 Found 73 hits with Last Name = 'peet' and Initial = 'c'
Found 73 hits with Last Name = 'peet' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259611
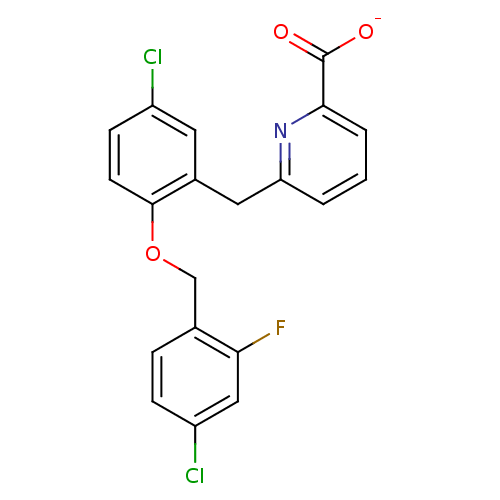
(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as inhibition of PGE2-mediated intracellular calcium mobilizati... |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50259611

(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of TP receptor |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259613

(CHEMBL467720 | sodium 6-(5-chloro-2-(2,4-dichlorob...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2Cl)n1 Show InChI InChI=1S/C20H14Cl3NO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as inhibition of PGE2-mediated intracellular calcium mobilizati... |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259612
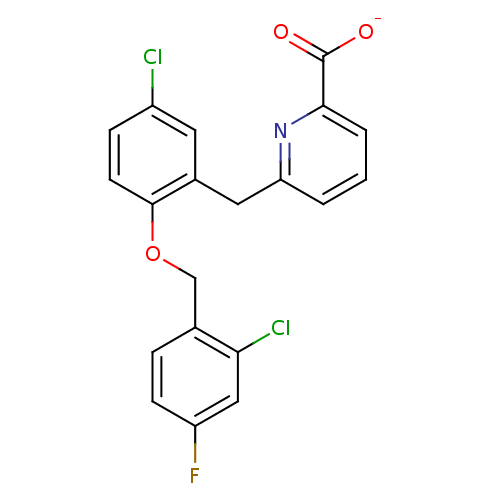
(CHEMBL513491 | sodium 6-(5-chloro-2-(2-chloro-4-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(F)cc2Cl)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-5-7-19(27-11-12-4-6-15(23)10-17(12)22)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as inhibition of PGE2-mediated intracellular calcium mobilizati... |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414022

(CHEMBL2110364 | GSK-180100B)Show SMILES Cc1cc(nn1Cc1cc(Br)ccc1OCc1ccc(Cl)cc1Cl)C([O-])=O Show InChI InChI=1S/C19H15BrCl2N2O3/c1-11-6-17(19(25)26)23-24(11)9-13-7-14(20)3-5-18(13)27-10-12-2-4-15(21)8-16(12)22/h2-8H,9-10H2,1H3,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50197898

(6-(2-(2-(2,4-difluorobenzyloxy)-5-chlorophenyl)cyc...)Show SMILES OC(=O)c1cccc(n1)C1=C(CCC1)c1cc(Cl)ccc1OCc1ccc(F)cc1F |t:10| Show InChI InChI=1S/C24H18ClF2NO3/c25-15-8-10-23(31-13-14-7-9-16(26)12-20(14)27)19(11-15)17-3-1-4-18(17)21-5-2-6-22(28-21)24(29)30/h2,5-12H,1,3-4,13H2,(H,29,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50121279
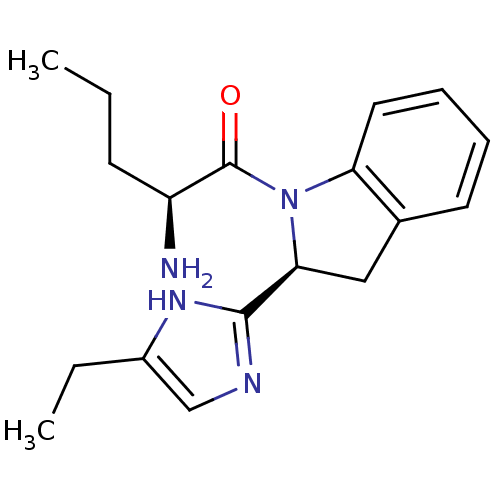
(2-Amino-1-[2-(5-ethyl-1H-imidazol-2-yl)-2,3-dihydr...)Show SMILES CCC[C@H](N)C(=O)N1[C@@H](Cc2ccccc12)c1ncc(CC)[nH]1 Show InChI InChI=1S/C18H24N4O/c1-3-7-14(19)18(23)22-15-9-6-5-8-12(15)10-16(22)17-20-11-13(4-2)21-17/h5-6,8-9,11,14,16H,3-4,7,10,19H2,1-2H3,(H,20,21)/t14-,16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liver |
J Med Chem 45: 5303-10 (2002)
BindingDB Entry DOI: 10.7270/Q2736Q7X |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50121284
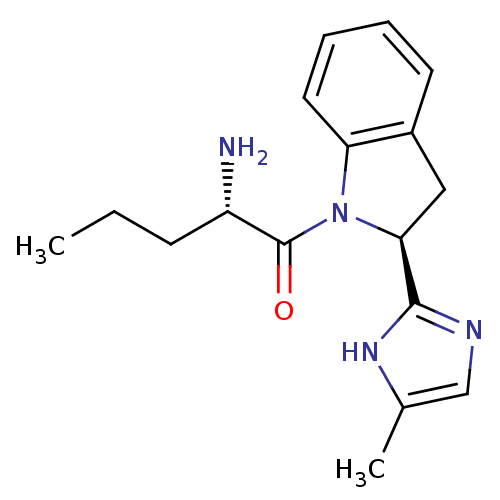
(2-Amino-1-[2-(5-methyl-1H-imidazol-2-yl)-2,3-dihyd...)Show SMILES CCC[C@H](N)C(=O)N1[C@@H](Cc2ccccc12)c1ncc(C)[nH]1 Show InChI InChI=1S/C17H22N4O/c1-3-6-13(18)17(22)21-14-8-5-4-7-12(14)9-15(21)16-19-10-11(2)20-16/h4-5,7-8,10,13,15H,3,6,9,18H2,1-2H3,(H,19,20)/t13-,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liver |
J Med Chem 45: 5303-10 (2002)
BindingDB Entry DOI: 10.7270/Q2736Q7X |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50121282
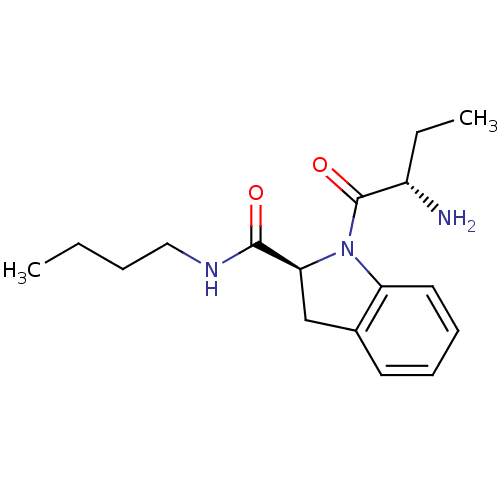
((S)-1-((S)-2-aminobutanoyl)-N-butylindoline-2-carb...)Show SMILES CCCCNC(=O)[C@@H]1Cc2ccccc2N1C(=O)[C@@H](N)CC Show InChI InChI=1S/C17H25N3O2/c1-3-5-10-19-16(21)15-11-12-8-6-7-9-14(12)20(15)17(22)13(18)4-2/h6-9,13,15H,3-5,10-11,18H2,1-2H3,(H,19,21)/t13-,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liver |
J Med Chem 45: 5303-10 (2002)
BindingDB Entry DOI: 10.7270/Q2736Q7X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50197899
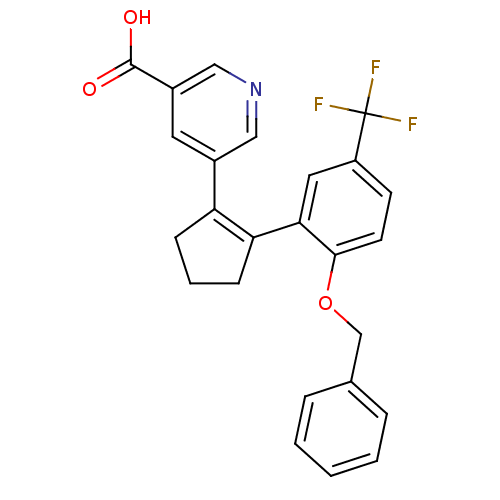
(5-(2-(2-(benzyloxy)-5-(trifluoromethyl)phenyl)cycl...)Show SMILES OC(=O)c1cncc(c1)C1=C(CCC1)c1cc(ccc1OCc1ccccc1)C(F)(F)F |t:10| Show InChI InChI=1S/C25H20F3NO3/c26-25(27,28)19-9-10-23(32-15-16-5-2-1-3-6-16)22(12-19)21-8-4-7-20(21)17-11-18(24(30)31)14-29-13-17/h1-3,5-6,9-14H,4,7-8,15H2,(H,30,31) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259613

(CHEMBL467720 | sodium 6-(5-chloro-2-(2,4-dichlorob...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2Cl)n1 Show InChI InChI=1S/C20H14Cl3NO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259612

(CHEMBL513491 | sodium 6-(5-chloro-2-(2-chloro-4-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(F)cc2Cl)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-5-7-19(27-11-12-4-6-15(23)10-17(12)22)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259611

(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50121280
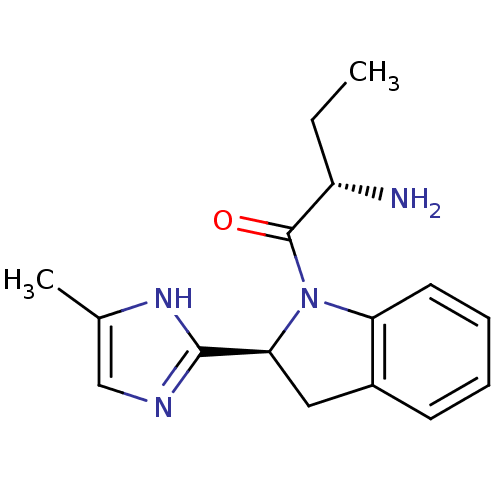
(2-Amino-1-[2-(5-methyl-1H-imidazol-2-yl)-2,3-dihyd...)Show SMILES CC[C@H](N)C(=O)N1[C@@H](Cc2ccccc12)c1ncc(C)[nH]1 Show InChI InChI=1S/C16H20N4O/c1-3-12(17)16(21)20-13-7-5-4-6-11(13)8-14(20)15-18-9-10(2)19-15/h4-7,9,12,14H,3,8,17H2,1-2H3,(H,18,19)/t12-,14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liver |
J Med Chem 45: 5303-10 (2002)
BindingDB Entry DOI: 10.7270/Q2736Q7X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414026
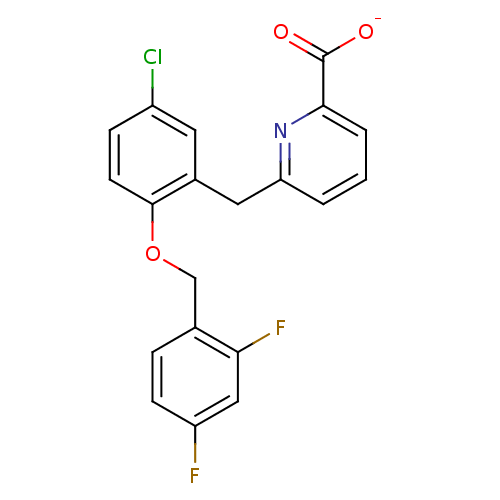
(CHEMBL466101)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(F)cc2F)n1 Show InChI InChI=1S/C20H14ClF2NO3/c21-14-5-7-19(27-11-12-4-6-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414032

(CHEMBL511252)Show InChI InChI=1S/C19H20ClNO3/c20-15-8-9-18(24-12-13-4-1-2-5-13)14(10-15)11-16-6-3-7-17(21-16)19(22)23/h3,6-10,13H,1-2,4-5,11-12H2,(H,22,23)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50121283
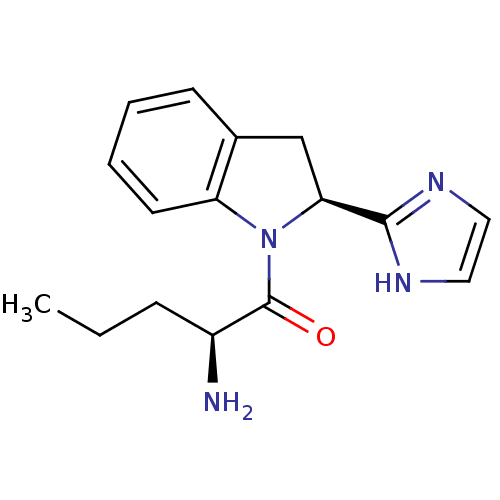
(2-Amino-1-[2-(1H-imidazol-2-yl)-2,3-dihydro-indol-...)Show SMILES CCC[C@H](N)C(=O)N1[C@@H](Cc2ccccc12)c1ncc[nH]1 Show InChI InChI=1S/C16H20N4O/c1-2-5-12(17)16(21)20-13-7-4-3-6-11(13)10-14(20)15-18-8-9-19-15/h3-4,6-9,12,14H,2,5,10,17H2,1H3,(H,18,19)/t12-,14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liver |
J Med Chem 45: 5303-10 (2002)
BindingDB Entry DOI: 10.7270/Q2736Q7X |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414028

(CHEMBL466103)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccccc2Cl)n1 Show InChI InChI=1S/C20H15Cl2NO3/c21-15-8-9-19(26-12-13-4-1-2-6-17(13)22)14(10-15)11-16-5-3-7-18(23-16)20(24)25/h1-10H,11-12H2,(H,24,25)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414030

(CHEMBL257998)Show InChI InChI=1S/C17H18ClNO3/c1-11(2)10-22-16-7-6-13(18)8-12(16)9-14-4-3-5-15(19-14)17(20)21/h3-8,11H,9-10H2,1-2H3,(H,20,21)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414029

(CHEMBL467113)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2)n1 Show InChI InChI=1S/C20H15Cl2NO3/c21-15-6-4-13(5-7-15)12-26-19-9-8-16(22)10-14(19)11-17-2-1-3-18(23-17)20(24)25/h1-10H,11-12H2,(H,24,25)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414027
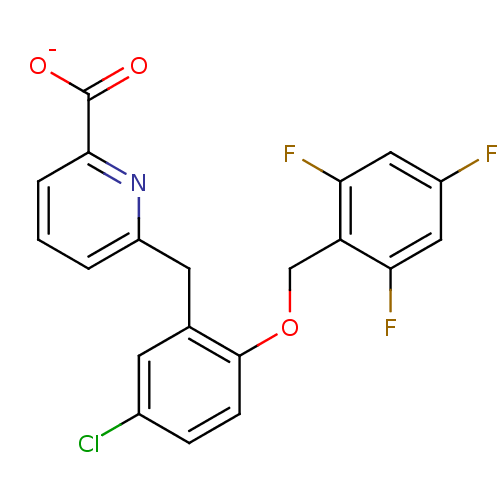
(CHEMBL466102)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2c(F)cc(F)cc2F)n1 Show InChI InChI=1S/C20H13ClF3NO3/c21-12-4-5-19(28-10-15-16(23)8-13(22)9-17(15)24)11(6-12)7-14-2-1-3-18(25-14)20(26)27/h1-6,8-9H,7,10H2,(H,26,27)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414024

(CHEMBL508276)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccccc2F)n1 Show InChI InChI=1S/C20H15ClFNO3/c21-15-8-9-19(26-12-13-4-1-2-6-17(13)22)14(10-15)11-16-5-3-7-18(23-16)20(24)25/h1-10H,11-12H2,(H,24,25)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414025
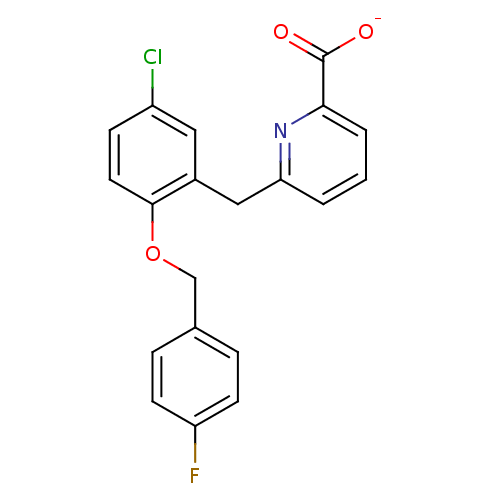
(CHEMBL511733)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(F)cc2)n1 Show InChI InChI=1S/C20H15ClFNO3/c21-15-6-9-19(26-12-13-4-7-16(22)8-5-13)14(10-15)11-17-2-1-3-18(23-17)20(24)25/h1-10H,11-12H2,(H,24,25)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414031
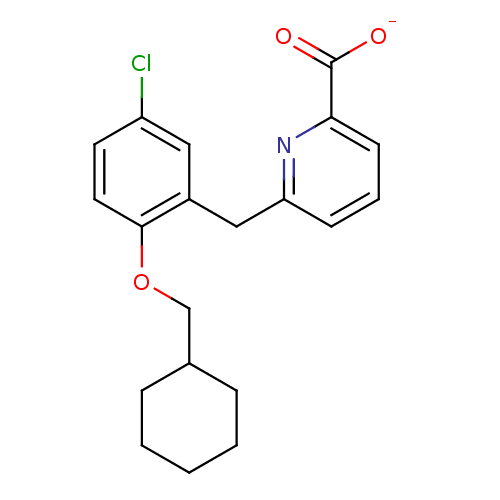
(CHEMBL468723)Show InChI InChI=1S/C20H22ClNO3/c21-16-9-10-19(25-13-14-5-2-1-3-6-14)15(11-16)12-17-7-4-8-18(22-17)20(23)24/h4,7-11,14H,1-3,5-6,12-13H2,(H,23,24)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414023
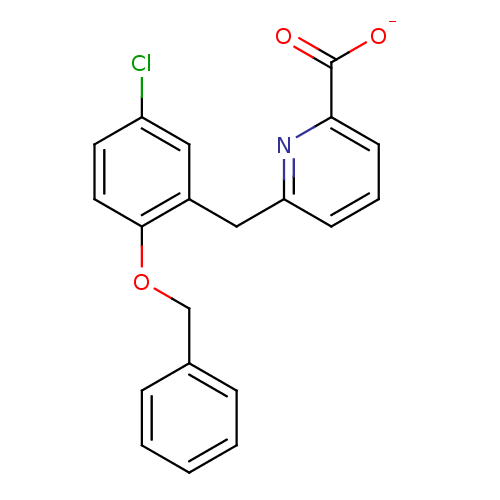
(CHEMBL258041)Show InChI InChI=1S/C20H16ClNO3/c21-16-9-10-19(25-13-14-5-2-1-3-6-14)15(11-16)12-17-7-4-8-18(22-17)20(23)24/h1-11H,12-13H2,(H,23,24)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50414033
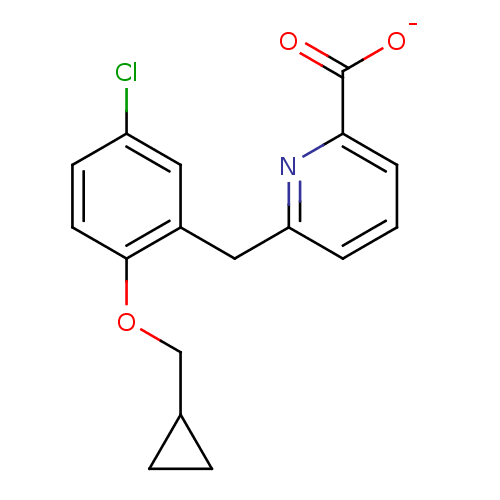
(CHEMBL511919)Show InChI InChI=1S/C17H16ClNO3/c18-13-6-7-16(22-10-11-4-5-11)12(8-13)9-14-2-1-3-15(19-14)17(20)21/h1-3,6-8,11H,4-5,9-10H2,(H,20,21)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50177043
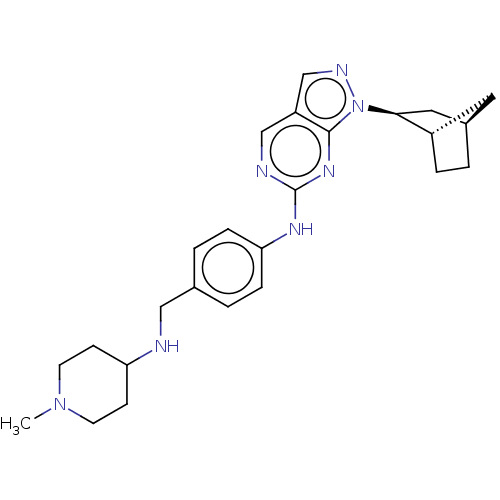
(CHEMBL3814671)Show SMILES [H][C@@]12CC[C@@]([H])(C1)[C@H](C2)n1ncc2cnc(Nc3ccc(CNC4CCN(C)CC4)cc3)nc12 |r| Show InChI InChI=1S/C25H33N7/c1-31-10-8-21(9-11-31)26-14-17-3-6-22(7-4-17)29-25-27-15-20-16-28-32(24(20)30-25)23-13-18-2-5-19(23)12-18/h3-4,6-7,15-16,18-19,21,23,26H,2,5,8-14H2,1H3,(H,27,29,30)/t18-,19+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 339 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50177042
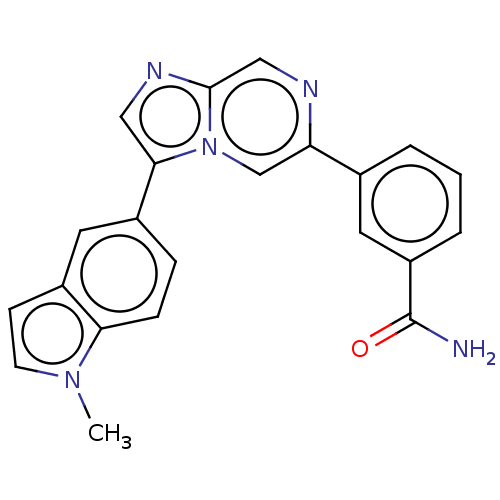
(CHEMBL3730906)Show SMILES Cn1ccc2cc(ccc12)-c1cnc2cnc(cn12)-c1cccc(c1)C(N)=O Show InChI InChI=1S/C22H17N5O/c1-26-8-7-16-10-15(5-6-19(16)26)20-11-25-21-12-24-18(13-27(20)21)14-3-2-4-17(9-14)22(23)28/h2-13H,1H3,(H2,23,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 427 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50259611

(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of IP receptor |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50259611

(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EP3 receptor by FLIPR assay |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50259611

(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of EP2 receptor |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50177033
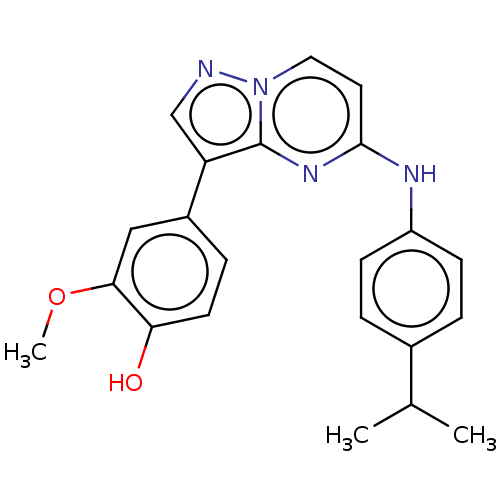
(CHEMBL3813839)Show SMILES COc1cc(ccc1O)-c1cnn2ccc(Nc3ccc(cc3)C(C)C)nc12 Show InChI InChI=1S/C22H22N4O2/c1-14(2)15-4-7-17(8-5-15)24-21-10-11-26-22(25-21)18(13-23-26)16-6-9-19(27)20(12-16)28-3/h4-14,27H,1-3H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma using PIP2 as substrate by HTRF assay in presence of biotin-PIP3 |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50177041

(CHEMBL3814491)Show SMILES NC(=O)c1nc(nc2n(CCc3ccccc3)c(=O)[nH]c12)-c1ccc2ncccc2c1 Show InChI InChI=1S/C23H18N6O2/c24-20(30)18-19-22(29(23(31)27-19)12-10-14-5-2-1-3-6-14)28-21(26-18)16-8-9-17-15(13-16)7-4-11-25-17/h1-9,11,13H,10,12H2,(H2,24,30)(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50429613
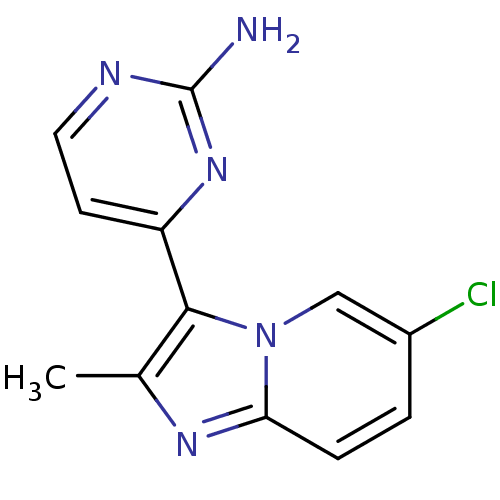
(CHEMBL2334590)Show InChI InChI=1S/C12H10ClN5/c1-7-11(9-4-5-15-12(14)17-9)18-6-8(13)2-3-10(18)16-7/h2-6H,1H3,(H2,14,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
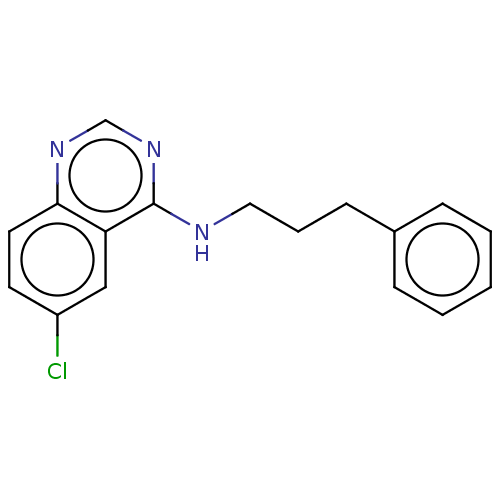
(Homo sapiens (Human)) | BDBM50177040
(CHEMBL3814642)Show InChI InChI=1S/C17H16ClN3/c18-14-8-9-16-15(11-14)17(21-12-20-16)19-10-4-7-13-5-2-1-3-6-13/h1-3,5-6,8-9,11-12H,4,7,10H2,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50177039
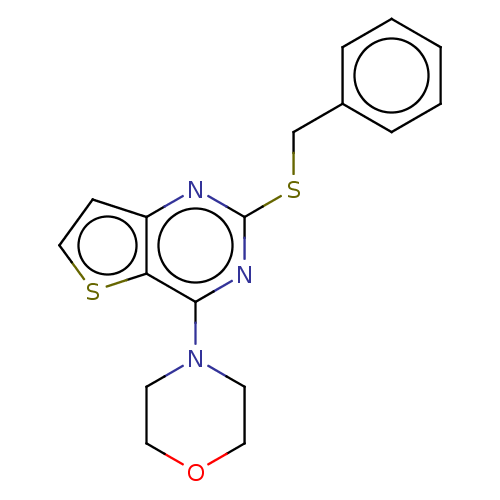
(CHEMBL3814308)Show InChI InChI=1S/C17H17N3OS2/c1-2-4-13(5-3-1)12-23-17-18-14-6-11-22-15(14)16(19-17)20-7-9-21-10-8-20/h1-6,11H,7-10,12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50259613

(CHEMBL467720 | sodium 6-(5-chloro-2-(2,4-dichlorob...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2Cl)n1 Show InChI InChI=1S/C20H14Cl3NO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50177038
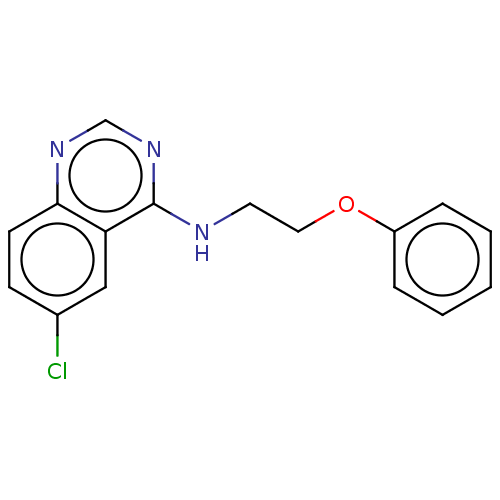
(CHEMBL3814655)Show InChI InChI=1S/C16H14ClN3O/c17-12-6-7-15-14(10-12)16(20-11-19-15)18-8-9-21-13-4-2-1-3-5-13/h1-7,10-11H,8-9H2,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50177032
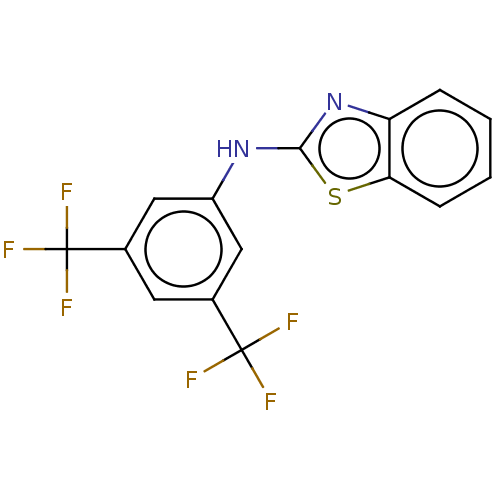
(CHEMBL3814407)Show InChI InChI=1S/C15H8F6N2S/c16-14(17,18)8-5-9(15(19,20)21)7-10(6-8)22-13-23-11-3-1-2-4-12(11)24-13/h1-7H,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma using PIP2 as substrate by HTRF assay in presence of biotin-PIP3 |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50177031
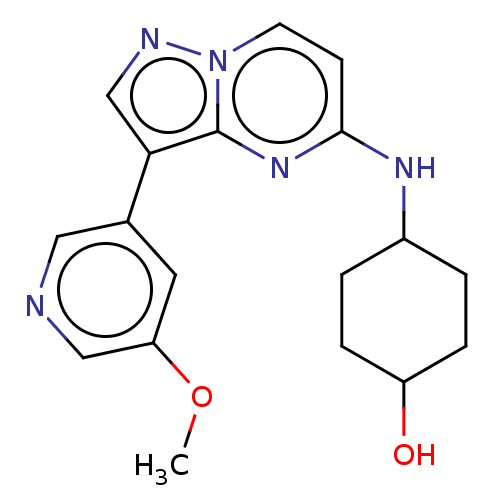
(CHEMBL3814969)Show SMILES COc1cncc(c1)-c1cnn2ccc(NC3CCC(O)CC3)nc12 |(5.87,-6.24,;5.57,-5.05,;4.09,-4.62,;2.98,-5.68,;1.5,-5.26,;1.13,-3.76,;2.24,-2.7,;3.72,-3.12,;1.76,-1.24,;2.66,.02,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-3.72,-1.53,;-3.72,-3.07,;-5.06,-3.84,;-5.06,-5.38,;-3.73,-6.15,;-3.74,-7.38,;-2.4,-5.39,;-2.39,-3.85,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C18H21N5O2/c1-25-15-8-12(9-19-10-15)16-11-20-23-7-6-17(22-18(16)23)21-13-2-4-14(24)5-3-13/h6-11,13-14,24H,2-5H2,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma using PIP2 as substrate by HTRF assay in presence of biotin-PIP3 |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50177044

(CHEMBL3814695)Show SMILES COc1cc(Nc2ccn3ncc(-c4cccc(c4)C(N)=O)c3n2)cc(OC)c1OC Show InChI InChI=1S/C22H21N5O4/c1-29-17-10-15(11-18(30-2)20(17)31-3)25-19-7-8-27-22(26-19)16(12-24-27)13-5-4-6-14(9-13)21(23)28/h4-12H,1-3H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma using PIP2 as substrate by HTRF assay in presence of biotin-PIP3 |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50177037

(CHEMBL3814438)Show InChI InChI=1S/C21H17N5S/c1-2-6-18-16(5-1)12-19(27-18)17-14-24-21-8-7-20(25-26(17)21)23-11-9-15-4-3-10-22-13-15/h1-8,10,12-14H,9,11H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50177045
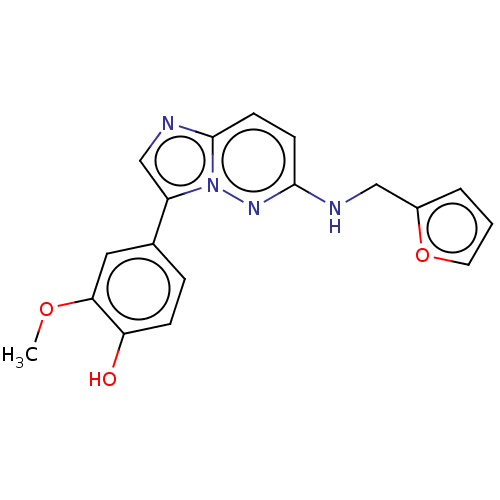
(CHEMBL3814733)Show InChI InChI=1S/C18H16N4O3/c1-24-16-9-12(4-5-15(16)23)14-11-20-18-7-6-17(21-22(14)18)19-10-13-3-2-8-25-13/h2-9,11,23H,10H2,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma using PIP2 as substrate by HTRF assay in presence of biotin-PIP3 |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50177036

(CHEMBL1528007)Show InChI InChI=1S/C16H14BrN3/c1-20(10-12-5-3-2-4-6-12)16-14-9-13(17)7-8-15(14)18-11-19-16/h2-9,11H,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50259611

(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50177035

(CHEMBL3814774)Show InChI InChI=1S/C16H15N5/c1-10-14-13(21(20-10)12-5-3-2-4-6-12)8-7-11-9-18-16(17)19-15(11)14/h2-6,9H,7-8H2,1H3,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50177034
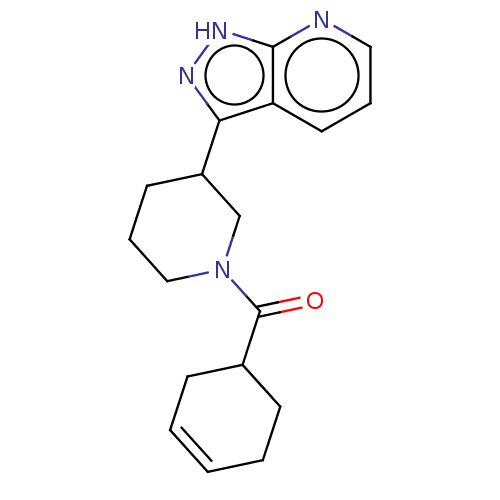
(CHEMBL3813732)Show SMILES O=C(C1CCC=CC1)N1CCCC(C1)c1n[nH]c2ncccc12 |c:5| Show InChI InChI=1S/C18H22N4O/c23-18(13-6-2-1-3-7-13)22-11-5-8-14(12-22)16-15-9-4-10-19-17(15)21-20-16/h1-2,4,9-10,13-14H,3,5-8,11-12H2,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human full length recombinant His-tagged PIK3CD/PIK3R1 expressed in baculovirus expression system after 60 mins by LanthaScreen Eu bind... |
J Med Chem 59: 4302-13 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01974
BindingDB Entry DOI: 10.7270/Q2DN46ZF |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50121278
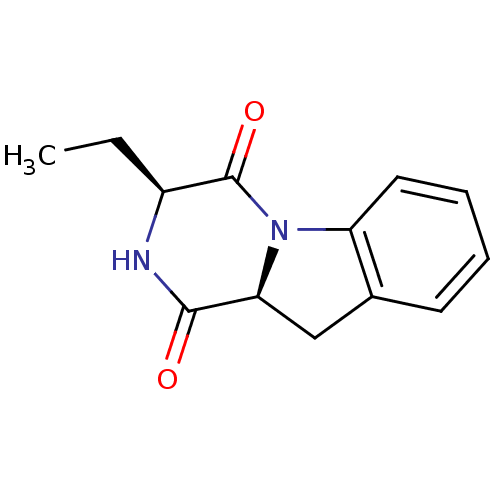
(3-Ethyl-2,3,10,10a-tetrahydro-pyrazino[1,2-a]indol...)Show InChI InChI=1S/C13H14N2O2/c1-2-9-13(17)15-10-6-4-3-5-8(10)7-11(15)12(16)14-9/h3-6,9,11H,2,7H2,1H3,(H,14,16)/t9-,11-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against tripeptidyl peptidase II purified from rat liver |
J Med Chem 45: 5303-10 (2002)
BindingDB Entry DOI: 10.7270/Q2736Q7X |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50259611

(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of FP receptor |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data