 Found 233 hits with Last Name = 'pang' and Initial = 'x'
Found 233 hits with Last Name = 'pang' and Initial = 'x' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610910

(CHEMBL5280844)Show SMILES OC[C@@H]1CCC(CO1)Nc1ncnc2[nH]cc(C(=O)c3ccc(Oc4ccccc4)cc3Cl)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50610910

(CHEMBL5280844)Show SMILES OC[C@@H]1CCC(CO1)Nc1ncnc2[nH]cc(C(=O)c3ccc(Oc4ccccc4)cc3Cl)c12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM291388

((R)-1-(3-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-...)Show SMILES Nc1ncnc(N[C@@H]2CCN(C2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C23H23N5O2/c1-2-20(29)28-13-12-17(14-28)27-23-21(22(24)25-15-26-23)16-8-10-19(11-9-16)30-18-6-4-3-5-7-18/h2-11,15,17H,1,12-14H2,(H3,24,25,26,27)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50161162
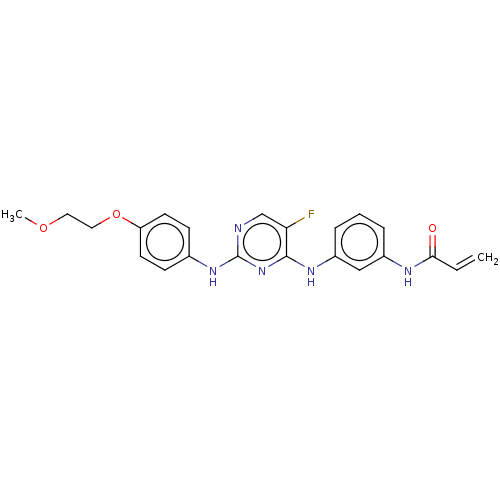
(AVL-292 | CC-292 | Spebrutinib | US10596172, Compo...)Show SMILES COCCOc1ccc(Nc2ncc(F)c(Nc3cccc(NC(=O)C=C)c3)n2)cc1 Show InChI InChI=1S/C19H18ClN3O/c20-13-9-11-7-8-21-14-6-5-10-3-1-2-4-12(10)16(14)15(11)18-17(13)22-19(24)23-18/h1-4,9,14,16,21H,5-8H2,(H2,22,23,24)/t14-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596018
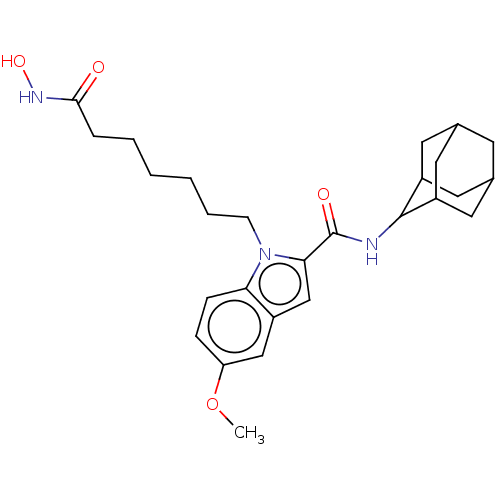
(CHEMBL5175796)Show SMILES COc1ccc2n(CCCCCCC(=O)NO)c(cc2c1)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:33:32:30:26.27.28,23:24:26.33.27:31.29.30,THB:33:27:24.32.31:30,28:27:24:31.29.30,28:29:24:26.33.27,23:24:30:26.27.28,(-6.83,6.65,;-6.83,5.17,;-5.55,4.43,;-5.55,2.87,;-4.2,2.11,;-2.87,2.88,;-1.41,2.42,;-1,.92,;.49,.52,;.89,-.97,;2.39,-1.37,;2.79,-2.87,;4.28,-3.27,;4.68,-4.76,;3.58,-5.86,;6.18,-5.16,;6.58,-6.65,;-.48,3.67,;-1.36,4.9,;-2.87,4.43,;-4.2,5.2,;1.03,3.66,;1.8,4.96,;1.78,2.34,;3.29,2.33,;4.37,3.48,;5.57,3.04,;6.82,3.35,;6.83,4.73,;5.58,5.25,;4.36,4.82,;4.64,4.13,;4.64,2.71,;5.91,2.2,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596004
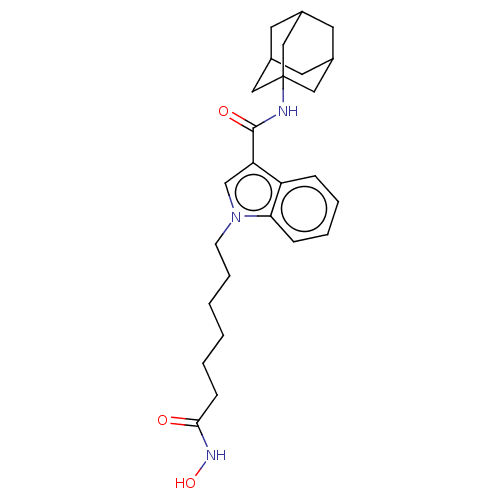
(CHEMBL5208269)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c2ccccc12 |TLB:19:20:17.18.23:24,THB:19:18:24:25.20.21,21:20:17:23.22.24,21:22:17:25.19.20| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596011
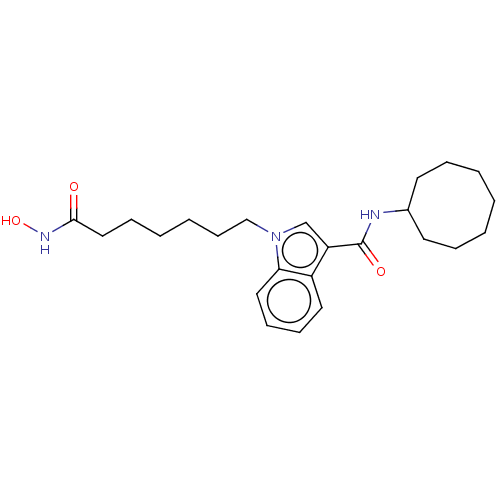
(CHEMBL5185898)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)NC2CCCCCCC2)c2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50398608
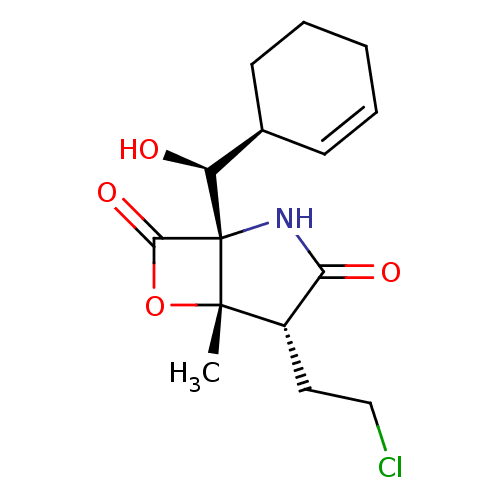
(MARIZOMIB)Show SMILES C[C@@]12OC(=O)[C@@]1(NC(=O)[C@@H]2CCCl)[C@@H](O)[C@H]1CCCC=C1 |r,c:21| Show InChI InChI=1S/C15H20ClNO4/c1-14-10(7-8-16)12(19)17-15(14,13(20)21-14)11(18)9-5-3-2-4-6-9/h3,5,9-11,18H,2,4,6-8H2,1H3,(H,17,19)/t9-,10+,11+,14+,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114117
BindingDB Entry DOI: 10.7270/Q2QR522B |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596009
(CHEMBL5197455) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM389631
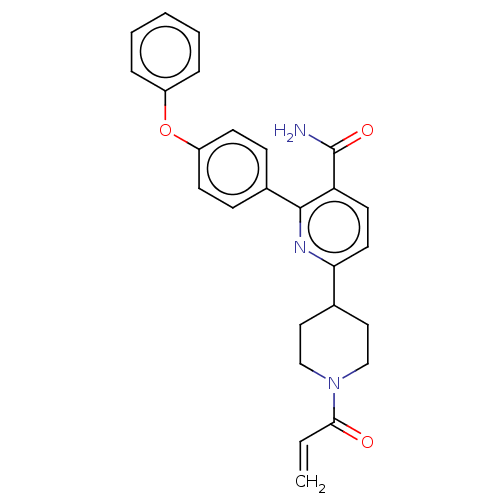
(6-(1-acryloylpiperidin-4-yl)-2-(4-phenoxyphenyl)ni...)Show SMILES NC(=O)c1ccc(nc1-c1ccc(Oc2ccccc2)cc1)C1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C26H25N3O3/c1-2-24(30)29-16-14-18(15-17-29)23-13-12-22(26(27)31)25(28-23)19-8-10-21(11-9-19)32-20-6-4-3-5-7-20/h2-13,18H,1,14-17H2,(H2,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
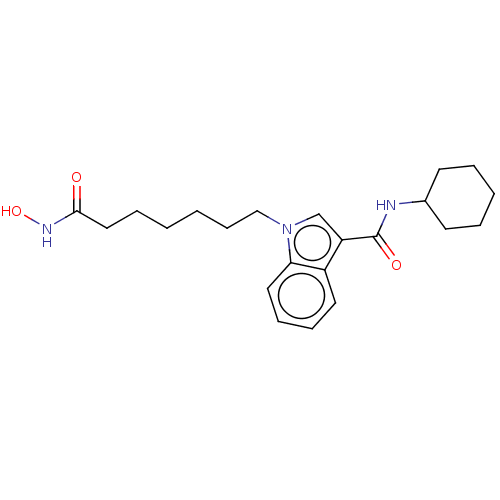
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596006
(CHEMBL5189457)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)NCc2ccc(Cl)cc2)c2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596005
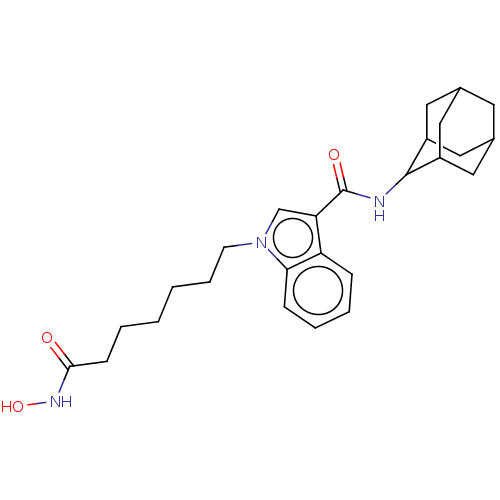
(CHEMBL5206239)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)NC2C3CC4CC(C3)CC2C4)c2ccccc12 |TLB:15:16:18:22.20.21,THB:20:19:16:22.21.23,20:21:18.19.25:16,23:21:18:25.24.16,23:24:18:22.20.21,(6.06,-7.06,;5.65,-5.57,;4.16,-5.17,;3.06,-6.27,;3.76,-3.68,;2.27,-3.28,;1.87,-1.79,;.37,-1.38,;-.02,.11,;-1.52,.51,;-1.92,2,;-1,3.25,;-1.88,4.48,;-1.48,5.97,;-2.57,7.06,;.01,6.37,;1.11,5.27,;1.12,4.23,;.28,3.34,;1.32,3.63,;2.31,3.24,;3.02,4.14,;2.04,3.89,;3.03,5.2,;2.05,5.61,;1.31,4.75,;-3.38,4.01,;-4.72,4.78,;-6.06,4.01,;-6.06,2.45,;-4.72,1.69,;-3.38,2.46,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596020
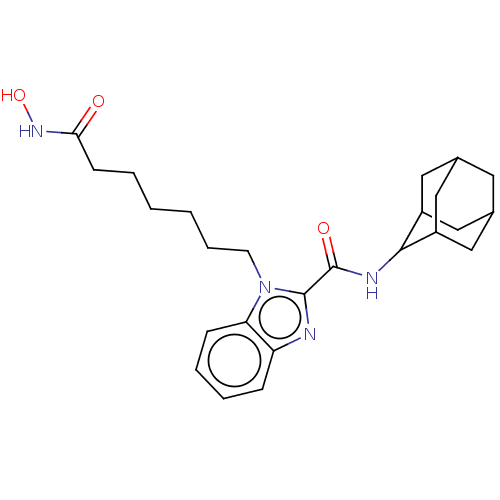
(CHEMBL5191345)Show SMILES ONC(=O)CCCCCCn1c(nc2ccccc12)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:31:30:28:24.25.26,21:22:24.31.25:29.27.28,THB:31:25:22.30.29:28,26:25:22:29.27.28,26:27:22:24.31.25,21:22:28:24.25.26,(5.94,-5.95,;5.54,-4.46,;4.04,-4.06,;2.94,-5.16,;3.64,-2.57,;2.15,-2.17,;1.75,-.67,;.25,-.27,;-.15,1.22,;-1.64,1.62,;-2.05,3.12,;-1.12,4.38,;-2,5.6,;-3.51,5.13,;-4.84,5.91,;-6.19,5.13,;-6.19,3.57,;-4.85,2.81,;-3.51,3.58,;.39,4.36,;1.16,5.66,;1.13,3.04,;2.65,3.03,;3.73,4.18,;4.92,3.74,;6.18,4.05,;6.19,5.43,;4.93,5.95,;3.72,5.52,;3.99,4.84,;4,3.41,;5.26,2.9,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596011

(CHEMBL5185898)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)NC2CCCCCCC2)c2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50588021

(CHEMBL4303247)Show SMILES Clc1cccc(NCC(=O)NC2CCCN(C2)c2ncnc3[nH]ccc23)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596004

(CHEMBL5208269)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c2ccccc12 |TLB:19:20:17.18.23:24,THB:19:18:24:25.20.21,21:20:17:23.22.24,21:22:17:25.19.20| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596010
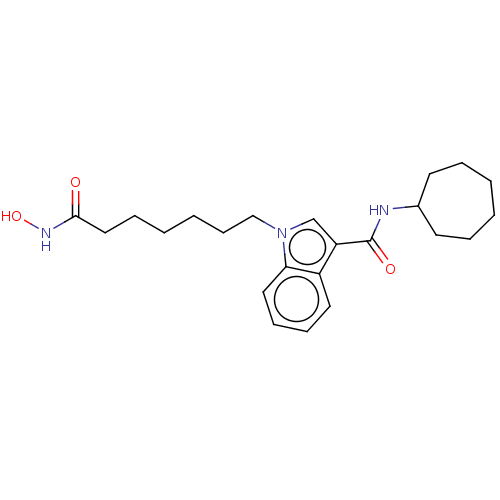
(CHEMBL5174843)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)NC2CCCCCC2)c2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596005

(CHEMBL5206239)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)NC2C3CC4CC(C3)CC2C4)c2ccccc12 |TLB:15:16:18:22.20.21,THB:20:19:16:22.21.23,20:21:18.19.25:16,23:21:18:25.24.16,23:24:18:22.20.21,(6.06,-7.06,;5.65,-5.57,;4.16,-5.17,;3.06,-6.27,;3.76,-3.68,;2.27,-3.28,;1.87,-1.79,;.37,-1.38,;-.02,.11,;-1.52,.51,;-1.92,2,;-1,3.25,;-1.88,4.48,;-1.48,5.97,;-2.57,7.06,;.01,6.37,;1.11,5.27,;1.12,4.23,;.28,3.34,;1.32,3.63,;2.31,3.24,;3.02,4.14,;2.04,3.89,;3.03,5.2,;2.05,5.61,;1.31,4.75,;-3.38,4.01,;-4.72,4.78,;-6.06,4.01,;-6.06,2.45,;-4.72,1.69,;-3.38,2.46,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 12
(Homo sapiens (Human)) | BDBM50239134

(CHEMBL4087516)Show InChI InChI=1S/C13H8F3N3O2S/c14-13(15,16)22(20,21)19-12-5-6-18-8-11(12)10-3-1-9(7-17)2-4-10/h1-6,8H,(H,18,19) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human URAT1 expressed in HEK293T cells assessed as reduction in [14C]uric acid uptake measured after 5 mins by liquid scintillation cou... |
Bioorg Med Chem Lett 27: 1919-1922 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.041
BindingDB Entry DOI: 10.7270/Q2NK3H6D |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596016
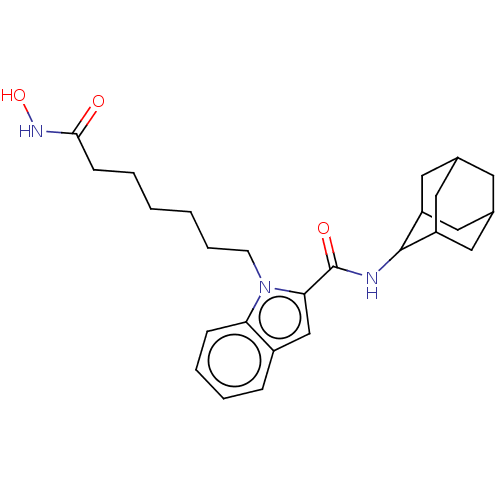
(CHEMBL5169341)Show SMILES ONC(=O)CCCCCCn1c(cc2ccccc12)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:31:30:28:24.25.26,21:22:24.31.25:29.27.28,THB:31:25:22.30.29:28,26:25:22:29.27.28,26:27:22:24.31.25,21:22:28:24.25.26,(5.94,-5.95,;5.54,-4.46,;4.04,-4.06,;2.94,-5.16,;3.64,-2.57,;2.15,-2.17,;1.75,-.67,;.25,-.27,;-.15,1.22,;-1.64,1.62,;-2.05,3.12,;-1.12,4.38,;-2,5.6,;-3.51,5.13,;-4.84,5.91,;-6.19,5.13,;-6.19,3.57,;-4.85,2.81,;-3.51,3.58,;.39,4.36,;1.16,5.66,;1.13,3.04,;2.65,3.03,;3.73,4.18,;4.93,3.74,;6.18,4.06,;6.19,5.43,;4.94,5.95,;3.72,5.52,;3.99,4.84,;4,3.41,;5.26,2.9,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50331095
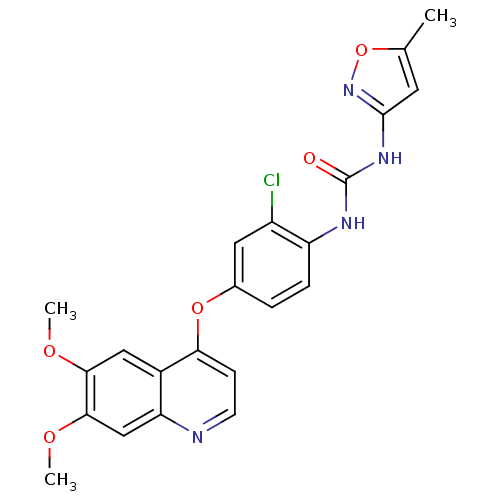
(CHEMBL1289494 | Tivozanib | US10464902, Tivozanib)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)Nc4cc(C)on4)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C22H19ClN4O5/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18/h4-11H,1-3H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 (unknown origin) by cell-free assay |
Bioorg Med Chem Lett 25: 2425-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.088
BindingDB Entry DOI: 10.7270/Q2KW5HRT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596010

(CHEMBL5174843)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)NC2CCCCCC2)c2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610909

(CHEMBL5285357)Show SMILES CC#CC(=O)N1CC[C@@H](C1)N1CN(c2c1ncnc2N)c1ccc(Oc2ccccc2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596017
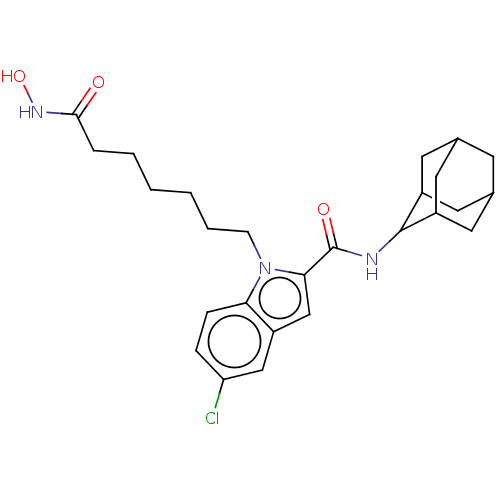
(CHEMBL5180181)Show SMILES ONC(=O)CCCCCCn1c(cc2cc(Cl)ccc12)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:32:31:29:25.26.27,22:23:25.32.26:30.28.29,THB:32:26:23.31.30:29,27:26:23:30.28.29,27:28:23:25.32.26,22:23:29:25.26.27,(6.58,-5.95,;6.18,-4.46,;4.68,-4.06,;3.58,-5.16,;4.28,-2.57,;2.79,-2.17,;2.39,-.67,;.89,-.27,;.49,1.22,;-1,1.62,;-1.41,3.12,;-.48,4.37,;-1.36,5.6,;-2.87,5.13,;-4.2,5.91,;-5.55,5.13,;-6.83,5.87,;-5.55,3.57,;-4.2,2.81,;-2.87,3.58,;1.03,4.36,;1.8,5.66,;1.78,3.04,;3.29,3.03,;4.37,4.18,;5.57,3.74,;6.82,4.05,;6.83,5.43,;5.58,5.95,;4.36,5.52,;4.63,4.83,;4.64,3.41,;5.9,2.9,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596018

(CHEMBL5175796)Show SMILES COc1ccc2n(CCCCCCC(=O)NO)c(cc2c1)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:33:32:30:26.27.28,23:24:26.33.27:31.29.30,THB:33:27:24.32.31:30,28:27:24:31.29.30,28:29:24:26.33.27,23:24:30:26.27.28,(-6.83,6.65,;-6.83,5.17,;-5.55,4.43,;-5.55,2.87,;-4.2,2.11,;-2.87,2.88,;-1.41,2.42,;-1,.92,;.49,.52,;.89,-.97,;2.39,-1.37,;2.79,-2.87,;4.28,-3.27,;4.68,-4.76,;3.58,-5.86,;6.18,-5.16,;6.58,-6.65,;-.48,3.67,;-1.36,4.9,;-2.87,4.43,;-4.2,5.2,;1.03,3.66,;1.8,4.96,;1.78,2.34,;3.29,2.33,;4.37,3.48,;5.57,3.04,;6.82,3.35,;6.83,4.73,;5.58,5.25,;4.36,4.82,;4.64,4.13,;4.64,2.71,;5.91,2.2,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
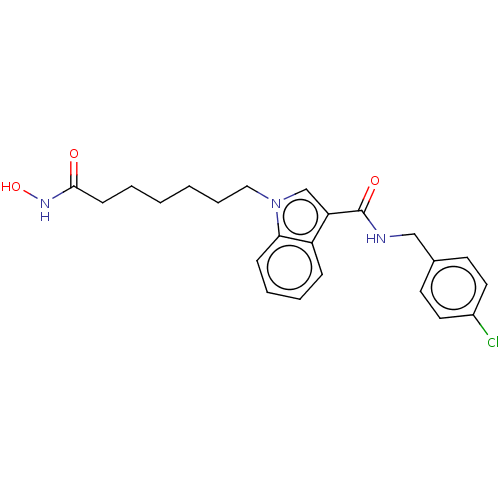
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596009

(CHEMBL5197455) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50331095

(CHEMBL1289494 | Tivozanib | US10464902, Tivozanib)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)Nc4cc(C)on4)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C22H19ClN4O5/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18/h4-11H,1-3H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PDGFRalpha (unknown origin) by cell-free assay |
Bioorg Med Chem Lett 25: 2425-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.088
BindingDB Entry DOI: 10.7270/Q2KW5HRT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596006

(CHEMBL5189457)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)NCc2ccc(Cl)cc2)c2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50331095

(CHEMBL1289494 | Tivozanib | US10464902, Tivozanib)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)Nc4cc(C)on4)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C22H19ClN4O5/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18/h4-11H,1-3H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR2 (unknown origin) by cell-free assay |
Bioorg Med Chem Lett 25: 2425-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.088
BindingDB Entry DOI: 10.7270/Q2KW5HRT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596016

(CHEMBL5169341)Show SMILES ONC(=O)CCCCCCn1c(cc2ccccc12)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:31:30:28:24.25.26,21:22:24.31.25:29.27.28,THB:31:25:22.30.29:28,26:25:22:29.27.28,26:27:22:24.31.25,21:22:28:24.25.26,(5.94,-5.95,;5.54,-4.46,;4.04,-4.06,;2.94,-5.16,;3.64,-2.57,;2.15,-2.17,;1.75,-.67,;.25,-.27,;-.15,1.22,;-1.64,1.62,;-2.05,3.12,;-1.12,4.38,;-2,5.6,;-3.51,5.13,;-4.84,5.91,;-6.19,5.13,;-6.19,3.57,;-4.85,2.81,;-3.51,3.58,;.39,4.36,;1.16,5.66,;1.13,3.04,;2.65,3.03,;3.73,4.18,;4.93,3.74,;6.18,4.06,;6.19,5.43,;4.94,5.95,;3.72,5.52,;3.99,4.84,;4,3.41,;5.26,2.9,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596007
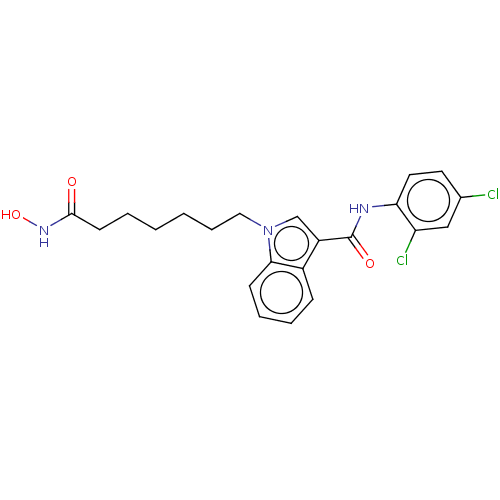
(CHEMBL5171183)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)Nc2ccc(Cl)cc2Cl)c2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 6
(Homo sapiens (Human)) | BDBM50610910

(CHEMBL5280844)Show SMILES OC[C@@H]1CCC(CO1)Nc1ncnc2[nH]cc(C(=O)c3ccc(Oc4ccccc4)cc3Cl)c12 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50610908
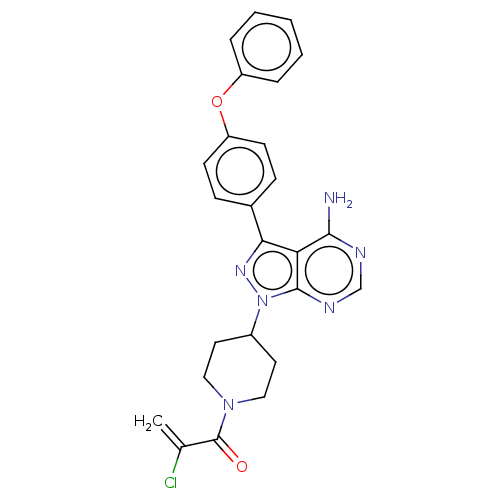
(CHEMBL5271514)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)C1CCN(CC1)C(=O)C(Cl)=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596021
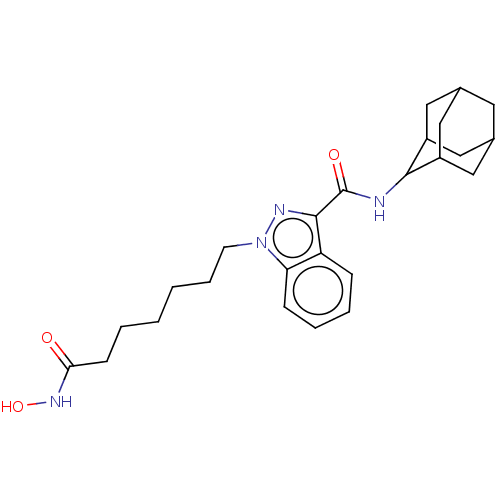
(CHEMBL5180263)Show SMILES ONC(=O)CCCCCCn1nc(C(=O)NC2C3CC4CC(C3)CC2C4)c2ccccc12 |TLB:25:24:22:18.19.20,15:16:22:18.19.20,THB:25:19:16.24.23:22,20:19:16:23.21.22,20:21:16:18.25.19,15:16:18.25.19:23.21.22,(6.06,-8.79,;5.66,-7.3,;4.16,-6.9,;3.07,-8,;3.76,-5.41,;2.27,-5.01,;1.87,-3.51,;.37,-3.11,;-.03,-1.62,;-1.52,-1.22,;-1.92,.28,;-.99,1.53,;-1.88,2.76,;-1.41,4.17,;-2.39,5.28,;.05,4.46,;.52,5.87,;1.59,7.02,;2.79,6.58,;4.05,6.89,;4.06,8.27,;2.8,8.79,;1.59,8.36,;1.86,7.67,;1.87,6.25,;3.13,5.74,;-3.38,2.29,;-4.72,3.06,;-6.06,2.29,;-6.06,.73,;-4.72,-.03,;-3.38,.74,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50175583
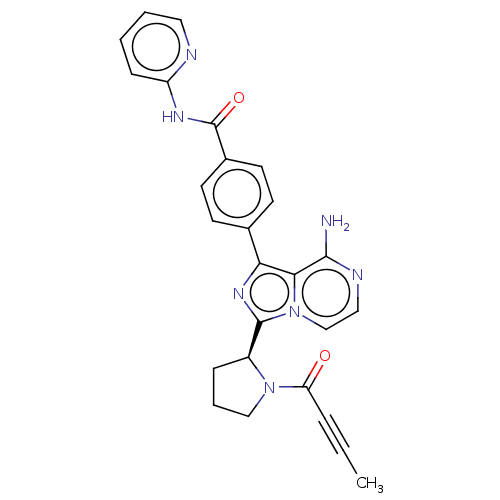
(ACP-196 | Acalabrutinib | US10239883, Example 6 | ...)Show SMILES [H][C@]1(CCCN1C(=O)C#CC)c1nc(-c2ccc(cc2)C(=O)Nc2ccccn2)c2c(N)nccn12 |r| Show InChI InChI=1S/C26H23N7O2/c1-2-6-21(34)32-15-5-7-19(32)25-31-22(23-24(27)29-14-16-33(23)25)17-9-11-18(12-10-17)26(35)30-20-8-3-4-13-28-20/h3-4,8-14,16,19H,5,7,15H2,1H3,(H2,27,29)(H,28,30,35)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596021

(CHEMBL5180263)Show SMILES ONC(=O)CCCCCCn1nc(C(=O)NC2C3CC4CC(C3)CC2C4)c2ccccc12 |TLB:25:24:22:18.19.20,15:16:22:18.19.20,THB:25:19:16.24.23:22,20:19:16:23.21.22,20:21:16:18.25.19,15:16:18.25.19:23.21.22,(6.06,-8.79,;5.66,-7.3,;4.16,-6.9,;3.07,-8,;3.76,-5.41,;2.27,-5.01,;1.87,-3.51,;.37,-3.11,;-.03,-1.62,;-1.52,-1.22,;-1.92,.28,;-.99,1.53,;-1.88,2.76,;-1.41,4.17,;-2.39,5.28,;.05,4.46,;.52,5.87,;1.59,7.02,;2.79,6.58,;4.05,6.89,;4.06,8.27,;2.8,8.79,;1.59,8.36,;1.86,7.67,;1.87,6.25,;3.13,5.74,;-3.38,2.29,;-4.72,3.06,;-6.06,2.29,;-6.06,.73,;-4.72,-.03,;-3.38,.74,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50331095

(CHEMBL1289494 | Tivozanib | US10464902, Tivozanib)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)Nc4cc(C)on4)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C22H19ClN4O5/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18/h4-11H,1-3H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR3 (unknown origin) by cell-free assay |
Bioorg Med Chem Lett 25: 2425-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.088
BindingDB Entry DOI: 10.7270/Q2KW5HRT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50596019
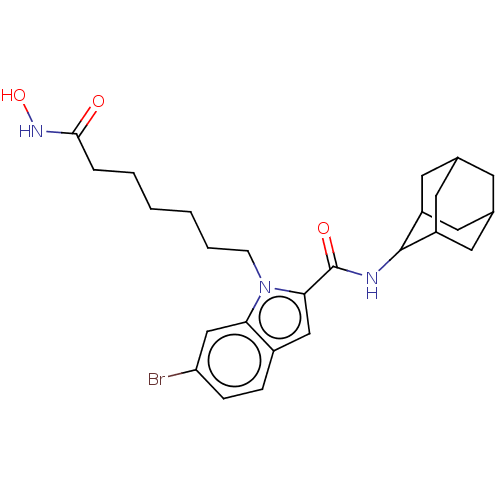
(CHEMBL5171482)Show SMILES ONC(=O)CCCCCCn1c(cc2ccc(Br)cc12)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:32:31:29:25.26.27,22:23:25.32.26:30.28.29,THB:32:26:23.31.30:29,27:26:23:30.28.29,27:28:23:25.32.26,22:23:29:25.26.27,(6.58,-5.95,;6.18,-4.46,;4.68,-4.06,;3.58,-5.16,;4.28,-2.57,;2.79,-2.17,;2.39,-.67,;.89,-.27,;.49,1.22,;-1,1.62,;-1.41,3.12,;-.48,4.38,;-1.36,5.6,;-2.87,5.13,;-4.2,5.91,;-5.55,5.13,;-5.55,3.57,;-6.83,2.82,;-4.21,2.81,;-2.87,3.58,;1.03,4.36,;1.8,5.66,;1.77,3.04,;3.29,3.03,;4.37,4.18,;5.56,3.74,;6.82,4.05,;6.83,5.43,;5.58,5.95,;4.36,5.52,;4.63,4.83,;4.64,3.41,;5.9,2.9,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50331095

(CHEMBL1289494 | Tivozanib | US10464902, Tivozanib)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)Nc4cc(C)on4)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C22H19ClN4O5/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18/h4-11H,1-3H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PDGFRalpha (unknown origin) by cell-free assay |
Bioorg Med Chem Lett 25: 2425-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.088
BindingDB Entry DOI: 10.7270/Q2KW5HRT |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596007

(CHEMBL5171183)Show SMILES ONC(=O)CCCCCCn1cc(C(=O)Nc2ccc(Cl)cc2Cl)c2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50610910

(CHEMBL5280844)Show SMILES OC[C@@H]1CCC(CO1)Nc1ncnc2[nH]cc(C(=O)c3ccc(Oc4ccccc4)cc3Cl)c12 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50596019

(CHEMBL5171482)Show SMILES ONC(=O)CCCCCCn1c(cc2ccc(Br)cc12)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:32:31:29:25.26.27,22:23:25.32.26:30.28.29,THB:32:26:23.31.30:29,27:26:23:30.28.29,27:28:23:25.32.26,22:23:29:25.26.27,(6.58,-5.95,;6.18,-4.46,;4.68,-4.06,;3.58,-5.16,;4.28,-2.57,;2.79,-2.17,;2.39,-.67,;.89,-.27,;.49,1.22,;-1,1.62,;-1.41,3.12,;-.48,4.38,;-1.36,5.6,;-2.87,5.13,;-4.2,5.91,;-5.55,5.13,;-5.55,3.57,;-6.83,2.82,;-4.21,2.81,;-2.87,3.58,;1.03,4.36,;1.8,5.66,;1.77,3.04,;3.29,3.03,;4.37,4.18,;5.56,3.74,;6.82,4.05,;6.83,5.43,;5.58,5.95,;4.36,5.52,;4.63,4.83,;4.64,3.41,;5.9,2.9,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50331095

(CHEMBL1289494 | Tivozanib | US10464902, Tivozanib)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)Nc4cc(C)on4)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C22H19ClN4O5/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18/h4-11H,1-3H3,(H2,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant VEGFR3 (unknown origin) by cell-free assay |
Bioorg Med Chem Lett 25: 2425-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.088
BindingDB Entry DOI: 10.7270/Q2KW5HRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50601461

(CHEMBL5203118)Show SMILES COc1cc(ccc1Nc1ncc2COC(=O)N(c3cccc(NC(=O)C=C)c3)c2n1)N1CCN(C)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113893
BindingDB Entry DOI: 10.7270/Q23R0XWZ |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50436850
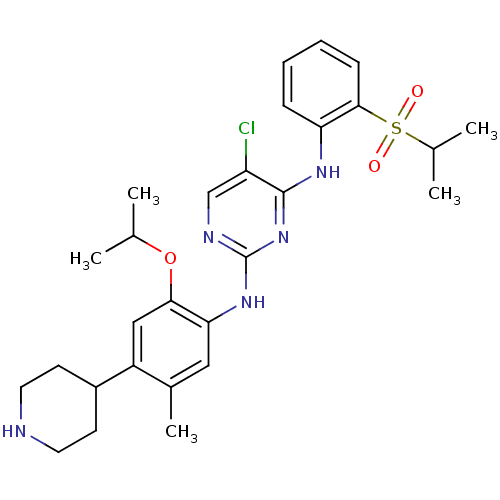
(CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...)Show SMILES CC(C)Oc1cc(C2CCNCC2)c(C)cc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C28H36ClN5O3S/c1-17(2)37-25-15-21(20-10-12-30-13-11-20)19(5)14-24(25)33-28-31-16-22(29)27(34-28)32-23-8-6-7-9-26(23)38(35,36)18(3)4/h6-9,14-18,20,30H,10-13H2,1-5H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Suzhou Zelgen Biopharmaceuticals Co., Ltd.
US Patent
| Assay Description
96-well plates was coated under 37° C. with coating buffer (125 μl/well) overnight, and coating buffer was polypeptide substrate [Poly (4:1 Glu,... |
US Patent US10618884 (2020)
BindingDB Entry DOI: 10.7270/Q2PV6PCX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50436850

(CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...)Show SMILES CC(C)Oc1cc(C2CCNCC2)c(C)cc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C28H36ClN5O3S/c1-17(2)37-25-15-21(20-10-12-30-13-11-20)19(5)14-24(25)33-28-31-16-22(29)27(34-28)32-23-8-6-7-9-26(23)38(35,36)18(3)4/h6-9,14-18,20,30H,10-13H2,1-5H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Suzhou Zelgen Biopharmaceuticals Co., Ltd.
US Patent
| Assay Description
The determination of IC50 of the inhibiting effect to kinase ALK 96-well plates was coated under 37° C. with coating buffer (125 ul/well) overnig... |
US Patent US9809572 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50436850

(CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...)Show SMILES CC(C)Oc1cc(C2CCNCC2)c(C)cc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C28H36ClN5O3S/c1-17(2)37-25-15-21(20-10-12-30-13-11-20)19(5)14-24(25)33-28-31-16-22(29)27(34-28)32-23-8-6-7-9-26(23)38(35,36)18(3)4/h6-9,14-18,20,30H,10-13H2,1-5H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Suzhou Zelgen Biopharmaceuticals Co., Ltd.
US Patent
| Assay Description
The determination of IC50 of the inhibiting effect to kinase ALK 96-well plates was coated under 37° C. with coating buffer (125 ul/well) overnig... |
US Patent US9809572 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50436850

(CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...)Show SMILES CC(C)Oc1cc(C2CCNCC2)c(C)cc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C28H36ClN5O3S/c1-17(2)37-25-15-21(20-10-12-30-13-11-20)19(5)14-24(25)33-28-31-16-22(29)27(34-28)32-23-8-6-7-9-26(23)38(35,36)18(3)4/h6-9,14-18,20,30H,10-13H2,1-5H3,(H2,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Suzhou Zelgen Biopharmaceuticals Co., Ltd.
US Patent
| Assay Description
The determination of IC50 of the inhibiting effect to kinase ALK 96-well plates was coated under 37° C. with coating buffer (125 ul/well) overnig... |
US Patent US9809572 (2017)
BindingDB Entry DOI: 10.7270/Q2RF5X4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data