 Found 278 hits with Last Name = 'pechulis' and Initial = 'ad'
Found 278 hits with Last Name = 'pechulis' and Initial = 'ad' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50290071
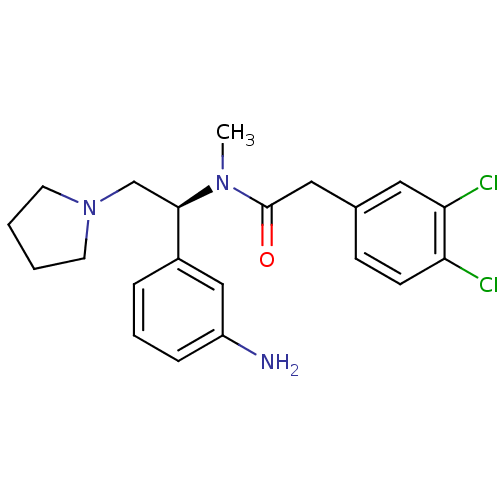
(CHEMBL542149 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(N)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H25Cl2N3O/c1-25(21(27)12-15-7-8-18(22)19(23)11-15)20(14-26-9-2-3-10-26)16-5-4-6-17(24)13-16/h4-8,11,13,20H,2-3,9-10,12,14,24H2,1H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0592 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50290076
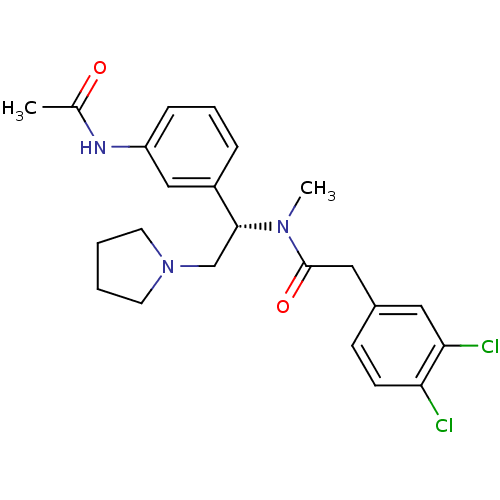
(CHEMBL558596 | N-[(S)-1-(3-Acetylamino-phenyl)-2-p...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(C)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C23H27Cl2N3O2/c1-16(29)26-19-7-5-6-18(14-19)22(15-28-10-3-4-11-28)27(2)23(30)13-17-8-9-20(24)21(25)12-17/h5-9,12,14,22H,3-4,10-11,13,15H2,1-2H3,(H,26,29)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0855 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50290072

(5-{3-[3-((S)-1-{[2-(3,4-Dichloro-phenyl)-acetyl]-m...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |wU:2.1,(9.81,-19.13,;9.81,-17.59,;11.14,-16.82,;12.48,-17.59,;13.81,-16.82,;13.97,-15.27,;15.48,-14.95,;16.25,-16.27,;15.23,-17.43,;11.14,-15.27,;9.81,-14.53,;9.81,-12.99,;11.14,-12.21,;12.48,-12.97,;13.81,-12.21,;13.81,-10.67,;12.48,-9.9,;15.14,-9.88,;15.14,-8.34,;13.81,-7.6,;13.8,-6.05,;15.14,-5.28,;16.48,-6.05,;16.48,-7.58,;18.02,-6.05,;18.78,-7.37,;18.78,-4.72,;15.14,-3.74,;13.81,-2.97,;12.49,-3.74,;11.16,-3,;11.14,-1.45,;9.81,-.7,;12.48,-.68,;13.8,-1.45,;15.14,-.66,;16.48,-1.43,;17.78,-.66,;19.11,-1.42,;20.43,-.65,;19.12,-2.96,;17.78,-3.72,;16.48,-2.96,;12.48,-14.52,;8.47,-16.82,;8.47,-15.27,;7.14,-17.6,;5.79,-16.83,;5.79,-15.27,;4.47,-14.51,;3.14,-15.3,;1.8,-14.51,;3.14,-16.83,;1.77,-17.6,;4.47,-17.6,)| Show InChI InChI=1S/C42H36Cl2N4O6S/c1-47(39(51)18-24-7-14-34(43)35(44)17-24)36(23-48-15-2-3-16-48)25-5-4-6-26(19-25)45-42(55)46-27-8-11-30(33(20-27)41(52)53)40-31-12-9-28(49)21-37(31)54-38-22-29(50)10-13-32(38)40/h4-14,17,19-22,36,49H,2-3,15-16,18,23H2,1H3,(H,52,53)(H2,45,46,55)/t36-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.898 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50290072

(5-{3-[3-((S)-1-{[2-(3,4-Dichloro-phenyl)-acetyl]-m...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |wU:2.1,(9.81,-19.13,;9.81,-17.59,;11.14,-16.82,;12.48,-17.59,;13.81,-16.82,;13.97,-15.27,;15.48,-14.95,;16.25,-16.27,;15.23,-17.43,;11.14,-15.27,;9.81,-14.53,;9.81,-12.99,;11.14,-12.21,;12.48,-12.97,;13.81,-12.21,;13.81,-10.67,;12.48,-9.9,;15.14,-9.88,;15.14,-8.34,;13.81,-7.6,;13.8,-6.05,;15.14,-5.28,;16.48,-6.05,;16.48,-7.58,;18.02,-6.05,;18.78,-7.37,;18.78,-4.72,;15.14,-3.74,;13.81,-2.97,;12.49,-3.74,;11.16,-3,;11.14,-1.45,;9.81,-.7,;12.48,-.68,;13.8,-1.45,;15.14,-.66,;16.48,-1.43,;17.78,-.66,;19.11,-1.42,;20.43,-.65,;19.12,-2.96,;17.78,-3.72,;16.48,-2.96,;12.48,-14.52,;8.47,-16.82,;8.47,-15.27,;7.14,-17.6,;5.79,-16.83,;5.79,-15.27,;4.47,-14.51,;3.14,-15.3,;1.8,-14.51,;3.14,-16.83,;1.77,-17.6,;4.47,-17.6,)| Show InChI InChI=1S/C42H36Cl2N4O6S/c1-47(39(51)18-24-7-14-34(43)35(44)17-24)36(23-48-15-2-3-16-48)25-5-4-6-26(19-25)45-42(55)46-27-8-11-30(33(20-27)41(52)53)40-31-12-9-28(49)21-37(31)54-38-22-29(50)10-13-32(38)40/h4-14,17,19-22,36,49H,2-3,15-16,18,23H2,1H3,(H,52,53)(H2,45,46,55)/t36-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50290070

(5-{3-[3-(1-{[2-(3,4-Dichloro-phenyl)-acetyl]-methy...)Show SMILES CN(C(CN1CCCC1)c1cccc(NC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |(8.59,-17.5,;8.59,-15.95,;9.92,-15.18,;11.25,-15.95,;12.59,-15.17,;12.75,-13.63,;14.26,-13.31,;15.03,-14.64,;14.01,-15.79,;9.92,-13.64,;8.59,-12.88,;8.58,-11.34,;9.92,-10.55,;11.25,-11.32,;12.59,-10.55,;12.59,-9.01,;11.25,-8.24,;13.92,-8.24,;13.92,-6.69,;12.59,-5.94,;12.58,-4.4,;13.92,-3.62,;15.25,-4.4,;15.25,-5.92,;16.8,-4.4,;17.57,-5.73,;17.57,-3.05,;13.92,-2.08,;12.59,-1.32,;11.27,-2.09,;9.94,-1.34,;9.92,.2,;8.59,.97,;11.25,.97,;12.58,.21,;13.91,1.01,;15.25,.23,;16.58,1,;17.89,.25,;19.23,1.02,;17.91,-1.29,;16.58,-2.07,;15.25,-1.29,;11.25,-12.87,;7.25,-15.18,;7.25,-13.64,;5.92,-15.95,;4.57,-15.18,;4.57,-13.64,;3.23,-12.87,;1.9,-13.64,;.57,-12.87,;1.9,-15.18,;.56,-15.95,;3.23,-15.95,)| Show InChI InChI=1S/C42H36Cl2N4O6S/c1-47(39(51)18-24-7-14-34(43)35(44)17-24)36(23-48-15-2-3-16-48)25-5-4-6-26(19-25)45-42(55)46-27-8-11-30(33(20-27)41(52)53)40-31-12-9-28(49)21-37(31)54-38-22-29(50)10-13-32(38)40/h4-14,17,19-22,36,49H,2-3,15-16,18,23H2,1H3,(H,52,53)(H2,45,46,55) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of rat NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401743
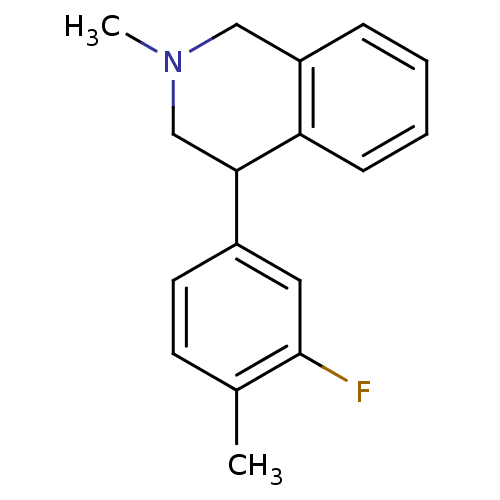
(CHEMBL2206526)Show InChI InChI=1S/C17H18FN/c1-12-7-8-13(9-17(12)18)16-11-19(2)10-14-5-3-4-6-15(14)16/h3-9,16H,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401732
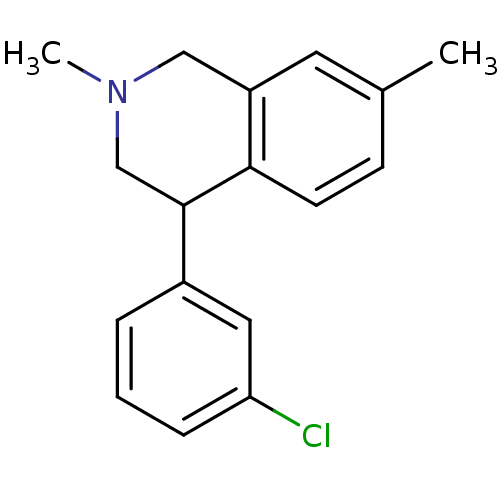
(CHEMBL2206507)Show InChI InChI=1S/C17H18ClN/c1-12-6-7-16-14(8-12)10-19(2)11-17(16)13-4-3-5-15(18)9-13/h3-9,17H,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401731
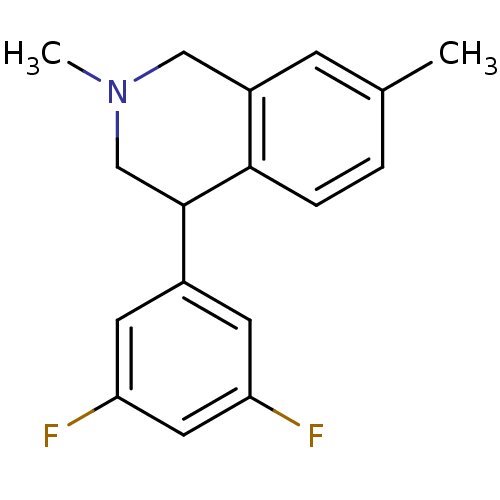
(CHEMBL2206508)Show InChI InChI=1S/C17H17F2N/c1-11-3-4-16-13(5-11)9-20(2)10-17(16)12-6-14(18)8-15(19)7-12/h3-8,17H,9-10H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 5.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50401730

(CHEMBL2206509)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3/t17-/m0/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of rat NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401730

(CHEMBL2206509)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401744
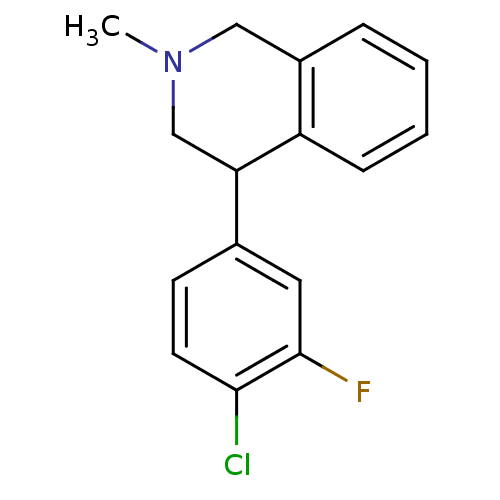
(CHEMBL2206525)Show InChI InChI=1S/C16H15ClFN/c1-19-9-12-4-2-3-5-13(12)14(10-19)11-6-7-15(17)16(18)8-11/h2-8,14H,9-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401737

(CHEMBL2206532)Show InChI InChI=1S/C18H21NO/c1-13-5-4-6-14(9-13)18-12-19(2)11-15-10-16(20-3)7-8-17(15)18/h4-10,18H,11-12H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50054539
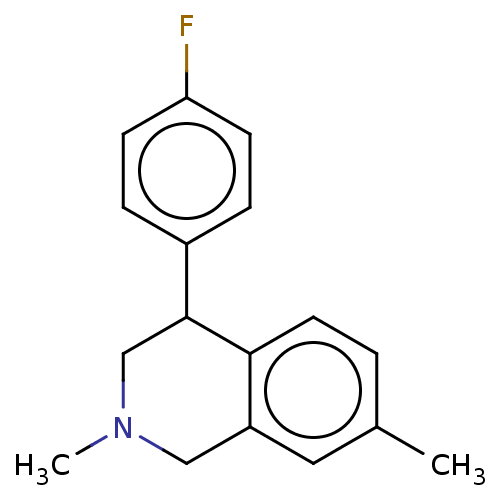
(CHEMBL3323088)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human NET expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50290071

(CHEMBL542149 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(N)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H25Cl2N3O/c1-25(21(27)12-15-7-8-18(22)19(23)11-15)20(14-26-9-2-3-10-26)16-5-4-6-17(24)13-16/h4-8,11,13,20H,2-3,9-10,12,14,24H2,1H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor mu 1 binding to guinea pig brain membranes, using [3H]- DAMGO as radioligand |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50290071

(CHEMBL542149 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(N)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H25Cl2N3O/c1-25(21(27)12-15-7-8-18(22)19(23)11-15)20(14-26-9-2-3-10-26)16-5-4-6-17(24)13-16/h4-8,11,13,20H,2-3,9-10,12,14,24H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50290070

(5-{3-[3-(1-{[2-(3,4-Dichloro-phenyl)-acetyl]-methy...)Show SMILES CN(C(CN1CCCC1)c1cccc(NC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |(8.59,-17.5,;8.59,-15.95,;9.92,-15.18,;11.25,-15.95,;12.59,-15.17,;12.75,-13.63,;14.26,-13.31,;15.03,-14.64,;14.01,-15.79,;9.92,-13.64,;8.59,-12.88,;8.58,-11.34,;9.92,-10.55,;11.25,-11.32,;12.59,-10.55,;12.59,-9.01,;11.25,-8.24,;13.92,-8.24,;13.92,-6.69,;12.59,-5.94,;12.58,-4.4,;13.92,-3.62,;15.25,-4.4,;15.25,-5.92,;16.8,-4.4,;17.57,-5.73,;17.57,-3.05,;13.92,-2.08,;12.59,-1.32,;11.27,-2.09,;9.94,-1.34,;9.92,.2,;8.59,.97,;11.25,.97,;12.58,.21,;13.91,1.01,;15.25,.23,;16.58,1,;17.89,.25,;19.23,1.02,;17.91,-1.29,;16.58,-2.07,;15.25,-1.29,;11.25,-12.87,;7.25,-15.18,;7.25,-13.64,;5.92,-15.95,;4.57,-15.18,;4.57,-13.64,;3.23,-12.87,;1.9,-13.64,;.57,-12.87,;1.9,-15.18,;.56,-15.95,;3.23,-15.95,)| Show InChI InChI=1S/C42H36Cl2N4O6S/c1-47(39(51)18-24-7-14-34(43)35(44)17-24)36(23-48-15-2-3-16-48)25-5-4-6-26(19-25)45-42(55)46-27-8-11-30(33(20-27)41(52)53)40-31-12-9-28(49)21-37(31)54-38-22-29(50)10-13-32(38)40/h4-14,17,19-22,36,49H,2-3,15-16,18,23H2,1H3,(H,52,53)(H2,45,46,55) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50290076

(CHEMBL558596 | N-[(S)-1-(3-Acetylamino-phenyl)-2-p...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(C)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C23H27Cl2N3O2/c1-16(29)26-19-7-5-6-18(14-19)22(15-28-10-3-4-11-28)27(2)23(30)13-17-8-9-20(24)21(25)12-17/h5-9,12,14,22H,3-4,10-11,13,15H2,1-2H3,(H,26,29)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor mu 1 binding to guinea pig brain membranes, using [3H]- DAMGO as radioligand |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401738
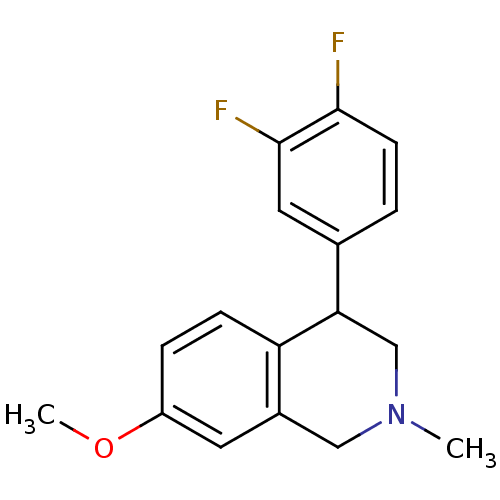
(CHEMBL2206531)Show InChI InChI=1S/C17H17F2NO/c1-20-9-12-7-13(21-2)4-5-14(12)15(10-20)11-3-6-16(18)17(19)8-11/h3-8,15H,9-10H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50401740

(CHEMBL2206529)Show InChI InChI=1S/C18H21N/c1-13-7-9-15(10-8-13)18-12-19(3)11-17-14(2)5-4-6-16(17)18/h4-10,18H,11-12H2,1-3H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401716
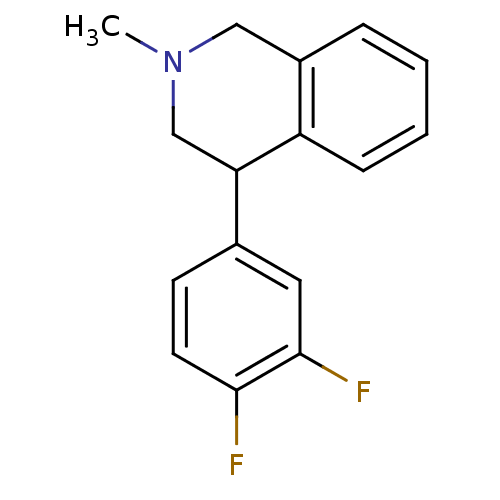
(CHEMBL2206524)Show InChI InChI=1S/C16H15F2N/c1-19-9-12-4-2-3-5-13(12)14(10-19)11-6-7-15(17)16(18)8-11/h2-8,14H,9-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401735
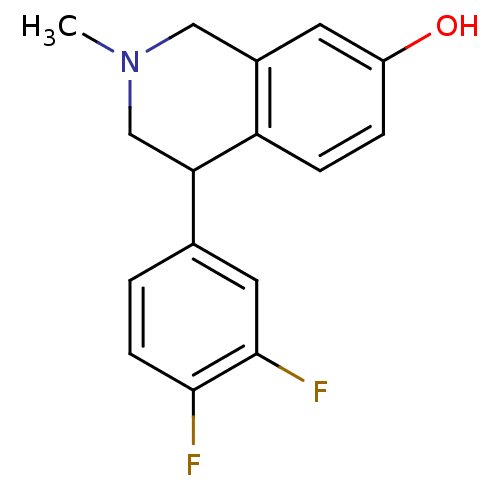
(CHEMBL2206534)Show InChI InChI=1S/C16H15F2NO/c1-19-8-11-6-12(20)3-4-13(11)14(9-19)10-2-5-15(17)16(18)7-10/h2-7,14,20H,8-9H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401742

(CHEMBL2206527)Show InChI InChI=1S/C17H18FN/c1-12-9-13(7-8-17(12)18)16-11-19(2)10-14-5-3-4-6-15(14)16/h3-9,16H,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401733
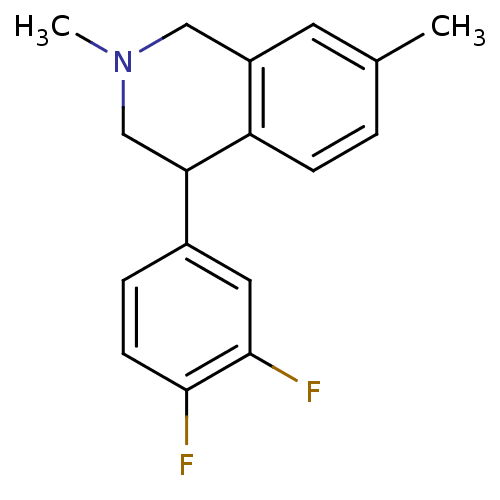
(CHEMBL2206536)Show InChI InChI=1S/C17H17F2N/c1-11-3-5-14-13(7-11)9-20(2)10-15(14)12-4-6-16(18)17(19)8-12/h3-8,15H,9-10H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50290076

(CHEMBL558596 | N-[(S)-1-(3-Acetylamino-phenyl)-2-p...)Show SMILES CN([C@H](CN1CCCC1)c1cccc(NC(C)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C23H27Cl2N3O2/c1-16(29)26-19-7-5-6-18(14-19)22(15-28-10-3-4-11-28)27(2)23(30)13-17-8-9-20(24)21(25)12-17/h5-9,12,14,22H,3-4,10-11,13,15H2,1-2H3,(H,26,29)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor delta 1 binding to guinea pig brain membranes, using 0.2 nM [3H]-naltrindole as radiolig... |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401734

(CHEMBL2206535)Show InChI InChI=1S/C18H21N/c1-13-4-7-15(8-5-13)18-12-19(3)11-16-10-14(2)6-9-17(16)18/h4-10,18H,11-12H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50290074
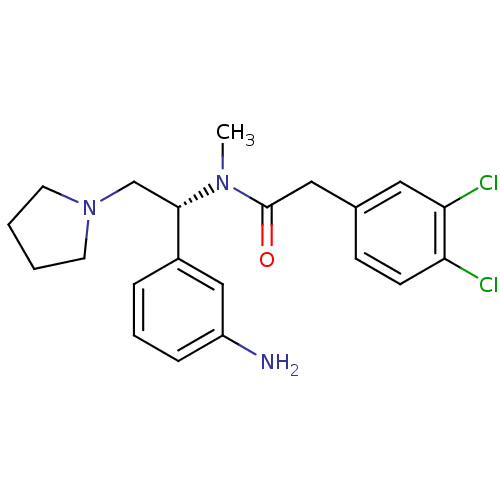
(CHEMBL558597 | N-[(R)-1-(3-Amino-phenyl)-2-pyrroli...)Show SMILES CN([C@@H](CN1CCCC1)c1cccc(N)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C21H25Cl2N3O/c1-25(21(27)12-15-7-8-18(22)19(23)11-15)20(14-26-9-2-3-10-26)16-5-4-6-17(24)13-16/h4-8,11,13,20H,2-3,9-10,12,14,24H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401736
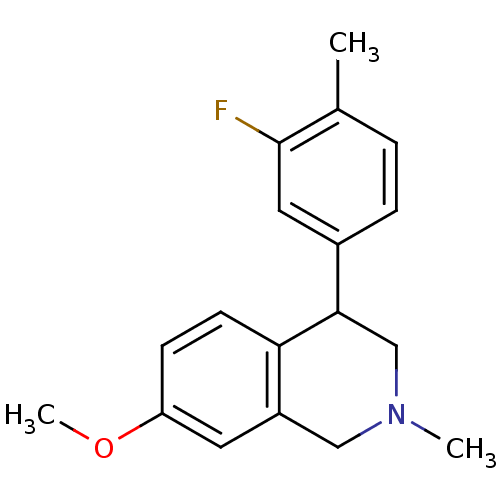
(CHEMBL2206533)Show InChI InChI=1S/C18H20FNO/c1-12-4-5-13(9-18(12)19)17-11-20(2)10-14-8-15(21-3)6-7-16(14)17/h4-9,17H,10-11H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50290075
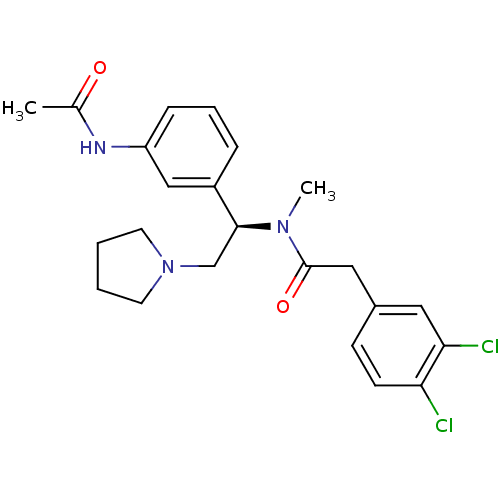
(CHEMBL542403 | N-[(R)-1-(3-Acetylamino-phenyl)-2-p...)Show SMILES CN([C@@H](CN1CCCC1)c1cccc(NC(C)=O)c1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C23H27Cl2N3O2/c1-16(29)26-19-7-5-6-18(14-19)22(15-28-10-3-4-11-28)27(2)23(30)13-17-8-9-20(24)21(25)12-17/h5-9,12,14,22H,3-4,10-11,13,15H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Opioid receptor kappa1 binding to guinea pig brain membranes, using 1 nM [3H]- U69,593 as radioligand |
Bioorg Med Chem Lett 7: 2271-2276 (1997)
Article DOI: 10.1016/S0960-894X(97)00406-X
BindingDB Entry DOI: 10.7270/Q2GB242Q |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401740

(CHEMBL2206529)Show InChI InChI=1S/C18H21N/c1-13-7-9-15(10-8-13)18-12-19(3)11-17-14(2)5-4-6-16(17)18/h4-10,18H,11-12H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50005548
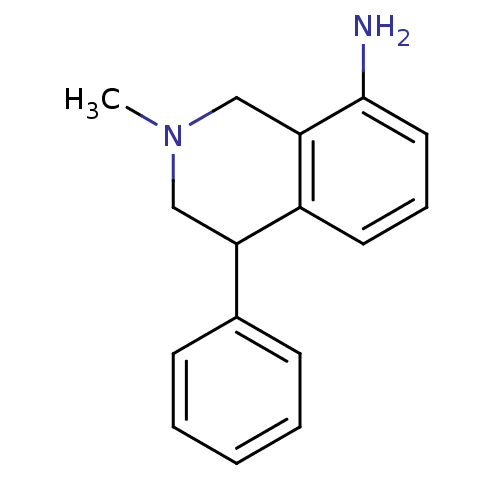
((+)-2-Methyl-4-phenyl-1,2,3,4-tetrahydro-isoquinol...)Show InChI InChI=1S/C16H18N2/c1-18-10-14(12-6-3-2-4-7-12)13-8-5-9-16(17)15(13)11-18/h2-9,14H,10-11,17H2,1H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of rat NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50401736

(CHEMBL2206533)Show InChI InChI=1S/C18H20FNO/c1-12-4-5-13(9-18(12)19)17-11-20(2)10-14-8-15(21-3)6-7-16(14)17/h4-9,17H,10-11H2,1-3H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50005548

((+)-2-Methyl-4-phenyl-1,2,3,4-tetrahydro-isoquinol...)Show InChI InChI=1S/C16H18N2/c1-18-10-14(12-6-3-2-4-7-12)13-8-5-9-16(17)15(13)11-18/h2-9,14H,10-11,17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50401717

(CHEMBL2206523)Show InChI InChI=1S/C16H16ClN/c1-18-10-13-4-2-3-5-15(13)16(11-18)12-6-8-14(17)9-7-12/h2-9,16H,10-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of SERT |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50401737

(CHEMBL2206532)Show InChI InChI=1S/C18H21NO/c1-13-5-4-6-14(9-13)18-12-19(2)11-15-10-16(20-3)7-8-17(15)18/h4-10,18H,11-12H2,1-3H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50401734

(CHEMBL2206535)Show InChI InChI=1S/C18H21N/c1-13-4-7-15(8-5-13)18-12-19(3)11-16-10-14(2)6-9-17(16)18/h4-10,18H,11-12H2,1-3H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401717

(CHEMBL2206523)Show InChI InChI=1S/C16H16ClN/c1-18-10-13-4-2-3-5-15(13)16(11-18)12-6-8-14(17)9-7-12/h2-9,16H,10-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401739
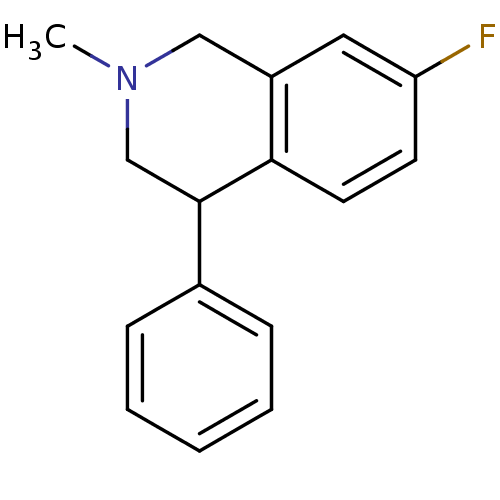
(CHEMBL2206530)Show InChI InChI=1S/C16H16FN/c1-18-10-13-9-14(17)7-8-15(13)16(11-18)12-5-3-2-4-6-12/h2-9,16H,10-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50401743

(CHEMBL2206526)Show InChI InChI=1S/C17H18FN/c1-12-7-8-13(9-17(12)18)16-11-19(2)10-14-5-3-4-6-15(14)16/h3-9,16H,10-11H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401721
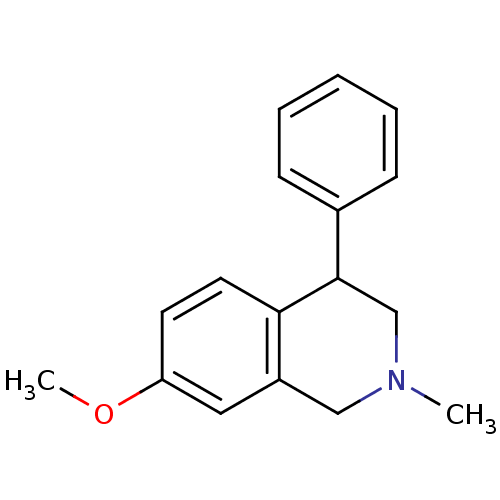
(CHEMBL2206518)Show InChI InChI=1S/C17H19NO/c1-18-11-14-10-15(19-2)8-9-16(14)17(12-18)13-6-4-3-5-7-13/h3-10,17H,11-12H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50062912
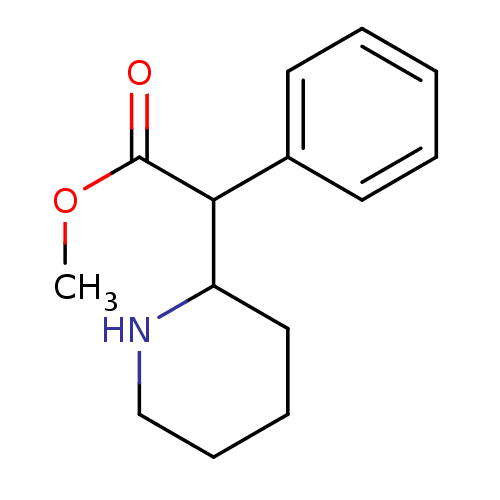
(CHEMBL796 | Methylphenidate | alpha-phenyl-2-piper...)Show InChI InChI=1S/C14H19NO2/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401723
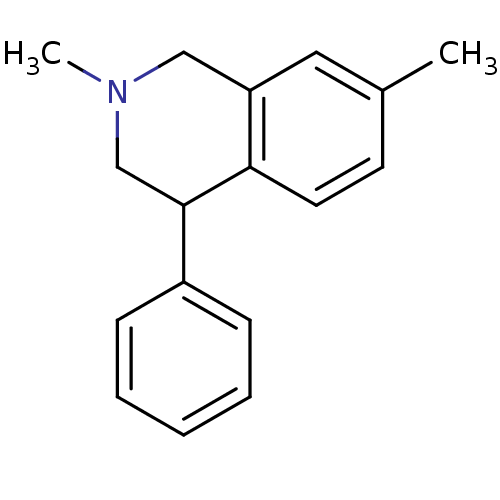
(CHEMBL2206516)Show InChI InChI=1S/C17H19N/c1-13-8-9-16-15(10-13)11-18(2)12-17(16)14-6-4-3-5-7-14/h3-10,17H,11-12H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401728
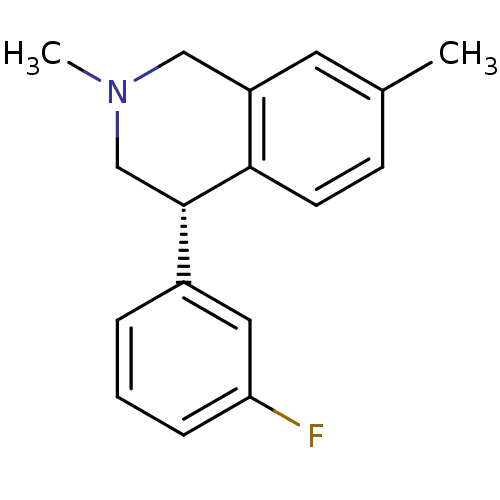
(CHEMBL2206511)Show InChI InChI=1S/C17H18FN/c1-12-6-7-16-14(8-12)10-19(2)11-17(16)13-4-3-5-15(18)9-13/h3-9,17H,10-11H2,1-2H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50401732

(CHEMBL2206507)Show InChI InChI=1S/C17H18ClN/c1-12-6-7-16-14(8-12)10-19(2)11-17(16)13-4-3-5-15(18)9-13/h3-9,17H,10-11H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50054539

(CHEMBL3323088)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN 35,428 from human DAT expressed in HEK293E cells after 1 hr by liquid scintillation counting |
ACS Med Chem Lett 5: 760-5 (2014)
Article DOI: 10.1021/ml500053b
BindingDB Entry DOI: 10.7270/Q2154JPR |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50401730

(CHEMBL2206509)Show InChI InChI=1S/C17H18FN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3/t17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50401741
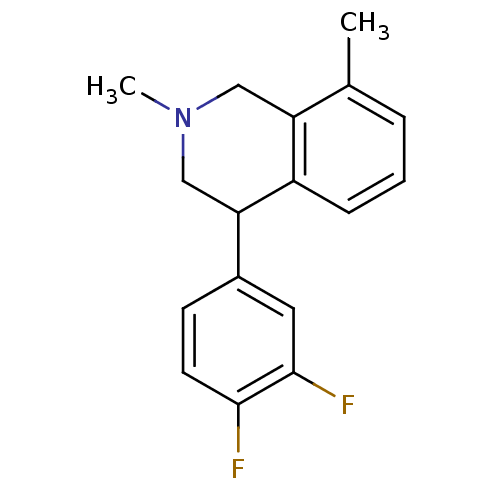
(CHEMBL2206528)Show InChI InChI=1S/C17H17F2N/c1-11-4-3-5-13-14(11)9-20(2)10-15(13)12-6-7-16(18)17(19)8-12/h3-8,15H,9-10H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of human NET |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50401717

(CHEMBL2206523)Show InChI InChI=1S/C16H16ClN/c1-18-10-13-4-2-3-5-15(13)16(11-18)12-6-8-14(17)9-7-12/h2-9,16H,10-11H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50401729
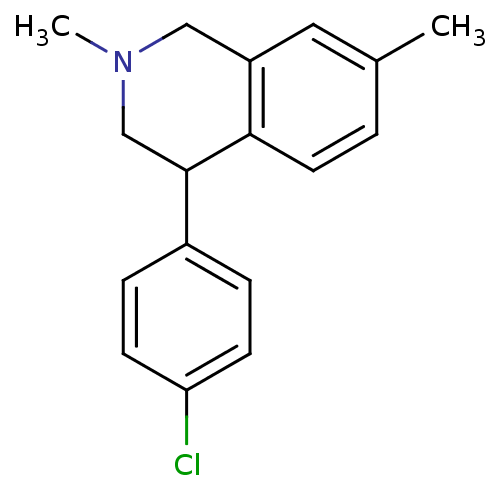
(CHEMBL2206510)Show InChI InChI=1S/C17H18ClN/c1-12-3-8-16-14(9-12)10-19(2)11-17(16)13-4-6-15(18)7-5-13/h3-9,17H,10-11H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Inhibition of DAT |
Bioorg Med Chem Lett 22: 7219-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.050
BindingDB Entry DOI: 10.7270/Q20K29QC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data