 Found 17 hits with Last Name = 'partridge' and Initial = 'jr'
Found 17 hits with Last Name = 'partridge' and Initial = 'jr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM151585

(US11739089, Compound Ketoconazole | US8987315, Ket...)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OCC2COC(Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using in human liver microsomes using testosterone as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM151585

(US11739089, Compound Ketoconazole | US8987315, Ket...)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OCC2COC(Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using in human liver microsomes using midazolam as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677
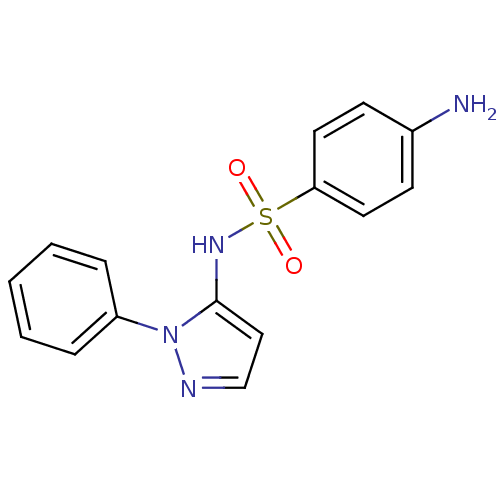
(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50236897
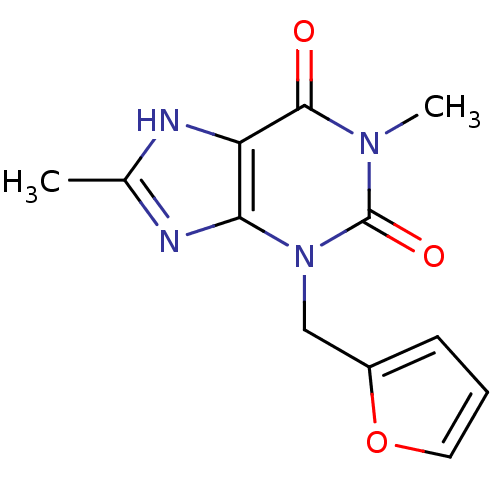
(3-(furan-2-ylmethyl)-1,8-dimethyl-1H-purine-2,6(3H...)Show InChI InChI=1S/C12H12N4O3/c1-7-13-9-10(14-7)16(6-8-4-3-5-19-8)12(18)15(2)11(9)17/h3-5H,6H2,1-2H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50235297

(CHEMBL4101807)Show InChI InChI=1S/C19H19N3O3/c1-13(2)22-16(8-10-21-22)19-14(5-4-9-20-19)12-25-18-7-3-6-17(24)15(18)11-23/h3-11,13,24H,12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50235297

(CHEMBL4101807)Show InChI InChI=1S/C19H19N3O3/c1-13(2)22-16(8-10-21-22)19-14(5-4-9-20-19)12-25-18-7-3-6-17(24)15(18)11-23/h3-11,13,24H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50240772

((1R,2S)-(-)-2-phenylcyclopropylamine | (1R,2S)-2-p...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using S-Mephentoin as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50235297

(CHEMBL4101807)Show InChI InChI=1S/C19H19N3O3/c1-13(2)22-16(8-10-21-22)19-14(5-4-9-20-19)12-25-18-7-3-6-17(24)15(18)11-23/h3-11,13,24H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using in human liver microsomes using testosterone as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50235297

(CHEMBL4101807)Show InChI InChI=1S/C19H19N3O3/c1-13(2)22-16(8-10-21-22)19-14(5-4-9-20-19)12-25-18-7-3-6-17(24)15(18)11-23/h3-11,13,24H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes using S-Mephentoin as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50235297

(CHEMBL4101807)Show InChI InChI=1S/C19H19N3O3/c1-13(2)22-16(8-10-21-22)19-14(5-4-9-20-19)12-25-18-7-3-6-17(24)15(18)11-23/h3-11,13,24H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50235297

(CHEMBL4101807)Show InChI InChI=1S/C19H19N3O3/c1-13(2)22-16(8-10-21-22)19-14(5-4-9-20-19)12-25-18-7-3-6-17(24)15(18)11-23/h3-11,13,24H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 using in human liver microsomes using midazolam as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50235297

(CHEMBL4101807)Show InChI InChI=1S/C19H19N3O3/c1-13(2)22-16(8-10-21-22)19-14(5-4-9-20-19)12-25-18-7-3-6-17(24)15(18)11-23/h3-11,13,24H,12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Global Blood Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate after 5 to 15 mins |
ACS Med Chem Lett 8: 321-326 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00491
BindingDB Entry DOI: 10.7270/Q21J9D2Q |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50358580
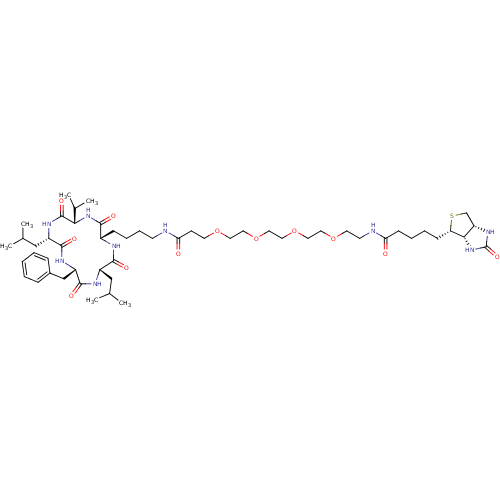
(CHEMBL1923743)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CCCCNC(=O)CCOCCOCCOCCOCCNC(=O)CCCC[C@@H]2SC[C@@H]3NC(=O)N[C@H]23)NC1=O)C(C)C |r| Show InChI InChI=1S/C53H87N9O12S/c1-34(2)30-39-49(66)56-38(48(65)61-46(36(5)6)52(69)59-40(31-35(3)4)50(67)58-41(51(68)57-39)32-37-14-8-7-9-15-37)16-12-13-20-54-45(64)19-22-71-24-26-73-28-29-74-27-25-72-23-21-55-44(63)18-11-10-17-43-47-42(33-75-43)60-53(70)62-47/h7-9,14-15,34-36,38-43,46-47H,10-13,16-33H2,1-6H3,(H,54,64)(H,55,63)(H,56,66)(H,57,68)(H,58,67)(H,59,69)(H,61,65)(H2,60,62,70)/t38-,39-,40-,41-,42-,43-,46-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Binding affinity to Hsp90 after 1 hrs by Western blotting |
Bioorg Med Chem Lett 21: 7068-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.096
BindingDB Entry DOI: 10.7270/Q22R3S3T |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50358579
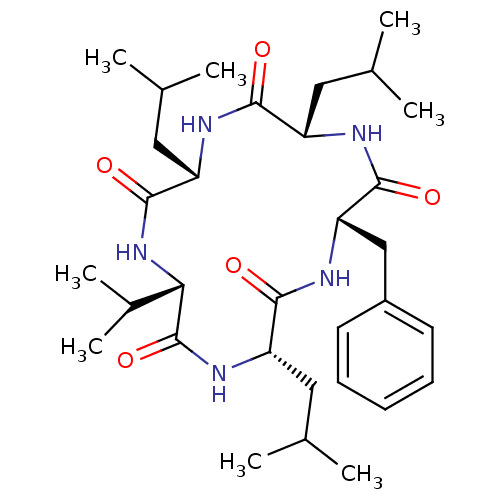
(CHEMBL192264)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC1=O)C(C)C |r| Show InChI InChI=1S/C32H51N5O5/c1-18(2)14-23-28(38)34-25(16-20(5)6)31(41)37-27(21(7)8)32(42)36-24(15-19(3)4)29(39)35-26(30(40)33-23)17-22-12-10-9-11-13-22/h9-13,18-21,23-27H,14-17H2,1-8H3,(H,33,40)(H,34,38)(H,35,39)(H,36,42)(H,37,41)/t23-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Binding affinity to human Hsp90 closed state by fluorescence polarization anisotropy |
Bioorg Med Chem Lett 21: 7068-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.096
BindingDB Entry DOI: 10.7270/Q22R3S3T |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM20732
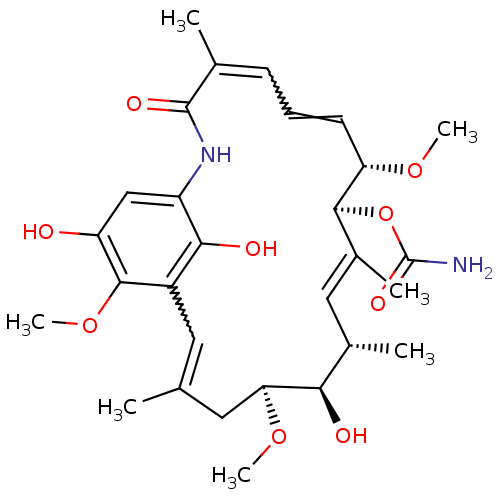
((4E,6Z,8S,9S,10E,12S,13R,14S,16R)-13-hydroxy-8,14,...)Show SMILES CO[C@H]1CC(C)=Cc2c(O)c(NC(=O)C(C)=CC=C[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@H]1O)cc(O)c2OC |r,w:16.16,6.6,t:28| Show InChI InChI=1S/C29H40N2O9/c1-15-11-19-25(34)20(14-21(32)27(19)39-7)31-28(35)16(2)9-8-10-22(37-5)26(40-29(30)36)18(4)13-17(3)24(33)23(12-15)38-6/h8-11,13-14,17,22-24,26,32-34H,12H2,1-7H3,(H2,30,36)(H,31,35)/b10-8?,15-11?,16-9?,18-13+/t17-,22-,23-,24+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a |
San Diego State University
Curated by ChEMBL
| Assay Description
Binding affinity to Hsp90 N-domain |
Bioorg Med Chem Lett 21: 7068-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.096
BindingDB Entry DOI: 10.7270/Q22R3S3T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data