

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound was tested for inhibition activity against rat liver lipophilic Dihydrofolate reductase (DHFR). | J Med Chem 41: 913-8 (1998) Article DOI: 10.1021/jm970614n BindingDB Entry DOI: 10.7270/Q2BR8R9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50029763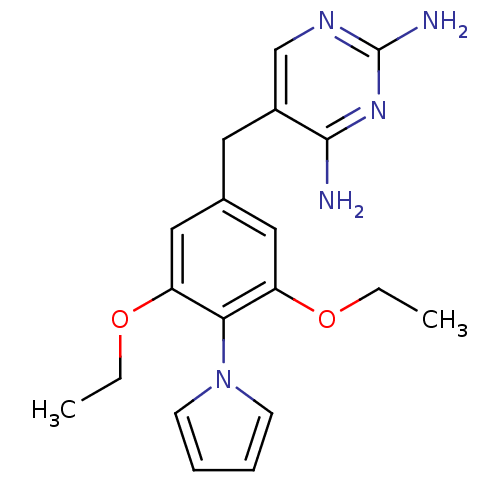 (5-(3,5-Diethoxy-4-pyrrol-1-yl-benzyl)-pyrimidine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of rat liver Dihydrofolate Reductase | J Med Chem 40: 3694-9 (1997) Article DOI: 10.1021/jm970399a BindingDB Entry DOI: 10.7270/Q2NG4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Rat liver | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound was tested for inhibition activity against toxoplasma gondii (T. gondii) Dihydrofolate reductase | J Med Chem 41: 913-8 (1998) Article DOI: 10.1021/jm970614n BindingDB Entry DOI: 10.7270/Q2BR8R9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Toxoplasma gondii. | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50029763 (5-(3,5-Diethoxy-4-pyrrol-1-yl-benzyl)-pyrimidine-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of T. gondii Dihydrofolate Reductase | J Med Chem 40: 3694-9 (1997) Article DOI: 10.1021/jm970399a BindingDB Entry DOI: 10.7270/Q2NG4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM18506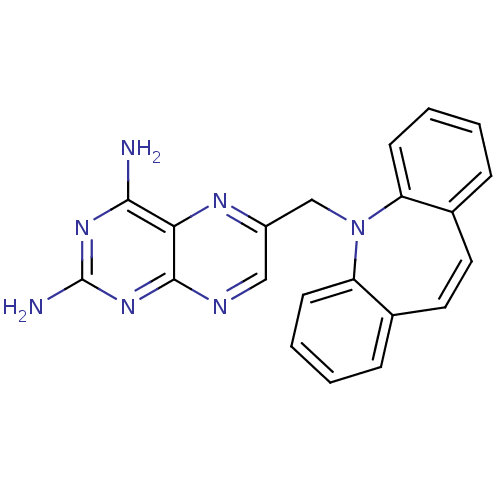 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Mycobacterium avium | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50082920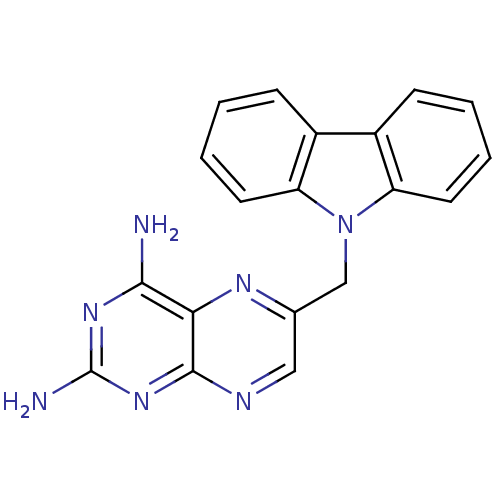 (6-Carbazol-9-ylmethyl-pteridine-2,4-diamine | CHEM...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Toxoplasma gondii. | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074735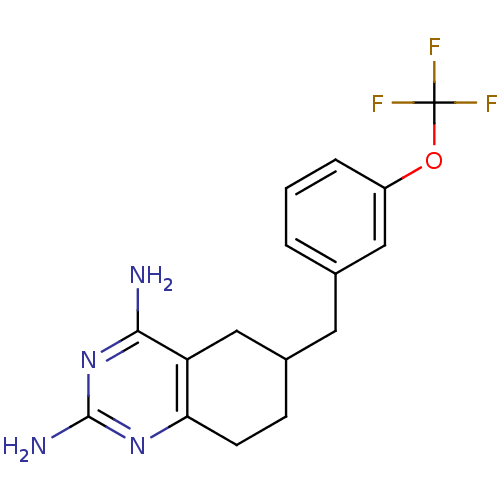 (6-(3-(trifluoromethoxy)benzyl)-5,6,7,8-tetrahydroq...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074724 (6-(2-Methoxy-benzyl)-5,6,7,8-tetrahydro-quinazolin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound was tested for inhibition activity against rat liver lipophilic Dihydrofolate reductase (DHFR). | J Med Chem 41: 913-8 (1998) Article DOI: 10.1021/jm970614n BindingDB Entry DOI: 10.7270/Q2BR8R9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50027970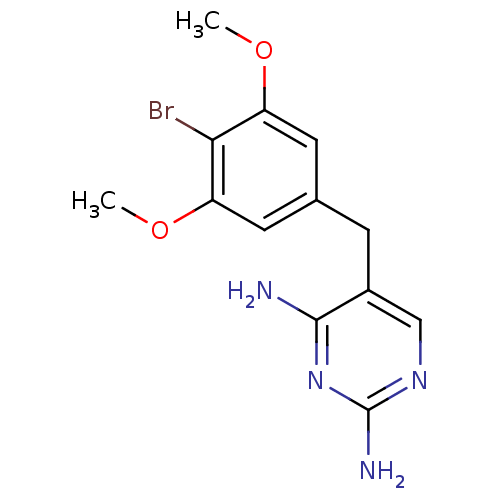 (5-(4-Bromo-3,5-dimethoxy-benzyl)-pyrimidine-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of rat liver Dihydrofolate Reductase | J Med Chem 40: 3694-9 (1997) Article DOI: 10.1021/jm970399a BindingDB Entry DOI: 10.7270/Q2NG4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit rat liver Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound was tested for inhibition activity against toxoplasma gondii (T. gondii) Dihydrofolate reductase | J Med Chem 41: 913-8 (1998) Article DOI: 10.1021/jm970614n BindingDB Entry DOI: 10.7270/Q2BR8R9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50027970 (5-(4-Bromo-3,5-dimethoxy-benzyl)-pyrimidine-2,4-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of T. gondii Dihydrofolate Reductase | J Med Chem 40: 3694-9 (1997) Article DOI: 10.1021/jm970399a BindingDB Entry DOI: 10.7270/Q2NG4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM50082923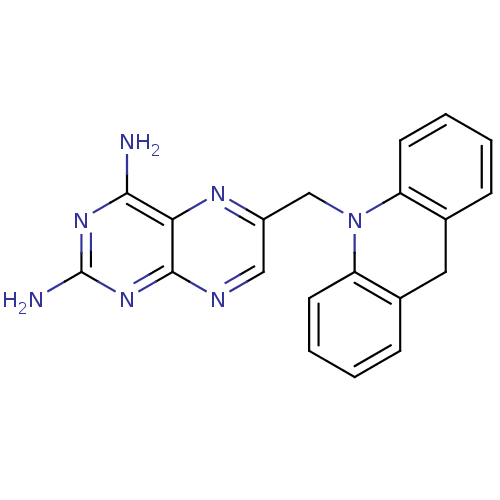 (6-(9H-Acridin-10-ylmethyl)-pteridine-2,4-diamine |...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Mycobacterium avium | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074725 (6-tert-Butyl-5,6,7,8-tetrahydro-quinazoline-2,4-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074718 (6-(3-Methoxy-benzyl)-5,6,7,8-tetrahydro-quinazolin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074720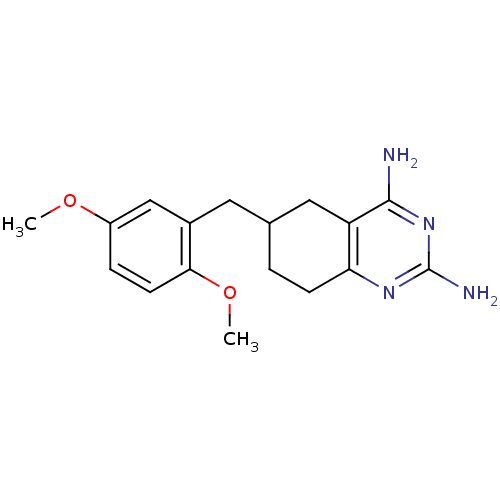 (6-(2,5-Dimethoxy-benzyl)-5,6,7,8-tetrahydro-quinaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074727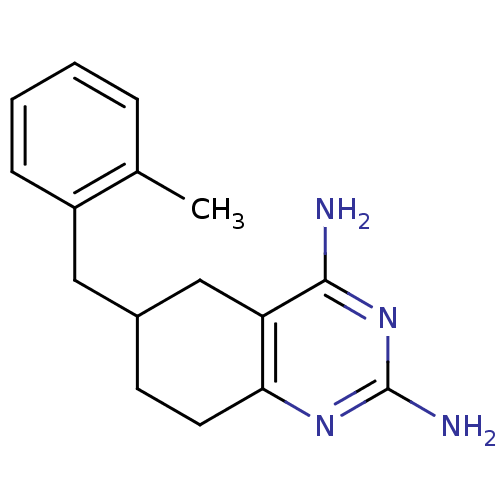 (6-(2-Methyl-benzyl)-5,6,7,8-tetrahydro-quinazoline...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074719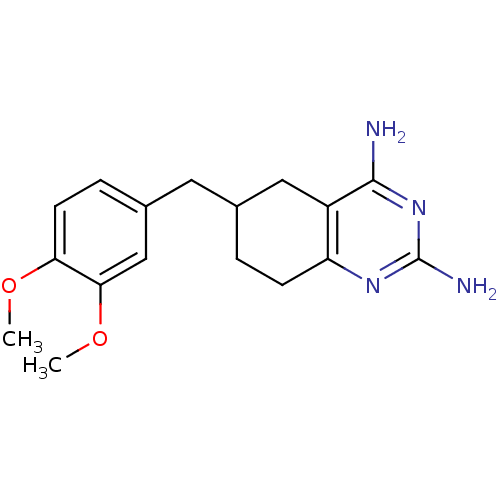 (6-(3,4-Dimethoxy-benzyl)-5,6,7,8-tetrahydro-quinaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074721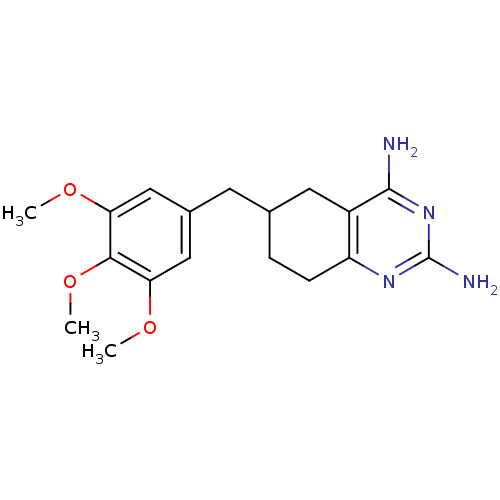 (6-(3,4,5-Trimethoxy-benzyl)-5,6,7,8-tetrahydro-qui...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50082923 (6-(9H-Acridin-10-ylmethyl)-pteridine-2,4-diamine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Rat liver | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mycobacterium avium) | BDBM50082922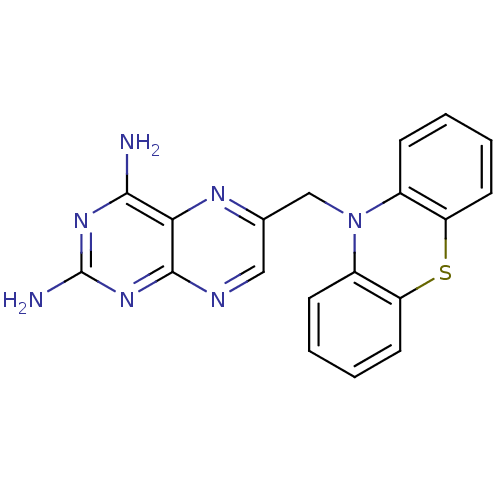 (6-Phenothiazin-10-ylmethyl-pteridine-2,4-diamine |...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Mycobacterium avium | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50082923 (6-(9H-Acridin-10-ylmethyl)-pteridine-2,4-diamine |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Toxoplasma gondii. | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound was tested for inhibition activity against pneumocystis carinii (P. carinii) Dihydrofolate reductase | J Med Chem 41: 913-8 (1998) Article DOI: 10.1021/jm970614n BindingDB Entry DOI: 10.7270/Q2BR8R9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM50027970 (5-(4-Bromo-3,5-dimethoxy-benzyl)-pyrimidine-2,4-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of P. carinii Dihydrofolate Reductase | J Med Chem 40: 3694-9 (1997) Article DOI: 10.1021/jm970399a BindingDB Entry DOI: 10.7270/Q2NG4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM18224 (6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit P. carinii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074723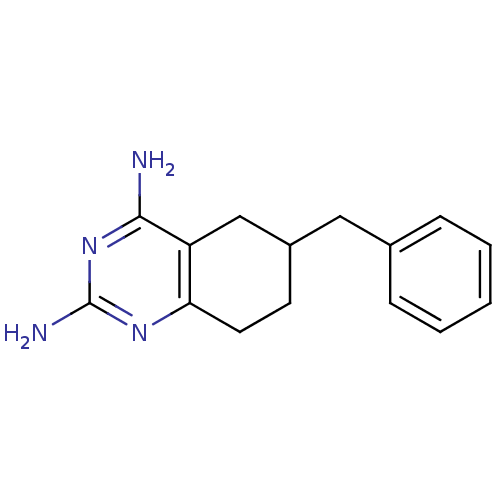 (6-Benzyl-5,6,7,8-tetrahydro-quinazoline-2,4-diamin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50074720 (6-(2,5-Dimethoxy-benzyl)-5,6,7,8-tetrahydro-quinaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit rat liver Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074722 (6-(3-Methyl-benzyl)-5,6,7,8-tetrahydro-quinazoline...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50074721 (6-(3,4,5-Trimethoxy-benzyl)-5,6,7,8-tetrahydro-qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit rat liver Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074732 (6-(Tetrahydro-furan-2-ylmethyl)-5,6,7,8-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM50029763 (5-(3,5-Diethoxy-4-pyrrol-1-yl-benzyl)-pyrimidine-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of P. carinii Dihydrofolate Reductase | J Med Chem 40: 3694-9 (1997) Article DOI: 10.1021/jm970399a BindingDB Entry DOI: 10.7270/Q2NG4PQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM50082923 (6-(9H-Acridin-10-ylmethyl)-pteridine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Pneumocystis carinii. | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Pneumocystis carinii. | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Compound was tested for inhibition activity against pneumocystis carinii (P. carinii) Dihydrofolate reductase | J Med Chem 41: 913-8 (1998) Article DOI: 10.1021/jm970614n BindingDB Entry DOI: 10.7270/Q2BR8R9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM18506 (6-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Toxoplasma gondii. | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074728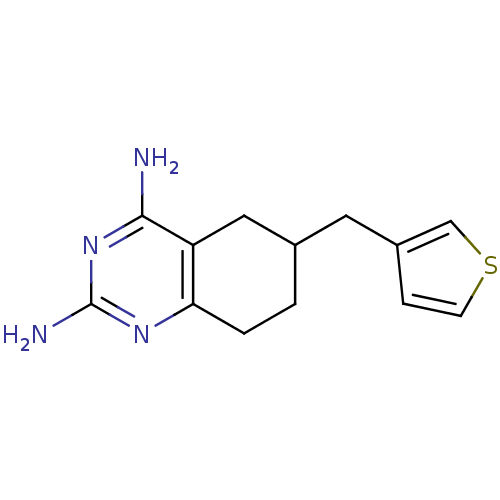 (6-(thiophen-3-ylmethyl)-5,6,7,8-tetrahydroquinazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074734 (6-(4-Methoxy-benzyl)-5,6,7,8-tetrahydro-quinazolin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50033539 (6-[(3,4,5-Trimethoxy-phenylamino)-methyl]-5,6,7,8-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50082920 (6-Carbazol-9-ylmethyl-pteridine-2,4-diamine | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity of the compound against DHFR (Dihydrofolate reductase) from Rat liver | J Med Chem 42: 4853-60 (1999) BindingDB Entry DOI: 10.7270/Q2S46R5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Pneumocystis carinii) | BDBM50074720 (6-(2,5-Dimethoxy-benzyl)-5,6,7,8-tetrahydro-quinaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit P. carinii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074737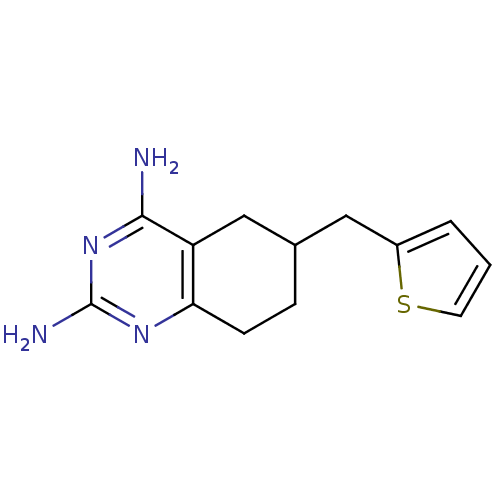 (6-Thiophen-2-ylmethyl-5,6,7,8-tetrahydro-quinazoli...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50074719 (6-(3,4-Dimethoxy-benzyl)-5,6,7,8-tetrahydro-quinaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit rat liver Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50074725 (6-tert-Butyl-5,6,7,8-tetrahydro-quinazoline-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit rat liver Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Toxoplasma gondii) | BDBM50074726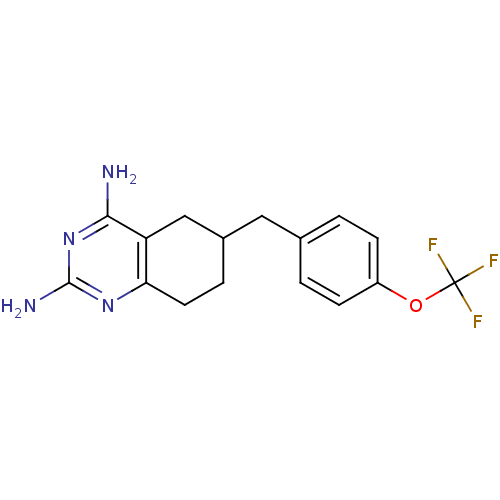 (6-(4-(trifluoromethoxy)benzyl)-5,6,7,8-tetrahydroq...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit T. gondii Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50074734 (6-(4-Methoxy-benzyl)-5,6,7,8-tetrahydro-quinazolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit rat liver Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Rattus norvegicus (rat)) | BDBM50074735 (6-(3-(trifluoromethoxy)benzyl)-5,6,7,8-tetrahydroq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description The ability of the compound to inhibit rat liver Dihydrofolate reductase was tested | J Med Chem 42: 1007-17 (1999) Article DOI: 10.1021/jm980572i BindingDB Entry DOI: 10.7270/Q2513XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 157 total ) | Next | Last >> |