 Found 607 hits with Last Name = 'piemontese' and Initial = 'l'
Found 607 hits with Last Name = 'piemontese' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from N-terminal His-tagged human PPARgamma ligand binding domain expressed in Escherichia coli BL21 DE3 cells by sc... |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28762
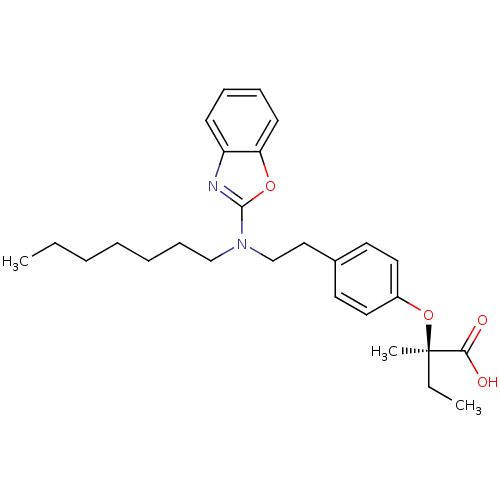
((2R)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...)Show SMILES CCCCCCCN(CCc1ccc(O[C@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C27H36N2O4/c1-4-6-7-8-11-19-29(26-28-23-12-9-10-13-24(23)32-26)20-18-21-14-16-22(17-15-21)33-27(3,5-2)25(30)31/h9-10,12-17H,4-8,11,18-20H2,1-3H3,(H,30,31)/t27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from N-terminal His-tagged human PPARgamma ligand binding domain expressed in Escherichia coli BL21 DE3 cells by sc... |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28763
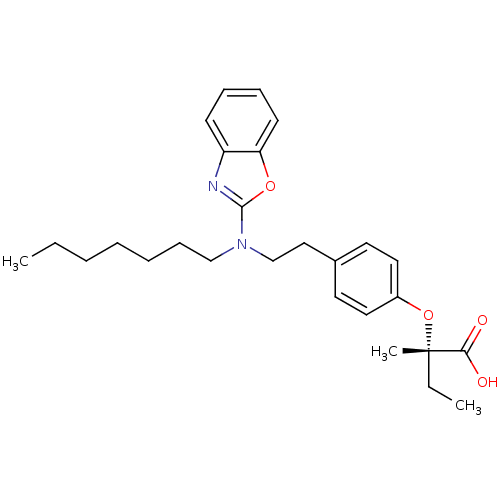
((2S)-2-(4-{2-[1,3-benzoxazol-2-yl(heptyl)amino]eth...)Show SMILES CCCCCCCN(CCc1ccc(O[C@@](C)(CC)C(O)=O)cc1)c1nc2ccccc2o1 |r| Show InChI InChI=1S/C27H36N2O4/c1-4-6-7-8-11-19-29(26-28-23-12-9-10-13-24(23)32-26)20-18-21-14-16-22(17-15-21)33-27(3,5-2)25(30)31/h9-10,12-17H,4-8,11,18-20H2,1-3H3,(H,30,31)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 971 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Tumori"Giovanni Paolo II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from N-terminal His-tagged human PPARgamma ligand binding domain expressed in Escherichia coli BL21 DE3 cells by sc... |
J Med Chem 55: 37-54 (2012)
Article DOI: 10.1021/jm201306q
BindingDB Entry DOI: 10.7270/Q23N24JP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro"
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in Wistar rat brain intact neurons assessed as reduction in [3H]anandamide hydrolysis using [3H]anandamide as substrate preincubat... |
J Med Chem 62: 10995-11003 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00885
BindingDB Entry DOI: 10.7270/Q26113K2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593777

(CHEMBL5185868)Show SMILES [O-][N+](=O)c1cc(Cl)ccc1OCC(=O)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593778

(CHEMBL5189408)Show SMILES [O-][N+](=O)c1ccc(F)cc1OCC(=O)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM26739

(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro"
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in Wistar rat brain membranes assessed as reduction in [3H]anandamide hydrolysis using [3H]anandamide as substrate preincubated fo... |
J Med Chem 62: 10995-11003 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00885
BindingDB Entry DOI: 10.7270/Q26113K2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50307083
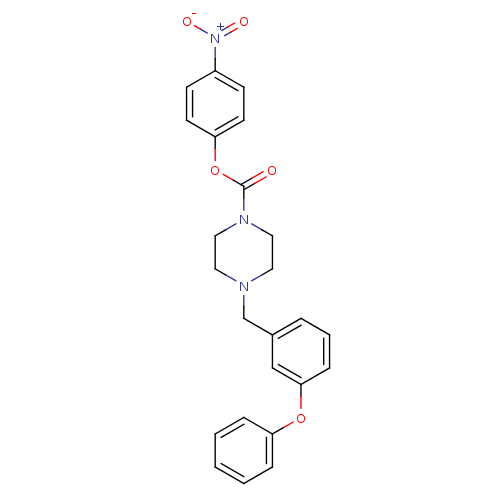
(4-nitrophenyl 4-(3-phenoxybenzyl)piperazine-1-carb...)Show SMILES [O-][N+](=O)c1ccc(OC(=O)N2CCN(Cc3cccc(Oc4ccccc4)c3)CC2)cc1 Show InChI InChI=1S/C24H23N3O5/c28-24(32-22-11-9-20(10-12-22)27(29)30)26-15-13-25(14-16-26)18-19-5-4-8-23(17-19)31-21-6-2-1-3-7-21/h1-12,17H,13-16,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593779
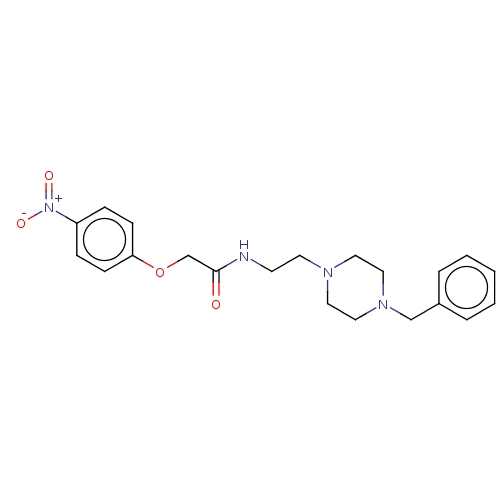
(CHEMBL5193520)Show SMILES [O-][N+](=O)c1ccc(OCC(=O)NCCN2CCN(Cc3ccccc3)CC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50505364
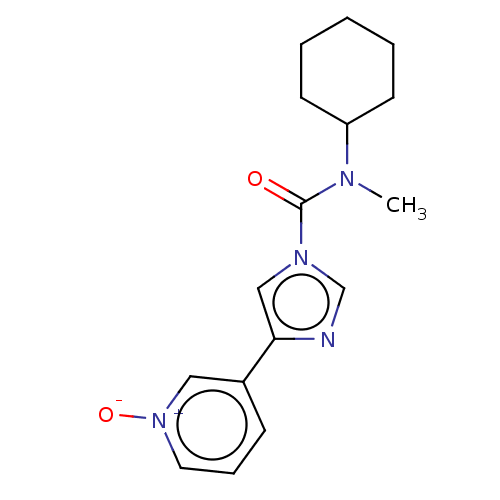
(CHEMBL4460898)Show SMILES CN(C1CCCCC1)C(=O)n1cnc(c1)-c1ccc[n+]([O-])c1 Show InChI InChI=1S/C16H20N4O2/c1-18(14-7-3-2-4-8-14)16(21)19-11-15(17-12-19)13-6-5-9-20(22)10-13/h5-6,9-12,14H,2-4,7-8H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro"
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged FAAH expressed in HEK293T cells preincubated for 4 hrs followed by addition of FP-TAMRA probe ... |
J Med Chem 62: 10995-11003 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00885
BindingDB Entry DOI: 10.7270/Q26113K2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593781

(CHEMBL5190969)Show SMILES Cc1cc(Br)cc(C)c1OCC(=O)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593768
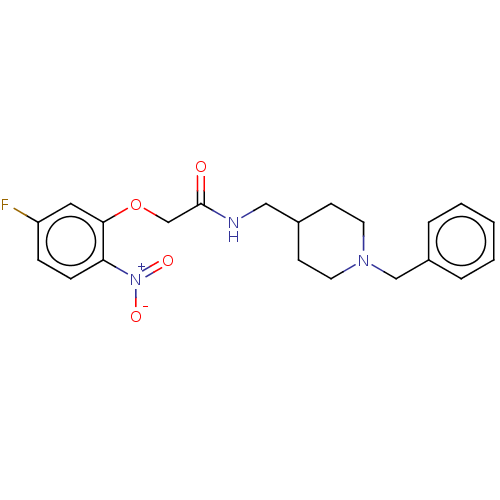
(CHEMBL5195980)Show SMILES [O-][N+](=O)c1ccc(F)cc1OCC(=O)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593767
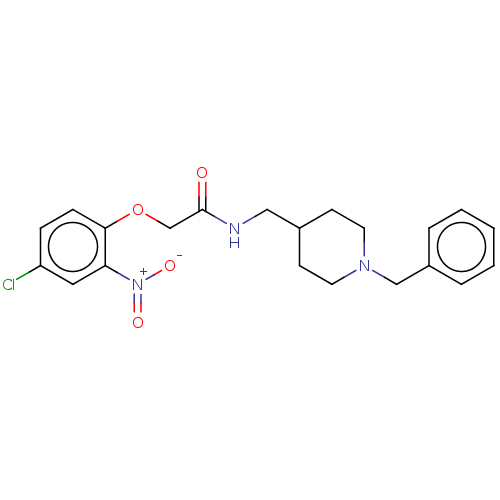
(CHEMBL5207120)Show SMILES [O-][N+](=O)c1cc(Cl)ccc1OCC(=O)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593773
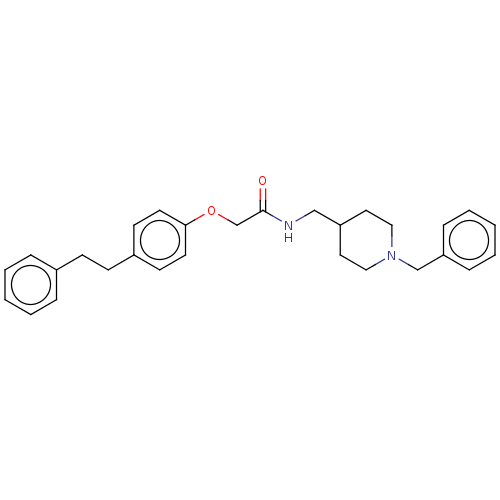
(CHEMBL5182803)Show SMILES O=C(COc1ccc(CCc2ccccc2)cc1)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593780

(CHEMBL5209013)Show SMILES FC(F)(F)c1ccccc1OCC(=O)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593785
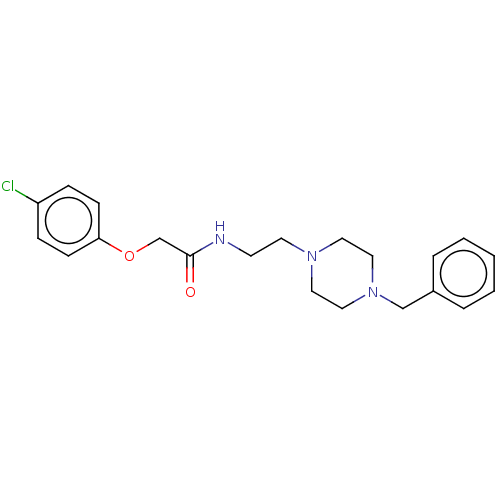
(CHEMBL5169781) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593784
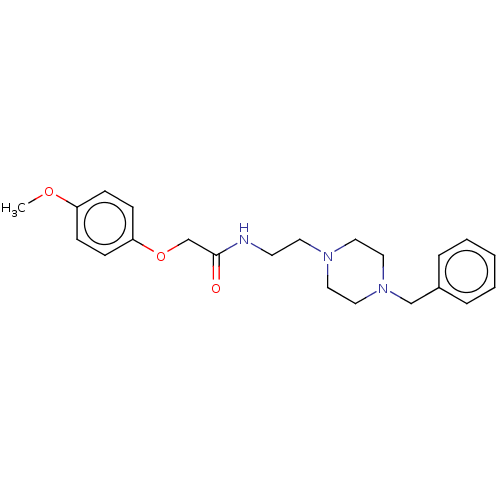
(CHEMBL5175715) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593780

(CHEMBL5209013)Show SMILES FC(F)(F)c1ccccc1OCC(=O)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 503 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593769
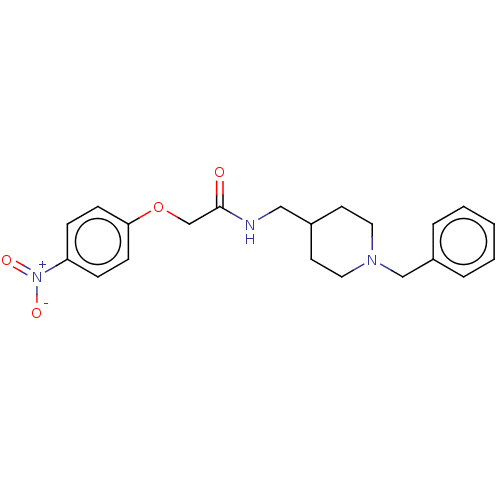
(CHEMBL5180014)Show SMILES [O-][N+](=O)c1ccc(OCC(=O)NCC2CCN(Cc3ccccc3)CC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 509 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593771
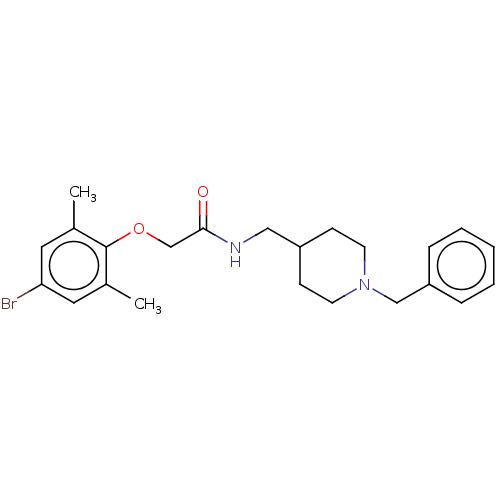
(CHEMBL5178350)Show SMILES Cc1cc(Br)cc(C)c1OCC(=O)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 595 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593776
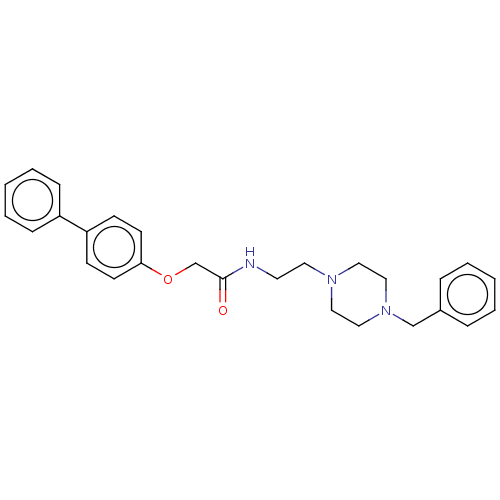
(CHEMBL5204532)Show SMILES O=C(COc1ccc(cc1)-c1ccccc1)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 735 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593770
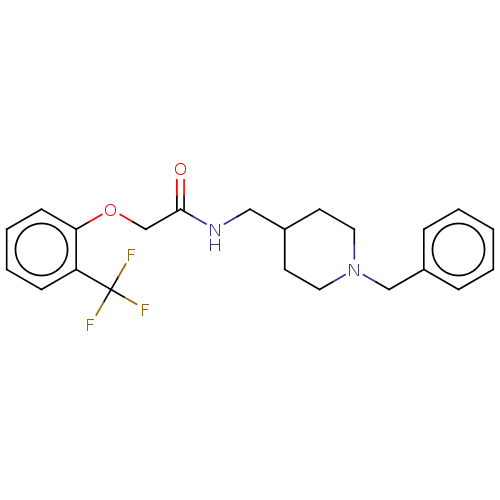
(CHEMBL5202267)Show SMILES FC(F)(F)c1ccccc1OCC(=O)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 767 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593774

(CHEMBL5177371) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593783
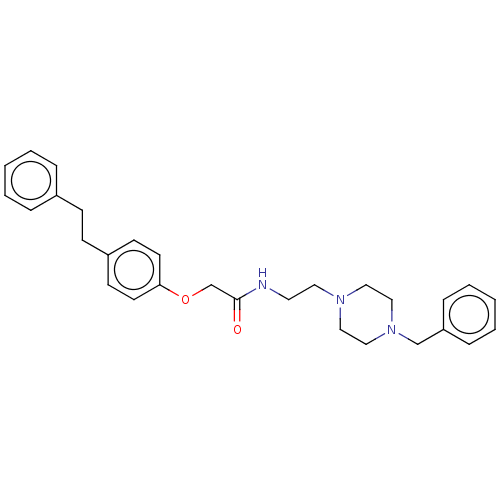
(CHEMBL5185234)Show SMILES O=C(COc1ccc(CCc2ccccc2)cc1)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593776

(CHEMBL5204532)Show SMILES O=C(COc1ccc(cc1)-c1ccccc1)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 969 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593782
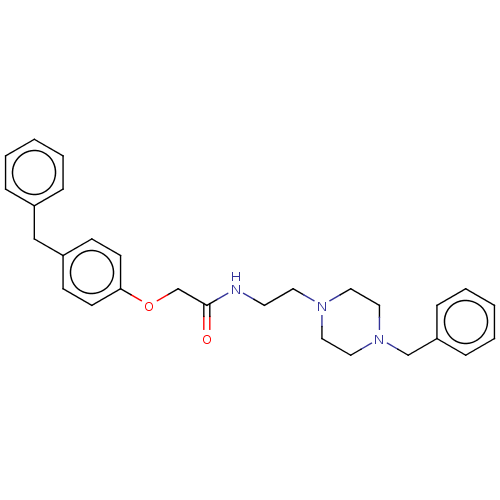
(CHEMBL5206245)Show SMILES O=C(COc1ccc(Cc2ccccc2)cc1)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 993 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593770

(CHEMBL5202267)Show SMILES FC(F)(F)c1ccccc1OCC(=O)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593772
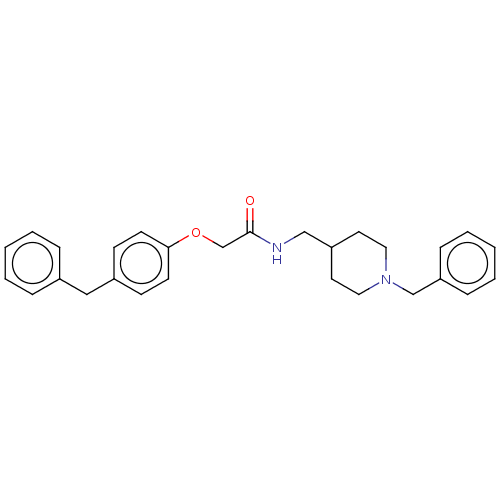
(CHEMBL5203325)Show SMILES O=C(COc1ccc(Cc2ccccc2)cc1)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593777

(CHEMBL5185868)Show SMILES [O-][N+](=O)c1cc(Cl)ccc1OCC(=O)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593775
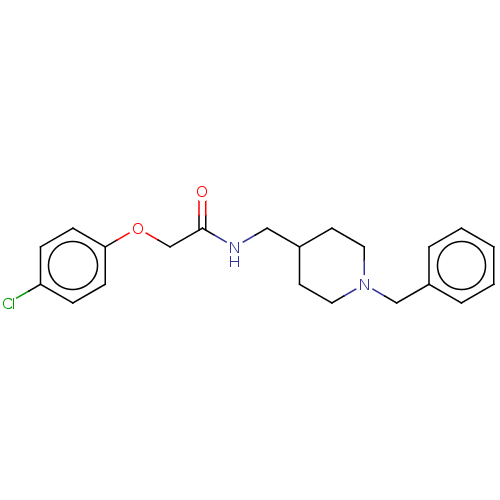
(CHEMBL5175950) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50593766

(CHEMBL5170984)Show SMILES O=C(COc1ccc(cc1)-c1ccccc1)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50541090
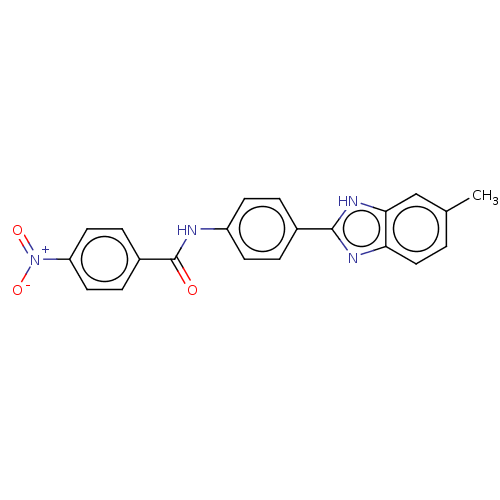
(CHEMBL4647123)Show SMILES Cc1ccc2nc([nH]c2c1)-c1ccc(NC(=O)c2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C21H16N4O3/c1-13-2-11-18-19(12-13)24-20(23-18)14-3-7-16(8-4-14)22-21(26)15-5-9-17(10-6-15)25(27)28/h2-12H,1H3,(H,22,26)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"A. Moro" University of Bari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MMP13 catalytic domain (Tyr104 to Asn274 residues) expressed in Escherichia coli cells using Mca-Pro-Leu-Gly-Leu-Dpa-... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115257
BindingDB Entry DOI: 10.7270/Q22B92J3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593785

(CHEMBL5169781) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50541091
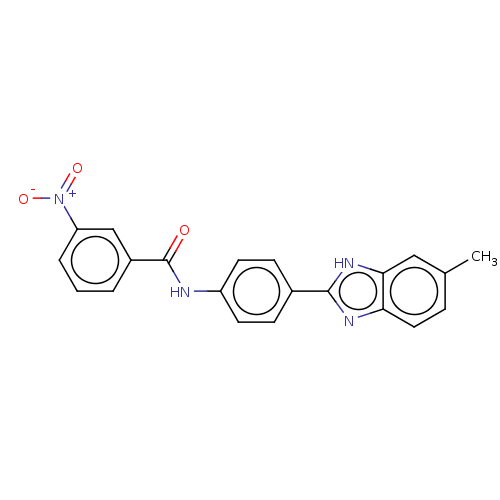
(CHEMBL4639311)Show SMILES Cc1ccc2nc([nH]c2c1)-c1ccc(NC(=O)c2cccc(c2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C21H16N4O3/c1-13-5-10-18-19(11-13)24-20(23-18)14-6-8-16(9-7-14)22-21(26)15-3-2-4-17(12-15)25(27)28/h2-12H,1H3,(H,22,26)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"A. Moro" University of Bari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MMP13 catalytic domain (Tyr104 to Asn274 residues) expressed in Escherichia coli cells using Mca-Pro-Leu-Gly-Leu-Dpa-... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115257
BindingDB Entry DOI: 10.7270/Q22B92J3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593771

(CHEMBL5178350)Show SMILES Cc1cc(Br)cc(C)c1OCC(=O)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50541092
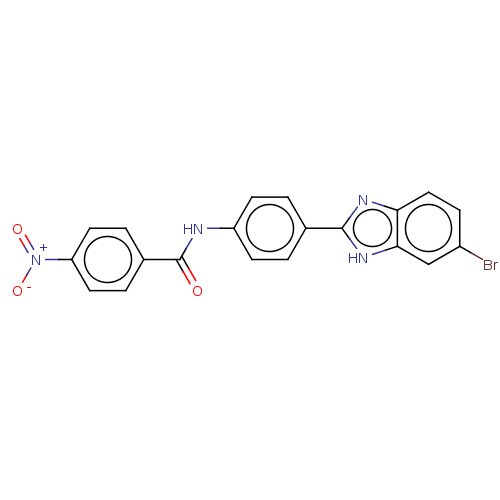
(CHEMBL4634914)Show SMILES [O-][N+](=O)c1ccc(cc1)C(=O)Nc1ccc(cc1)-c1nc2ccc(Br)cc2[nH]1 Show InChI InChI=1S/C20H13BrN4O3/c21-14-5-10-17-18(11-14)24-19(23-17)12-1-6-15(7-2-12)22-20(26)13-3-8-16(9-4-13)25(27)28/h1-11H,(H,22,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"A. Moro" University of Bari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MMP2 catalytic domain (Tyr110 to Asp452 residues) expressed in yeast cells using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2/... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115257
BindingDB Entry DOI: 10.7270/Q22B92J3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50541090

(CHEMBL4647123)Show SMILES Cc1ccc2nc([nH]c2c1)-c1ccc(NC(=O)c2ccc(cc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C21H16N4O3/c1-13-2-11-18-19(12-13)24-20(23-18)14-3-7-16(8-4-14)22-21(26)15-5-9-17(10-6-15)25(27)28/h2-12H,1H3,(H,22,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"A. Moro" University of Bari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MMP2 catalytic domain (Tyr110 to Asp452 residues) expressed in yeast cells using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2/... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115257
BindingDB Entry DOI: 10.7270/Q22B92J3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50541091

(CHEMBL4639311)Show SMILES Cc1ccc2nc([nH]c2c1)-c1ccc(NC(=O)c2cccc(c2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C21H16N4O3/c1-13-5-10-18-19(11-13)24-20(23-18)14-6-8-16(9-7-14)22-21(26)15-3-2-4-17(12-15)25(27)28/h2-12H,1H3,(H,22,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"A. Moro" University of Bari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MMP2 catalytic domain (Tyr110 to Asp452 residues) expressed in yeast cells using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2/... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115257
BindingDB Entry DOI: 10.7270/Q22B92J3 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50541093
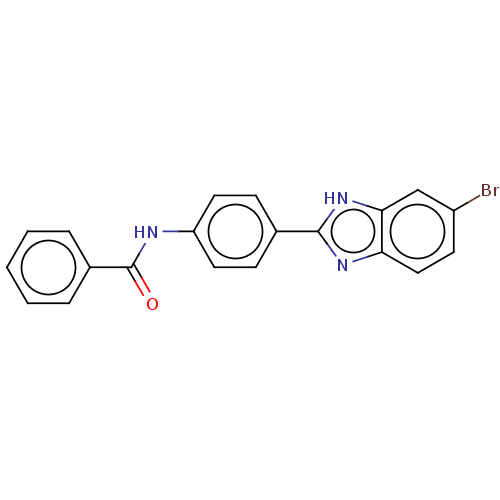
(CHEMBL4640667)Show SMILES Brc1ccc2nc([nH]c2c1)-c1ccc(NC(=O)c2ccccc2)cc1 Show InChI InChI=1S/C20H14BrN3O/c21-15-8-11-17-18(12-15)24-19(23-17)13-6-9-16(10-7-13)22-20(25)14-4-2-1-3-5-14/h1-12H,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"A. Moro" University of Bari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MMP13 catalytic domain (Tyr104 to Asn274 residues) expressed in Escherichia coli cells using Mca-Pro-Leu-Gly-Leu-Dpa-... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115257
BindingDB Entry DOI: 10.7270/Q22B92J3 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50541092

(CHEMBL4634914)Show SMILES [O-][N+](=O)c1ccc(cc1)C(=O)Nc1ccc(cc1)-c1nc2ccc(Br)cc2[nH]1 Show InChI InChI=1S/C20H13BrN4O3/c21-14-5-10-17-18(11-14)24-19(23-17)12-1-6-15(7-2-12)22-20(26)13-3-8-16(9-4-13)25(27)28/h1-11H,(H,22,26)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"A. Moro" University of Bari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MMP13 catalytic domain (Tyr104 to Asn274 residues) expressed in Escherichia coli cells using Mca-Pro-Leu-Gly-Leu-Dpa-... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115257
BindingDB Entry DOI: 10.7270/Q22B92J3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593781

(CHEMBL5190969)Show SMILES Cc1cc(Br)cc(C)c1OCC(=O)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50541104
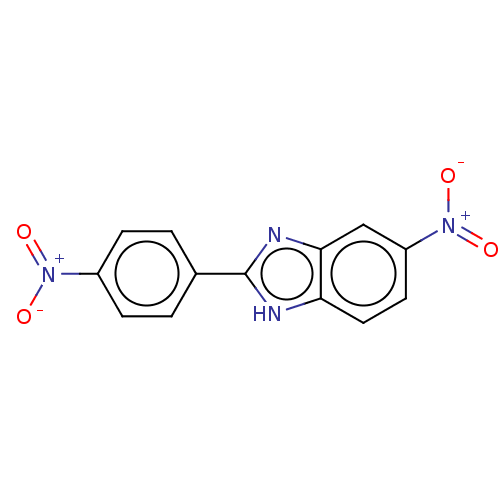
(CHEMBL1483847)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nc2cc(ccc2[nH]1)[N+]([O-])=O Show InChI InChI=1S/C13H8N4O4/c18-16(19)9-3-1-8(2-4-9)13-14-11-6-5-10(17(20)21)7-12(11)15-13/h1-7H,(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
"A. Moro" University of Bari
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MMP2 catalytic domain (Tyr110 to Asp452 residues) expressed in yeast cells using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2/... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115257
BindingDB Entry DOI: 10.7270/Q22B92J3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593767

(CHEMBL5207120)Show SMILES [O-][N+](=O)c1cc(Cl)ccc1OCC(=O)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593778

(CHEMBL5189408)Show SMILES [O-][N+](=O)c1ccc(F)cc1OCC(=O)NCCN1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593773

(CHEMBL5182803)Show SMILES O=C(COc1ccc(CCc2ccccc2)cc1)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593766

(CHEMBL5170984)Show SMILES O=C(COc1ccc(cc1)-c1ccccc1)NCC1CCN(Cc2ccccc2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM22987
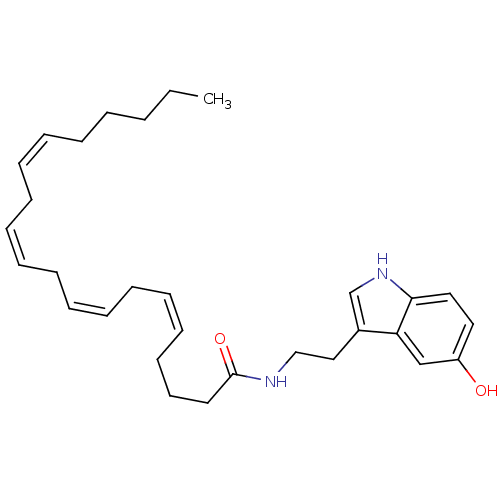
((5Z,8Z,11Z,14Z)-N-[2-(5-hydroxy-1H-indol-3-yl)ethy...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCc1c[nH]c2ccc(O)cc12 Show InChI InChI=1S/C30H42N2O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-30(34)31-23-22-26-25-32-29-21-20-27(33)24-28(26)29/h6-7,9-10,12-13,15-16,20-21,24-25,32-33H,2-5,8,11,14,17-19,22-23H2,1H3,(H,31,34)/b7-6-,10-9-,13-12-,16-15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro"
Curated by ChEMBL
| Assay Description
Inhibition of FAAH (unknown origin) |
J Med Chem 62: 10995-11003 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00885
BindingDB Entry DOI: 10.7270/Q26113K2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50593775

(CHEMBL5175950) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114358
BindingDB Entry DOI: 10.7270/Q2JS9VF3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data