

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM15234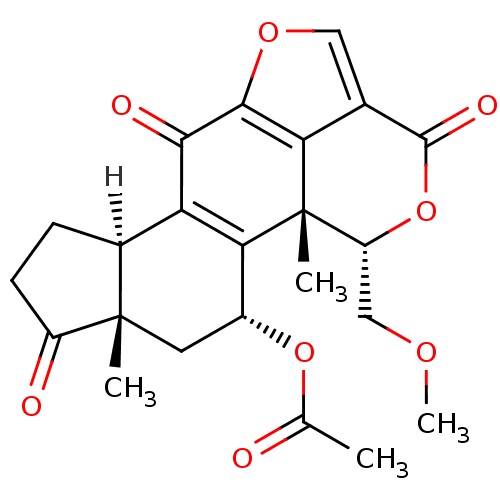 ((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 7.4 | 22 |
MRC | Assay Description Ptdlns 3-kinase enzyme activity was assayed by its converting Ptdlns(4,5)P2 into PtdIns(3,4,5)P3 in the presence of radioactive ATP. After reaction,... | Mol Cell 6: 909-19 (2000) Article DOI: 10.1016/s1097-2765(05)00089-4 BindingDB Entry DOI: 10.7270/Q22F7KPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM15234 ((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262092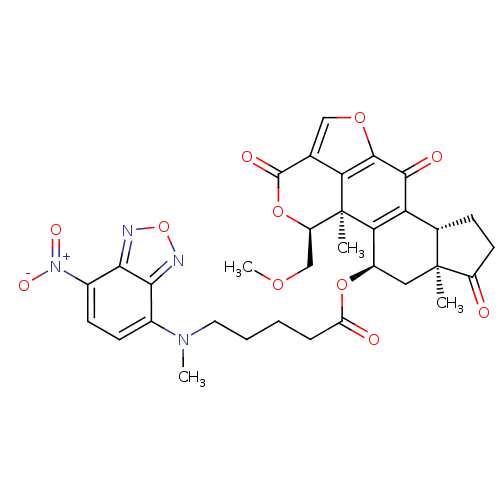 (5-[Methyl-(7-nitro-benzo[1,2,5]oxadiazol-4-yl)-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262097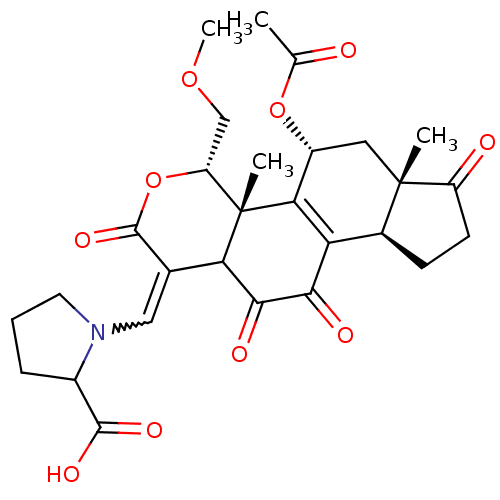 (1-((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a,6a-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262093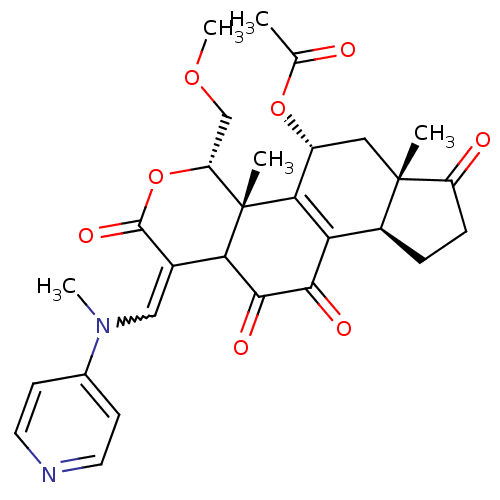 (11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-1-((me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262095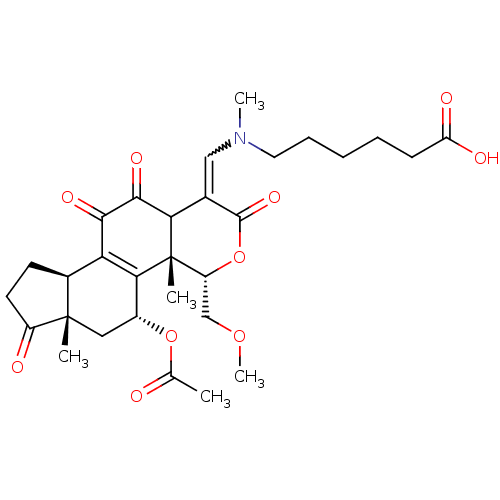 ((Z)-6-(((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262096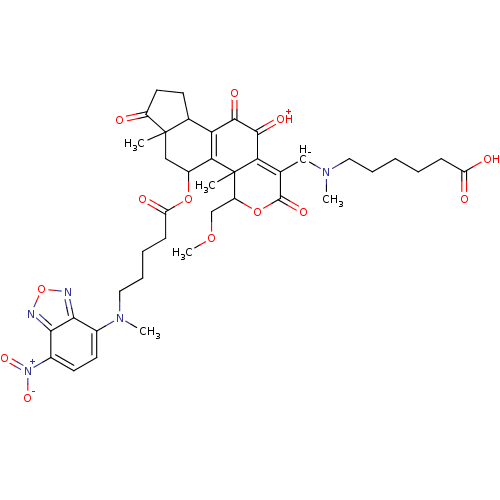 (6-(((11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262094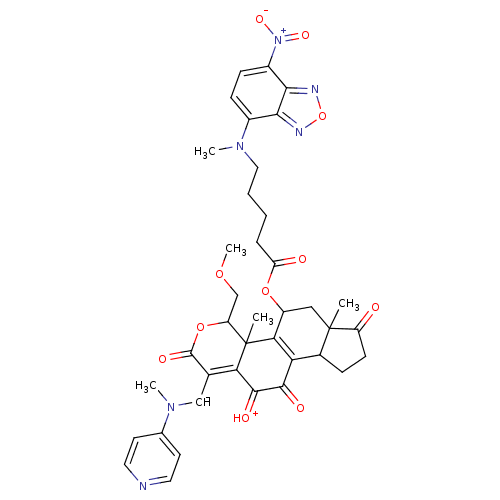 (11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-1-((me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM12915 (2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.40E+3 | 210 | n/a | n/a | n/a | 7.2 | 20 |
MRC | Assay Description Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit... | Mol Cell 6: 909-19 (2000) Article DOI: 10.1016/s1097-2765(05)00089-4 BindingDB Entry DOI: 10.7270/Q22F7KPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.80E+3 | 170 | n/a | n/a | n/a | 7.2 | 20 |
MRC | Assay Description Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit... | Mol Cell 6: 909-19 (2000) Article DOI: 10.1016/s1097-2765(05)00089-4 BindingDB Entry DOI: 10.7270/Q22F7KPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM195606 (NCT-503) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM195606 (NCT-503) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Whitehead Institute for Biomedical Research | Assay Description PHGDH assay buffer contained 50 mM TEA pH 8.0, 10 mM MgCl2, 0.05% BSA, and 0.01% Tween-20. PHGDH enzyme buffer consisted of assay buffer with 20 nM P... | Nat Chem Biol 12: 452-8 (2016) Article DOI: 10.1038/nchembio.2070 BindingDB Entry DOI: 10.7270/Q2H70DN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM195605 (NCT-502 | US11225469, Compound 72) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM195605 (NCT-502 | US11225469, Compound 72) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Whitehead Institute for Biomedical Research | Assay Description PHGDH assay buffer contained 50 mM TEA pH 8.0, 10 mM MgCl2, 0.05% BSA, and 0.01% Tween-20. PHGDH enzyme buffer consisted of assay buffer with 20 nM P... | Nat Chem Biol 12: 452-8 (2016) Article DOI: 10.1038/nchembio.2070 BindingDB Entry DOI: 10.7270/Q2H70DN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.80E+3 | 280 | n/a | n/a | n/a | 7.2 | 20 |
MRC | Assay Description Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit... | Mol Cell 6: 909-19 (2000) Article DOI: 10.1016/s1097-2765(05)00089-4 BindingDB Entry DOI: 10.7270/Q22F7KPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271477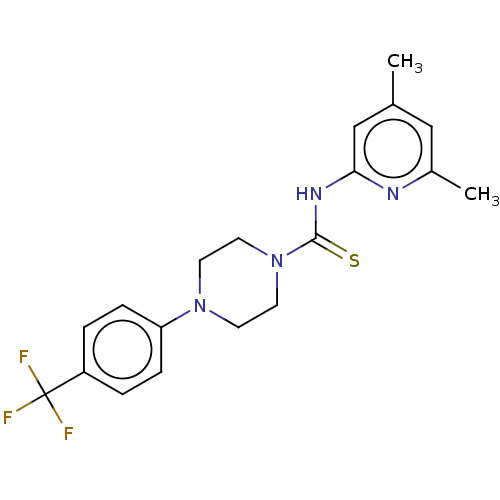 (CHEMBL4129748) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271521 (CHEMBL2140870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM58473 (MLS001172437 | N-(4-methyl-2-pyridinyl)-4-[3-(trif...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate in pres... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271523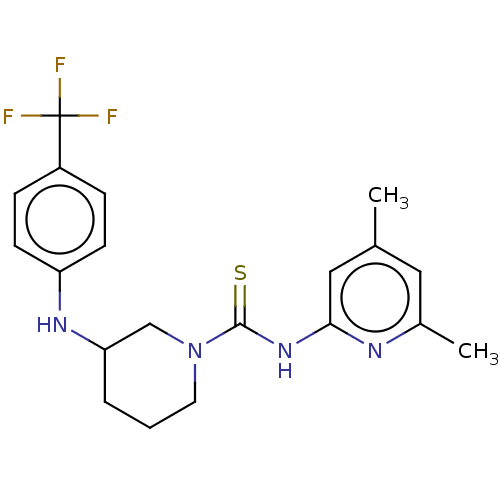 (CHEMBL4129229 | US11225469, Compound 252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271429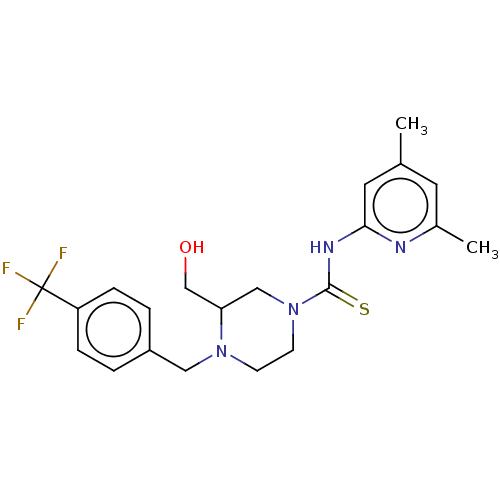 (CHEMBL4125702 | US11225469, Compound 294) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271412 (CHEMBL4125871 | US11225469, Compound 278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50232861 (CHEMBL2131647 | US11225469, Compound 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271461 (CHEMBL2143378 | US11225469, Compound 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271417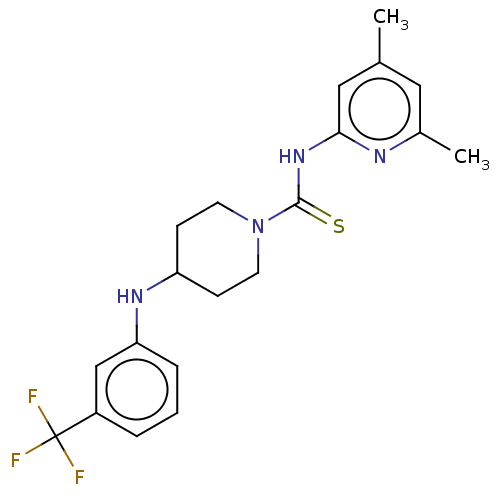 (CHEMBL4127018 | US11225469, Compound 250) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271438 (CHEMBL2137247 | US11225469, Compound 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271522 (CHEMBL4126002 | US11225469, Compound 251) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271425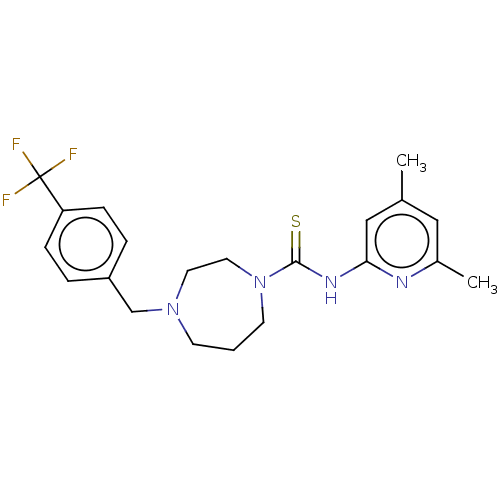 (CHEMBL4126752) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271396 (CHEMBL4129360) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271418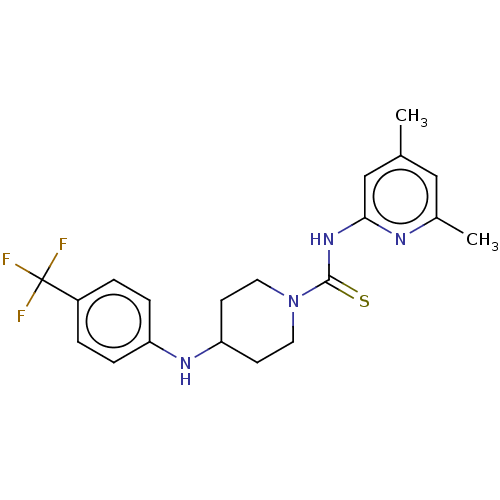 (CHEMBL4128391 | US11225469, Compound 258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271426 (CHEMBL4129099) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271427 (CHEMBL4127154 | US11225469, Compound 275) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271476 (CHEMBL4127403) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271415 (CHEMBL4125786 | US11225469, Compound 264) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271458 (CHEMBL3559789 | US11225469, Compound 30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9.00E+3 | 290 | n/a | n/a | n/a | 7.2 | 20 |
MRC | Assay Description Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit... | Mol Cell 6: 909-19 (2000) Article DOI: 10.1016/s1097-2765(05)00089-4 BindingDB Entry DOI: 10.7270/Q22F7KPC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271434 (CHEMBL4128309 | US11225469, Compound 276) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271422 (CHEMBL4126183) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271398 (CHEMBL4126631 | US11225469, Compound 297) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271420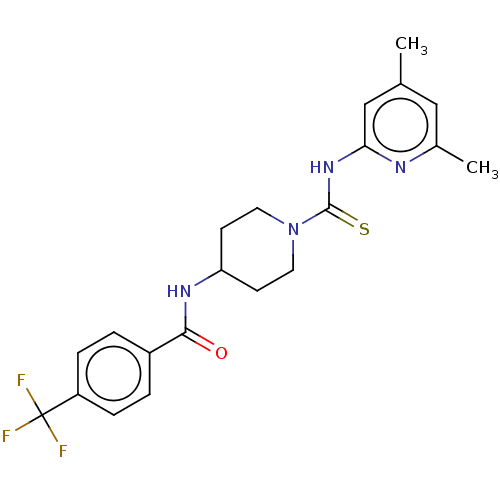 (CHEMBL4129623 | US11225469, Compound 266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271405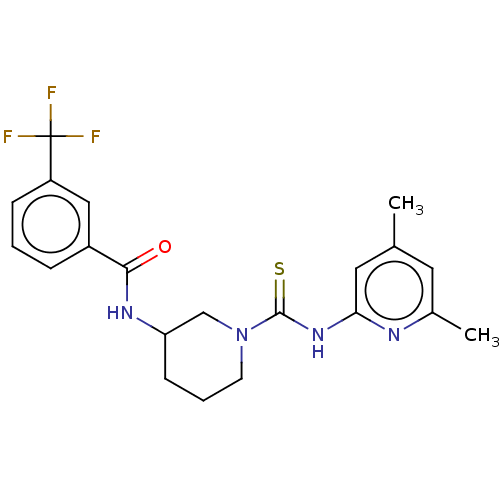 (CHEMBL4125915 | US11225469, Compound 262) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262138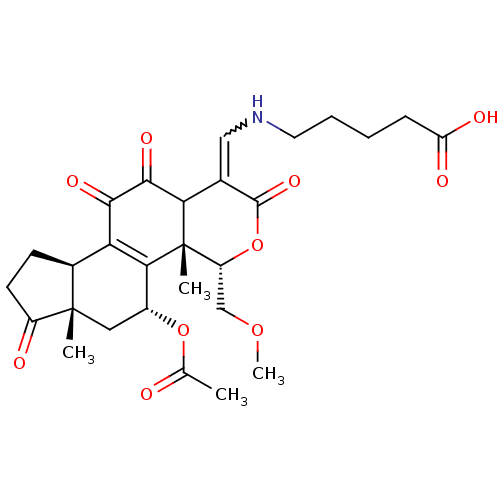 (5-((5-acetoxy-11-hydroxy-4-(methoxymethyl)-4a,6a-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271474 (CHEMBL2144036 | US11225469, Compound 34) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262139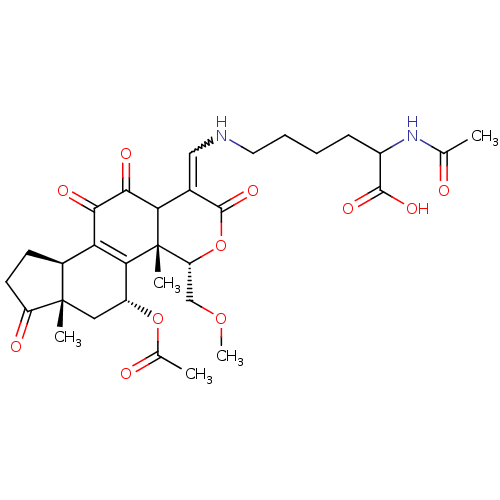 (2-acetamido-6-((5-acetoxy-11-hydroxy-4-(methoxymet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50262043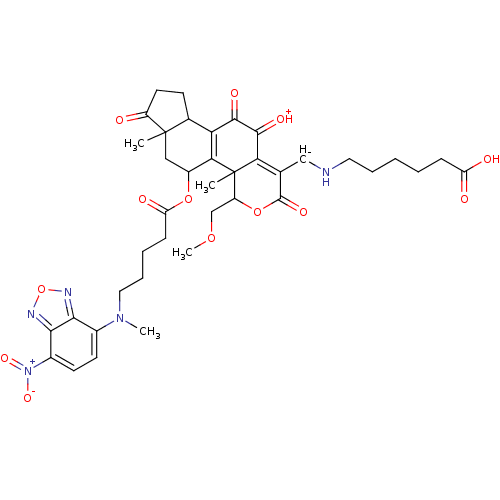 (6-((11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of PI3K (unknown origin) by fluorescence energy transfer assay | J Med Chem 51: 4699-707 (2008) Article DOI: 10.1021/jm800374f BindingDB Entry DOI: 10.7270/Q22B8XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271506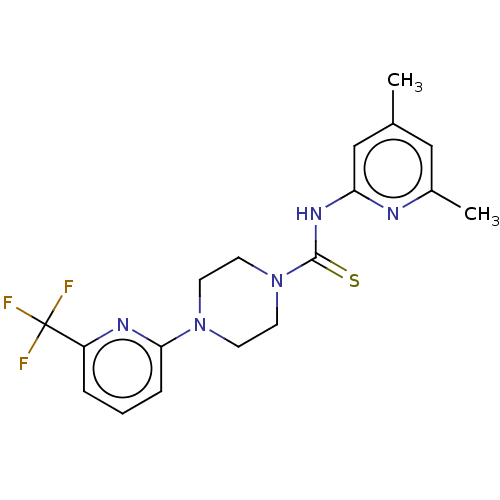 (CHEMBL2135778 | US11225469, Compound 116) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271410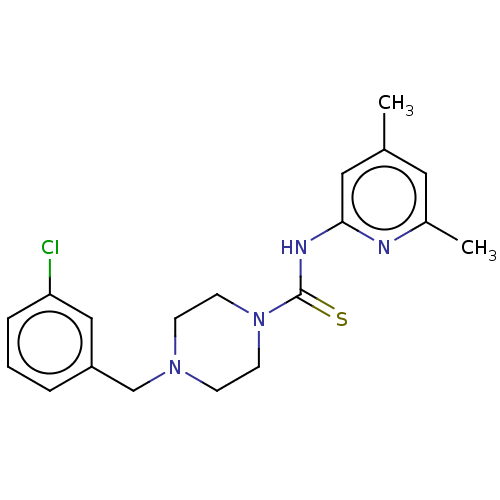 (CHEMBL4127362 | US11225469, Compound 284) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271414 (CHEMBL4128834 | US11225469, Compound 296) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271419 (CHEMBL4128252 | US11225469, Compound 263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271428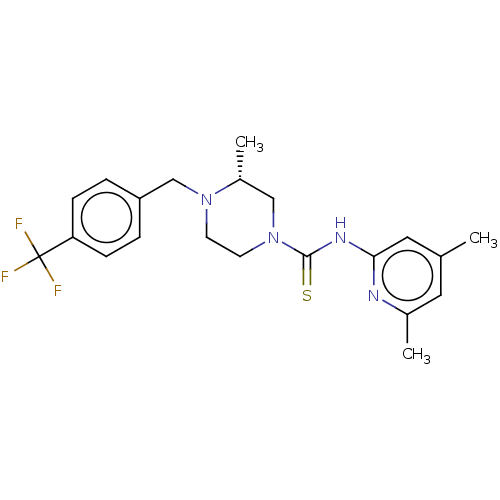 (CHEMBL4128967 | US11225469, Compound 298) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-3-phosphoglycerate dehydrogenase (Homo sapiens (Human)) | BDBM50271416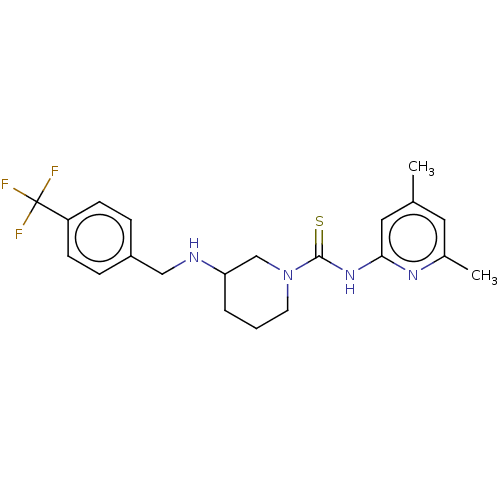 (CHEMBL4127149) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human liver PHGDH expressed in Escherichia coli Rosetta (DE3)pLysS using 3-phosphoglycerate as substrate after 2... | Bioorg Med Chem 26: 1727-1739 (2018) Article DOI: 10.1016/j.bmc.2018.02.016 BindingDB Entry DOI: 10.7270/Q2542R3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |