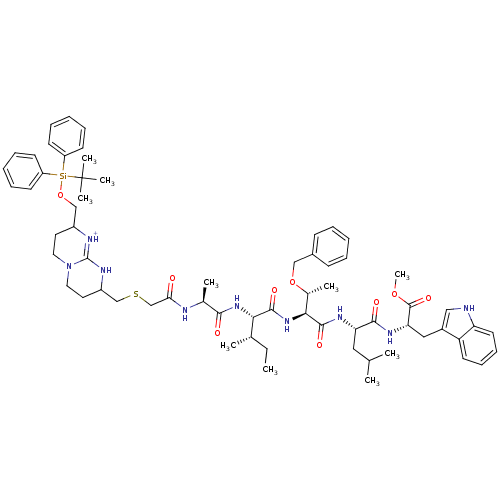 (Bicyclic Guanidinium Subunit | CHEMBL404936) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Binding affinity to VDR assessed as inhibition of fluorescent ligand by fluorescence polarization competition assay | J Med Chem 55: 8642-56 (2012) Article DOI: 10.1021/jm3008272 BindingDB Entry DOI: 10.7270/Q24Q7W49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397221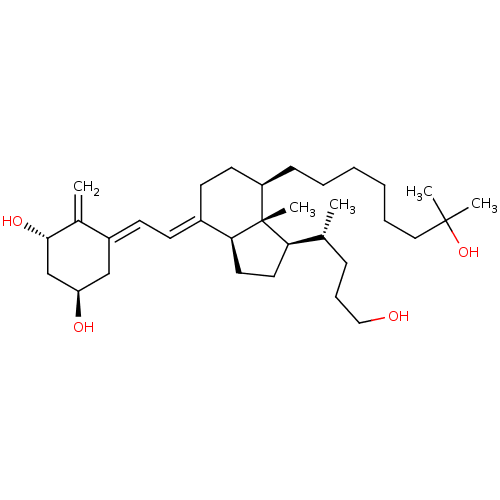 (CHEMBL2172537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Binding affinity to VDR assessed as inhibition of fluorescent ligand by fluorescence polarization competition assay | J Med Chem 55: 8642-56 (2012) Article DOI: 10.1021/jm3008272 BindingDB Entry DOI: 10.7270/Q24Q7W49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397223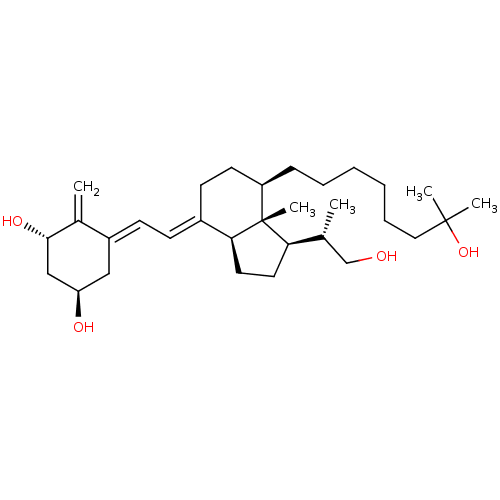 (CHEMBL2172539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Binding affinity to VDR assessed as inhibition of fluorescent ligand by fluorescence polarization competition assay | J Med Chem 55: 8642-56 (2012) Article DOI: 10.1021/jm3008272 BindingDB Entry DOI: 10.7270/Q24Q7W49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397222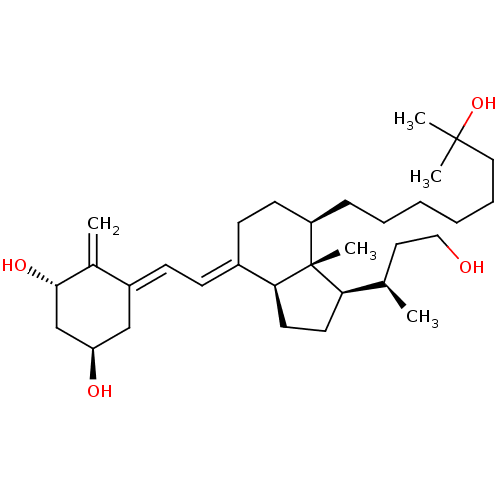 (CHEMBL2172538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Binding affinity to VDR assessed as inhibition of fluorescent ligand by fluorescence polarization competition assay | J Med Chem 55: 8642-56 (2012) Article DOI: 10.1021/jm3008272 BindingDB Entry DOI: 10.7270/Q24Q7W49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135845 (Bicyclic Guanidinium Subunit | CHEMBL266754) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135843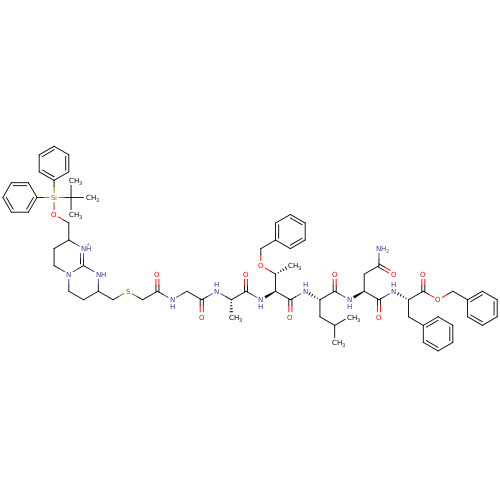 (Bicyclic Guanidinium Subunit | CHEMBL216653) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135843 (Bicyclic Guanidinium Subunit | CHEMBL216653) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135844 ((2S,8S)-2-[({[({[(1S)-1-{[(1S,2R)-2-(benzyloxy)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135846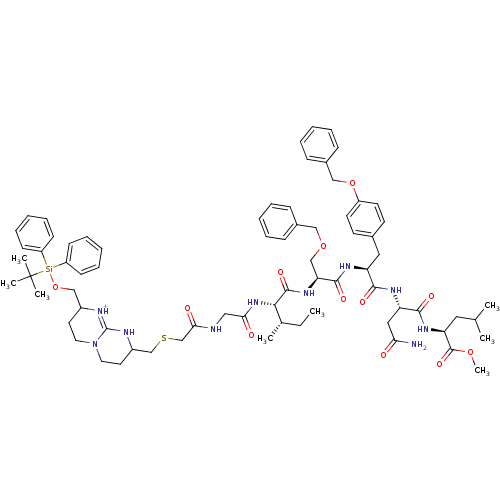 (Bicyclic Guanidinium Subunit | CHEMBL428283) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135847 (Bicyclic Guanidinium Subunit | CHEMBL404936) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135842 (Bicyclic Guanidinium Subunit | CHEMBL386994) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135848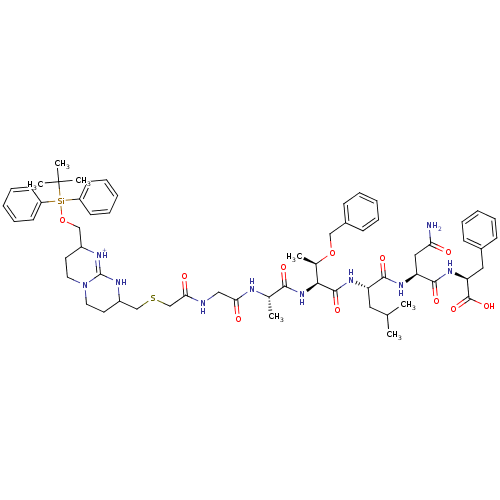 (Bicyclic Guanidinium Subunit | CHEMBL414980) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 3.28 | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Agonist activity at VDR in human MCF7 cells assessed as transcription of CYP24A1 gene after 24 hrs by luciferase reporter gene assay | J Med Chem 55: 8642-56 (2012) Article DOI: 10.1021/jm3008272 BindingDB Entry DOI: 10.7270/Q24Q7W49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397223 (CHEMBL2172539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.19 | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Agonist activity at VDR in human MCF7 cells assessed as transcription of CYP24A1 gene after 24 hrs by luciferase reporter gene assay | J Med Chem 55: 8642-56 (2012) Article DOI: 10.1021/jm3008272 BindingDB Entry DOI: 10.7270/Q24Q7W49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397222 (CHEMBL2172538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.04 | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Agonist activity at VDR in human MCF7 cells assessed as transcription of CYP24A1 gene after 24 hrs by luciferase reporter gene assay | J Med Chem 55: 8642-56 (2012) Article DOI: 10.1021/jm3008272 BindingDB Entry DOI: 10.7270/Q24Q7W49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50397221 (CHEMBL2172537) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.74 | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Agonist activity at VDR in human MCF7 cells assessed as transcription of CYP24A1 gene after 24 hrs by luciferase reporter gene assay | J Med Chem 55: 8642-56 (2012) Article DOI: 10.1021/jm3008272 BindingDB Entry DOI: 10.7270/Q24Q7W49 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||