 Found 664 hits with Last Name = 'pivnichny' and Initial = 'jv'
Found 664 hits with Last Name = 'pivnichny' and Initial = 'jv' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141931

((R)-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl-2...)Show SMILES CCn1nc(Cc2ccc(cc2)-c2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C2CCCCC2)C(O)=O)CC1 Show InChI InChI=1S/C42H51FN4O2/c1-2-47-40(26-38(44-47)24-30-16-18-32(19-17-30)31-10-5-3-6-11-31)33-20-22-45(23-21-33)27-36-28-46(29-39(36)35-14-9-15-37(43)25-35)41(42(48)49)34-12-7-4-8-13-34/h3,5-6,9-11,14-19,25-26,33-34,36,39,41H,2,4,7-8,12-13,20-24,27-29H2,1H3,(H,48,49)/t36-,39+,41+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141911

((R)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethyl-2H...)Show SMILES CCn1nc(Cc2ccc(cc2)C#N)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C35H44FN5O2/c1-5-41-32(19-30(38-41)17-24-9-11-25(20-37)12-10-24)26-13-15-39(16-14-26)21-28-22-40(33(34(42)43)35(2,3)4)23-31(28)27-7-6-8-29(36)18-27/h6-12,18-19,26,28,31,33H,5,13-17,21-23H2,1-4H3,(H,42,43)/t28-,31+,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141908

((R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butyl-benzyl)-2-eth...)Show SMILES CCn1nc(Cc2ccc(cc2)C(C)(C)C)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C38H53FN4O2/c1-8-43-34(22-32(40-43)20-26-12-14-30(15-13-26)37(2,3)4)27-16-18-41(19-17-27)23-29-24-42(35(36(44)45)38(5,6)7)25-33(29)28-10-9-11-31(39)21-28/h9-15,21-22,27,29,33,35H,8,16-20,23-25H2,1-7H3,(H,44,45)/t29-,33+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141905
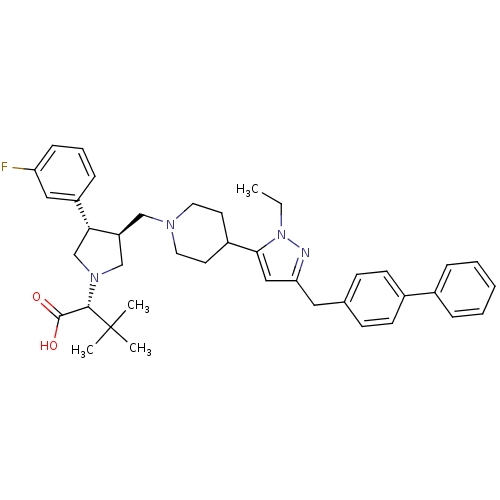
((R)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl...)Show SMILES CCn1nc(Cc2ccc(cc2)-c2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C40H49FN4O2/c1-5-45-37(24-35(42-45)22-28-14-16-30(17-15-28)29-10-7-6-8-11-29)31-18-20-43(21-19-31)25-33-26-44(38(39(46)47)40(2,3)4)27-36(33)32-12-9-13-34(41)23-32/h6-17,23-24,31,33,36,38H,5,18-22,25-27H2,1-4H3,(H,46,47)/t33-,36+,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141978
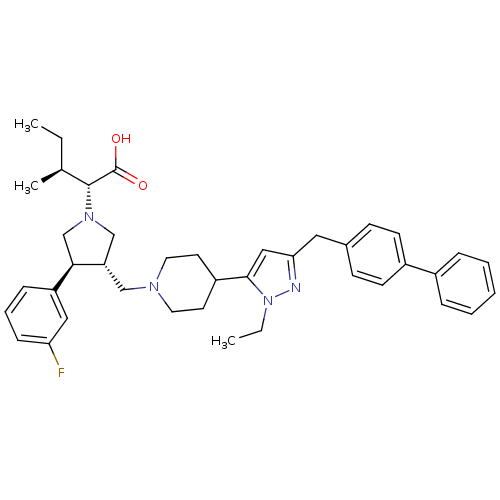
((2R,4S)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-e...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)-c3ccccc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C40H49FN4O2/c1-4-28(3)39(40(46)47)44-26-34(37(27-44)33-12-9-13-35(41)23-33)25-43-20-18-32(19-21-43)38-24-36(42-45(38)5-2)22-29-14-16-31(17-15-29)30-10-7-6-8-11-30/h6-17,23-24,28,32,34,37,39H,4-5,18-22,25-27H2,1-3H3,(H,46,47)/t28-,34-,37+,39+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141935
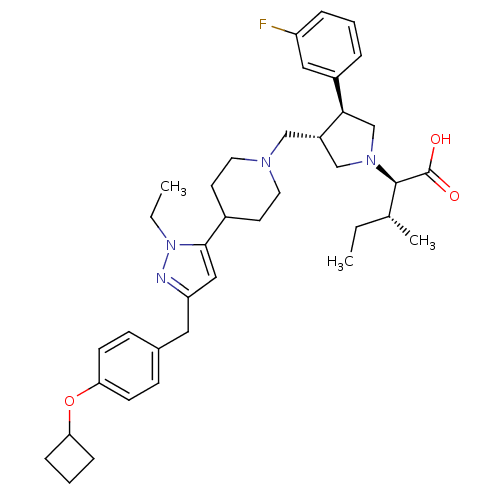
((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC4CCC4)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H51FN4O3/c1-4-26(3)37(38(44)45)42-24-30(35(25-42)29-8-6-9-31(39)21-29)23-41-18-16-28(17-19-41)36-22-32(40-43(36)5-2)20-27-12-14-34(15-13-27)46-33-10-7-11-33/h6,8-9,12-15,21-22,26,28,30,33,35,37H,4-5,7,10-11,16-20,23-25H2,1-3H3,(H,44,45)/t26-,30+,35-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141951
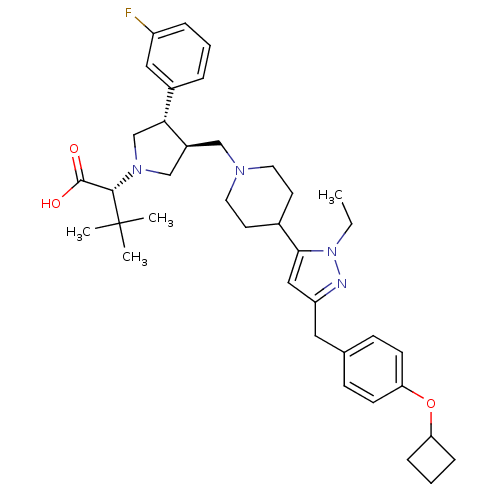
((R)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-2-et...)Show SMILES CCn1nc(Cc2ccc(OC3CCC3)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C38H51FN4O3/c1-5-43-35(22-31(40-43)20-26-12-14-33(15-13-26)46-32-10-7-11-32)27-16-18-41(19-17-27)23-29-24-42(36(37(44)45)38(2,3)4)25-34(29)28-8-6-9-30(39)21-28/h6,8-9,12-15,21-22,27,29,32,34,36H,5,7,10-11,16-20,23-25H2,1-4H3,(H,44,45)/t29-,34+,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141913
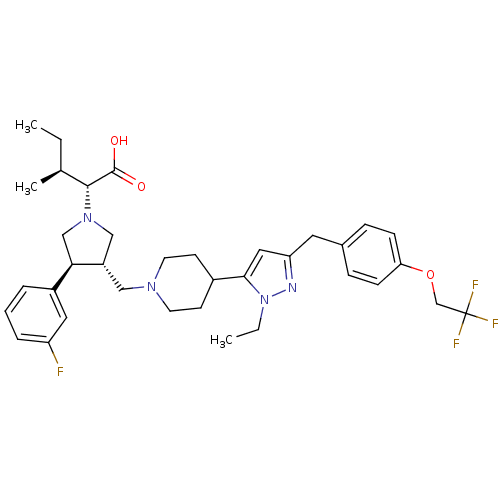
((2R,4S)-2-[(2S,3S)-3-(4-{2-Ethyl-5-[4-(2,2,2-trifl...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OCC(F)(F)F)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C36H46F4N4O3/c1-4-24(3)34(35(45)46)43-21-28(32(22-43)27-7-6-8-29(37)18-27)20-42-15-13-26(14-16-42)33-19-30(41-44(33)5-2)17-25-9-11-31(12-10-25)47-23-36(38,39)40/h6-12,18-19,24,26,28,32,34H,4-5,13-17,20-23H2,1-3H3,(H,45,46)/t24-,28-,32+,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141941
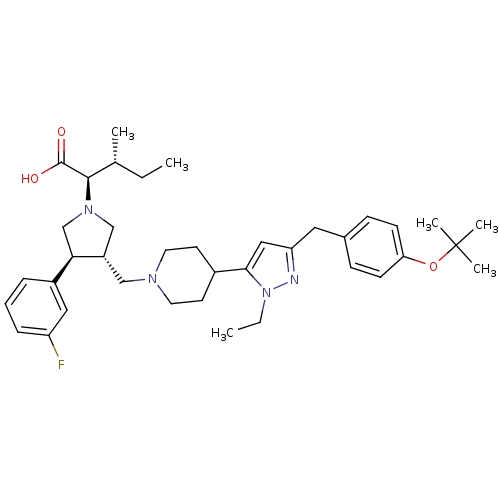
((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butoxy-benzyl)-...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC(C)(C)C)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H53FN4O3/c1-7-26(3)36(37(44)45)42-24-30(34(25-42)29-10-9-11-31(39)21-29)23-41-18-16-28(17-19-41)35-22-32(40-43(35)8-2)20-27-12-14-33(15-13-27)46-38(4,5)6/h9-15,21-22,26,28,30,34,36H,7-8,16-20,23-25H2,1-6H3,(H,44,45)/t26-,30+,34-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141906
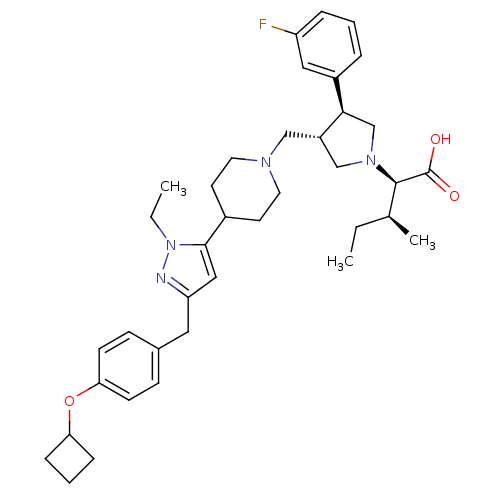
((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyclobutoxy-benzyl)-...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC4CCC4)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H51FN4O3/c1-4-26(3)37(38(44)45)42-24-30(35(25-42)29-8-6-9-31(39)21-29)23-41-18-16-28(17-19-41)36-22-32(40-43(36)5-2)20-27-12-14-34(15-13-27)46-33-10-7-11-33/h6,8-9,12-15,21-22,26,28,30,33,35,37H,4-5,7,10-11,16-20,23-25H2,1-3H3,(H,44,45)/t26-,30-,35+,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141973
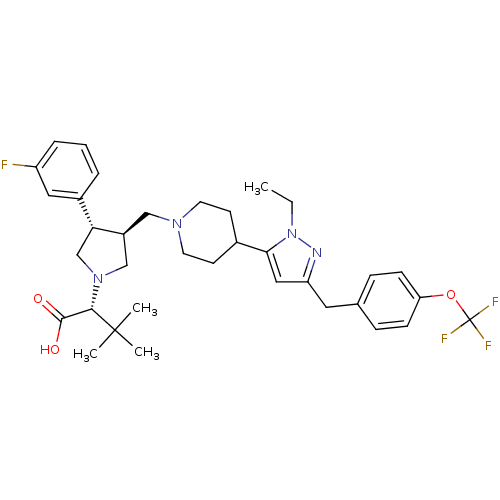
(2-[3-{4-[2-Ethyl-5-(4-trifluoromethoxy-benzyl)-2H-...)Show SMILES CCn1nc(Cc2ccc(OC(F)(F)F)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C35H44F4N4O3/c1-5-43-31(19-28(40-43)17-23-9-11-29(12-10-23)46-35(37,38)39)24-13-15-41(16-14-24)20-26-21-42(32(33(44)45)34(2,3)4)22-30(26)25-7-6-8-27(36)18-25/h6-12,18-19,24,26,30,32H,5,13-17,20-22H2,1-4H3,(H,44,45)/t26-,30+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141910
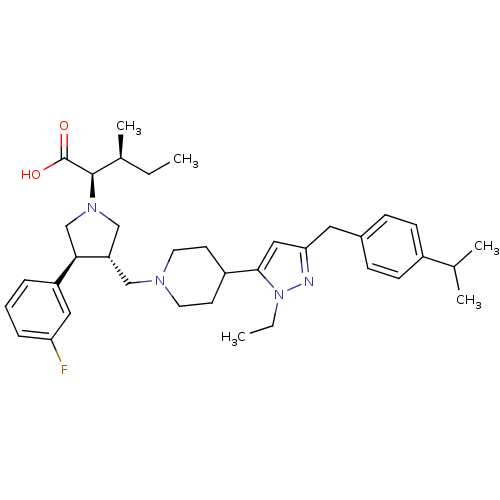
((2R,4S)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropyl-be...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)C(C)C)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C37H51FN4O2/c1-6-26(5)36(37(43)44)41-23-31(34(24-41)30-9-8-10-32(38)20-30)22-40-17-15-29(16-18-40)35-21-33(39-42(35)7-2)19-27-11-13-28(14-12-27)25(3)4/h8-14,20-21,25-26,29,31,34,36H,6-7,15-19,22-24H2,1-5H3,(H,43,44)/t26-,31-,34+,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141979
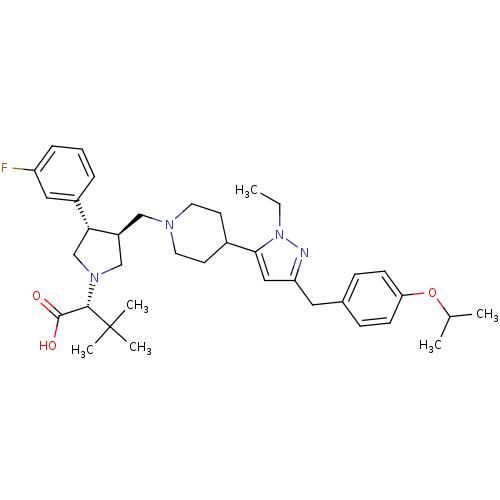
((R)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropoxy-benzy...)Show SMILES CCn1nc(Cc2ccc(OC(C)C)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C37H51FN4O3/c1-7-42-34(21-31(39-42)19-26-11-13-32(14-12-26)45-25(2)3)27-15-17-40(18-16-27)22-29-23-41(35(36(43)44)37(4,5)6)24-33(29)28-9-8-10-30(38)20-28/h8-14,20-21,25,27,29,33,35H,7,15-19,22-24H2,1-6H3,(H,43,44)/t29-,33+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141883
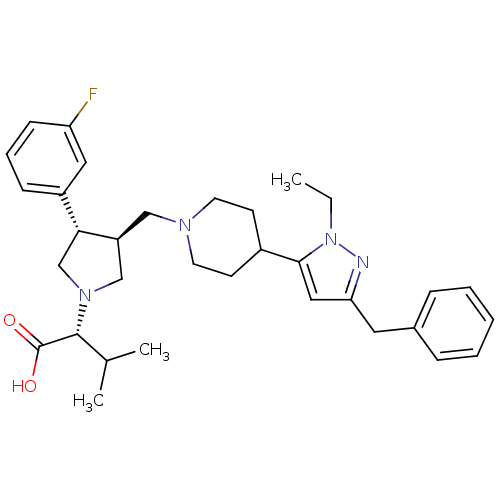
((R)-2-((3S,4S)-3-((4-(3-benzyl-1-ethyl-1H-pyrazol-...)Show SMILES CCn1nc(Cc2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C(C)C)C(O)=O)CC1 |r| Show InChI InChI=1S/C33H43FN4O2/c1-4-38-31(19-29(35-38)17-24-9-6-5-7-10-24)25-13-15-36(16-14-25)20-27-21-37(32(23(2)3)33(39)40)22-30(27)26-11-8-12-28(34)18-26/h5-12,18-19,23,25,27,30,32H,4,13-17,20-22H2,1-3H3,(H,39,40)/t27-,30+,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141984
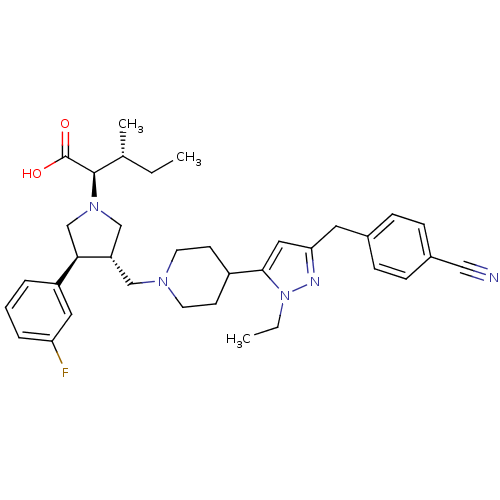
((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyano-benzyl)-2-ethy...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)C#N)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C35H44FN5O2/c1-4-24(3)34(35(42)43)40-22-29(32(23-40)28-7-6-8-30(36)18-28)21-39-15-13-27(14-16-39)33-19-31(38-41(33)5-2)17-25-9-11-26(20-37)12-10-25/h6-12,18-19,24,27,29,32,34H,4-5,13-17,21-23H2,1-3H3,(H,42,43)/t24-,29+,32-,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141972
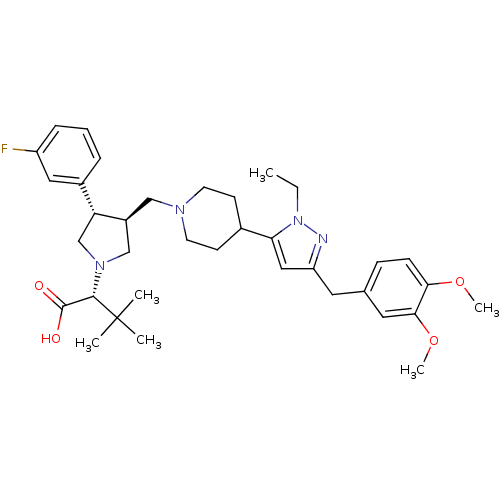
((R)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-2-et...)Show SMILES CCn1nc(Cc2ccc(OC)c(OC)c2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C36H49FN4O4/c1-7-41-31(20-29(38-41)17-24-11-12-32(44-5)33(18-24)45-6)25-13-15-39(16-14-25)21-27-22-40(34(35(42)43)36(2,3)4)23-30(27)26-9-8-10-28(37)19-26/h8-12,18-20,25,27,30,34H,7,13-17,21-23H2,1-6H3,(H,42,43)/t27-,30+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141874
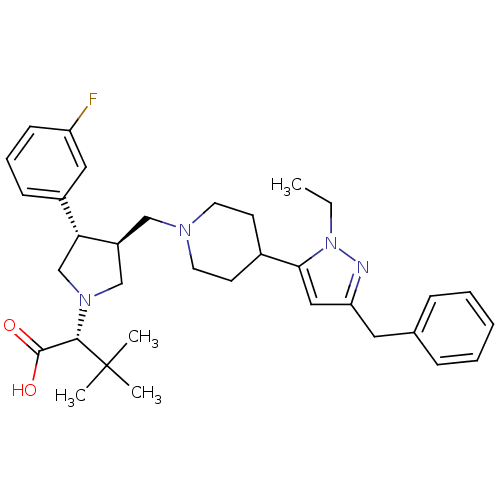
((R)-2-((3S,4S)-3-((4-(3-benzyl-1-ethyl-1H-pyrazol-...)Show SMILES CCn1nc(Cc2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 |r| Show InChI InChI=1S/C34H45FN4O2/c1-5-39-31(20-29(36-39)18-24-10-7-6-8-11-24)25-14-16-37(17-15-25)21-27-22-38(32(33(40)41)34(2,3)4)23-30(27)26-12-9-13-28(35)19-26/h6-13,19-20,25,27,30,32H,5,14-18,21-23H2,1-4H3,(H,40,41)/t27-,30+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141945
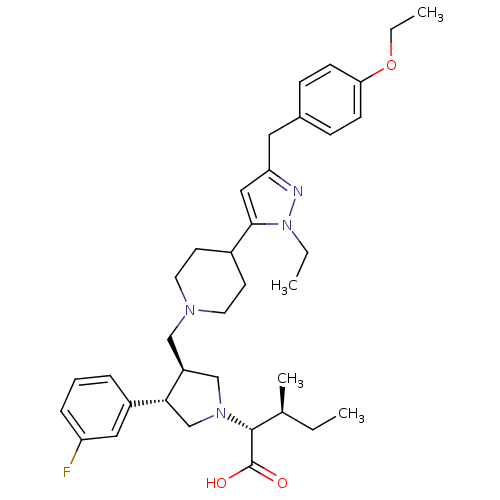
((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Ethoxy-benzyl)-2-eth...)Show SMILES CCOc1ccc(Cc2cc(C3CCN(C[C@H]4CN(C[C@@H]4c4cccc(F)c4)[C@H]([C@@H](C)CC)C(O)=O)CC3)n(CC)n2)cc1 Show InChI InChI=1S/C36H49FN4O3/c1-5-25(4)35(36(42)43)40-23-29(33(24-40)28-9-8-10-30(37)20-28)22-39-17-15-27(16-18-39)34-21-31(38-41(34)6-2)19-26-11-13-32(14-12-26)44-7-3/h8-14,20-21,25,27,29,33,35H,5-7,15-19,22-24H2,1-4H3,(H,42,43)/t25-,29-,33+,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141914
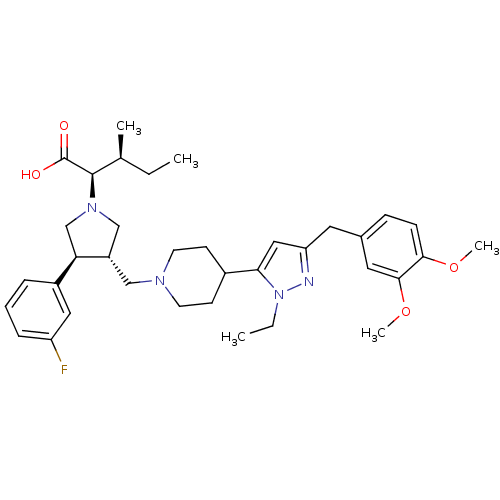
((2R,4S)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC)c(OC)c3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C36H49FN4O4/c1-6-24(3)35(36(42)43)40-22-28(31(23-40)27-9-8-10-29(37)19-27)21-39-15-13-26(14-16-39)32-20-30(38-41(32)7-2)17-25-11-12-33(44-4)34(18-25)45-5/h8-12,18-20,24,26,28,31,35H,6-7,13-17,21-23H2,1-5H3,(H,42,43)/t24-,28-,31+,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141875
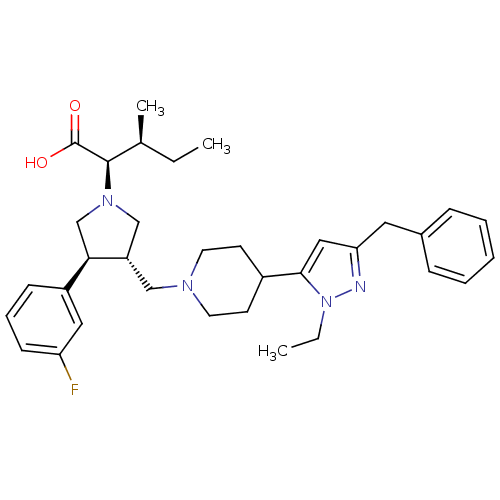
((2R,3S)-2-[(2S,3S)-3-[4-(5-Benzyl-2-ethyl-2H-pyraz...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccccc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C34H45FN4O2/c1-4-24(3)33(34(40)41)38-22-28(31(23-38)27-12-9-13-29(35)19-27)21-37-16-14-26(15-17-37)32-20-30(36-39(32)5-2)18-25-10-7-6-8-11-25/h6-13,19-20,24,26,28,31,33H,4-5,14-18,21-23H2,1-3H3,(H,40,41)/t24-,28-,31+,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50141887
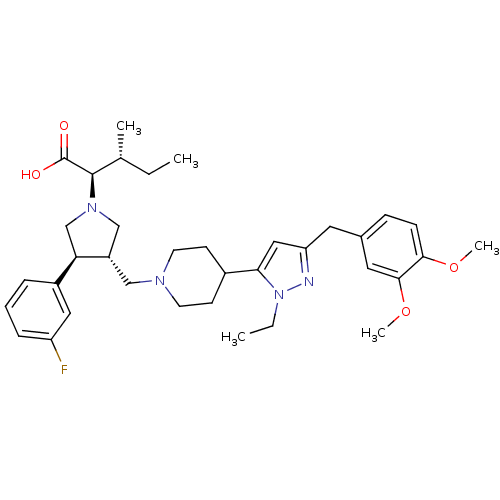
((2R,4R)-2-[(2S,3S)-3-{4-[5-(3,4-Dimethoxy-benzyl)-...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC)c(OC)c3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C36H49FN4O4/c1-6-24(3)35(36(42)43)40-22-28(31(23-40)27-9-8-10-29(37)19-27)21-39-15-13-26(14-16-39)32-20-30(38-41(32)7-2)17-25-11-12-33(44-4)34(18-25)45-5/h8-12,18-20,24,26,28,31,35H,6-7,13-17,21-23H2,1-5H3,(H,42,43)/t24-,28+,31-,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1 |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122385
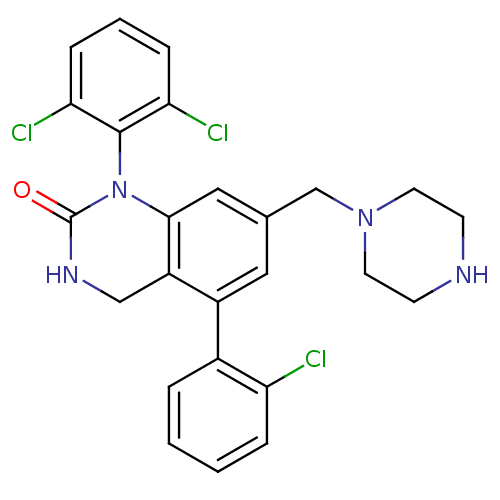
(5-(2-Chloro-phenyl)-1-(2,6-dichloro-phenyl)-7-pipe...)Show SMILES Clc1ccccc1-c1cc(CN2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H23Cl3N4O/c26-20-5-2-1-4-17(20)18-12-16(15-31-10-8-29-9-11-31)13-23-19(18)14-30-25(33)32(23)24-21(27)6-3-7-22(24)28/h1-7,12-13,29H,8-11,14-15H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122387

(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(OC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H21Cl3FN3O2/c26-20-2-1-3-21(27)24(20)32-23-12-16(34-15-6-8-30-9-7-15)11-18(19(23)13-31-25(32)33)17-5-4-14(29)10-22(17)28/h1-5,10-12,15,30H,6-9,13H2,(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122386
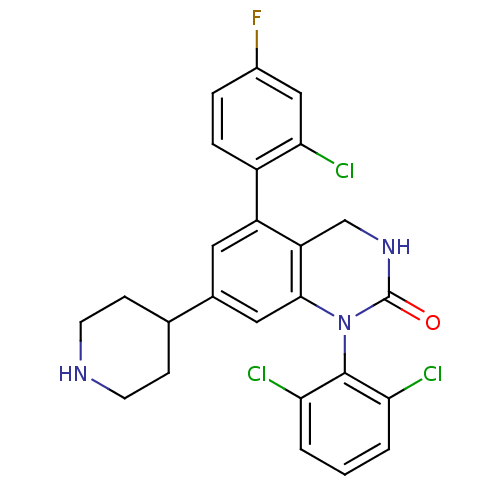
(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCNCC1 Show InChI InChI=1S/C25H21Cl3FN3O/c26-20-2-1-3-21(27)24(20)32-23-11-15(14-6-8-30-9-7-14)10-18(19(23)13-31-25(32)33)17-5-4-16(29)12-22(17)28/h1-5,10-12,14,30H,6-9,13H2,(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175747
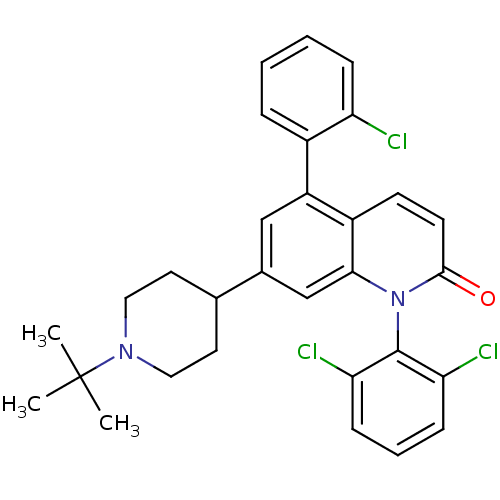
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chlorophenyl)-...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(19.17,-37.34,;17.84,-38.12,;18.62,-39.45,;17.07,-36.79,;16.51,-38.89,;16.51,-40.43,;15.18,-41.2,;13.84,-40.43,;13.83,-38.89,;15.17,-38.12,;12.51,-41.21,;12.51,-42.76,;11.17,-43.53,;11.18,-45.07,;9.84,-45.84,;9.84,-47.37,;11.17,-48.15,;12.51,-47.37,;12.51,-45.83,;13.84,-45.06,;9.84,-42.76,;8.51,-43.54,;7.17,-42.76,;7.17,-41.22,;5.84,-40.45,;8.51,-40.45,;8.51,-38.92,;7.18,-38.15,;5.85,-38.92,;7.18,-36.61,;8.52,-35.84,;9.85,-36.61,;9.85,-38.15,;11.18,-38.92,;9.84,-41.22,;11.17,-40.45,)| Show InChI InChI=1S/C30H29Cl3N2O/c1-30(2,3)34-15-13-19(14-16-34)20-17-23(21-7-4-5-8-24(21)31)22-11-12-28(36)35(27(22)18-20)29-25(32)9-6-10-26(29)33/h4-12,17-19H,13-16H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122390
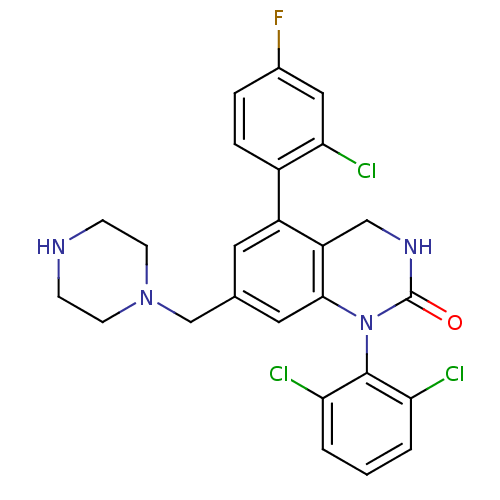
(5-(2-Chloro-4-fluoro-phenyl)-1-(2,6-dichloro-pheny...)Show SMILES Fc1ccc(c(Cl)c1)-c1cc(CN2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H22Cl3FN4O/c26-20-2-1-3-21(27)24(20)33-23-11-15(14-32-8-6-30-7-9-32)10-18(19(23)13-31-25(33)34)17-5-4-16(29)12-22(17)28/h1-5,10-12,30H,6-9,13-14H2,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
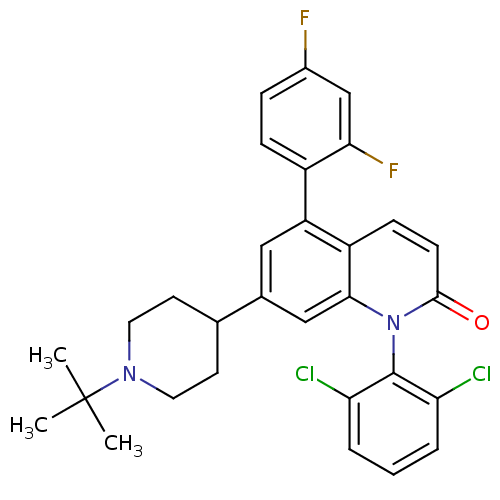
(Homo sapiens (Human)) | BDBM50175745
(7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2F)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(5.3,.54,;3.97,-.23,;4.75,-1.56,;3.2,1.1,;2.64,-1,;2.64,-2.54,;1.31,-3.31,;-.03,-2.54,;-.04,-1.01,;1.3,-.23,;-1.36,-3.31,;-1.35,-4.86,;-2.69,-5.64,;-2.69,-7.17,;-4.03,-7.94,;-4.03,-9.48,;-2.69,-10.25,;-2.69,-11.79,;-1.35,-9.47,;-1.36,-7.94,;-.03,-7.16,;-4.03,-4.86,;-5.36,-5.63,;-6.69,-4.85,;-6.69,-3.31,;-8.02,-2.53,;-5.34,-2.55,;-5.33,-1.01,;-6.66,-.24,;-8,-1,;-6.66,1.3,;-5.31,2.07,;-3.98,1.29,;-3.99,-.25,;-2.67,-1.03,;-4.02,-3.32,;-2.69,-2.55,)| Show InChI InChI=1S/C30H28Cl2F2N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(33)17-26(21)34)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141937
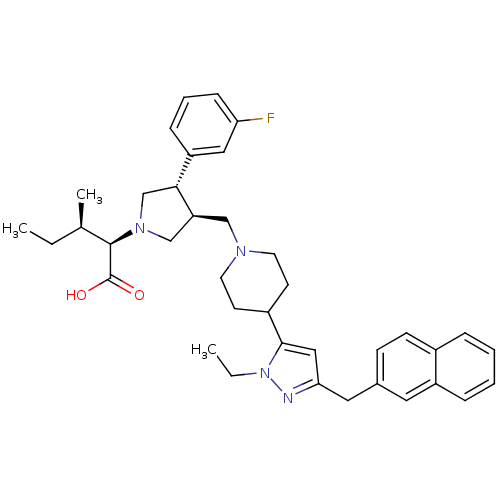
((2R,4R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-2-yl...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc4ccccc4c3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H47FN4O2/c1-4-26(3)37(38(44)45)42-24-32(35(25-42)31-11-8-12-33(39)21-31)23-41-17-15-29(16-18-41)36-22-34(40-43(36)5-2)20-27-13-14-28-9-6-7-10-30(28)19-27/h6-14,19,21-22,26,29,32,35,37H,4-5,15-18,20,23-25H2,1-3H3,(H,44,45)/t26-,32+,35-,37-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141948

((R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-1-ylmeth...)Show SMILES CCn1nc(Cc2cccc3ccccc23)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C38H47FN4O2/c1-5-43-35(22-32(40-43)21-29-12-8-11-26-10-6-7-15-33(26)29)27-16-18-41(19-17-27)23-30-24-42(36(37(44)45)38(2,3)4)25-34(30)28-13-9-14-31(39)20-28/h6-15,20,22,27,30,34,36H,5,16-19,21,23-25H2,1-4H3,(H,44,45)/t30-,34+,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122384
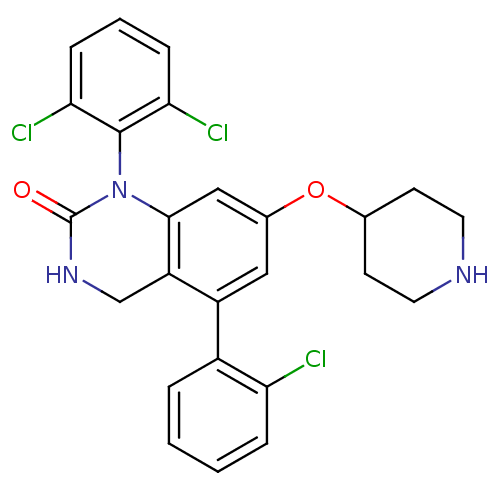
(5-(2-Chloro-phenyl)-1-(2,6-dichloro-phenyl)-7-(pip...)Show SMILES Clc1ccccc1-c1cc(OC2CCNCC2)cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H22Cl3N3O2/c26-20-5-2-1-4-17(20)18-12-16(33-15-8-10-29-11-9-15)13-23-19(18)14-30-25(32)31(23)24-21(27)6-3-7-22(24)28/h1-7,12-13,15,29H,8-11,14H2,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122379

(7-(1-tert-Butyl-piperidin-4-yl)-5-(2-chloro-4-fluo...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C29H29Cl3FN3O/c1-29(2,3)35-11-9-17(10-12-35)18-13-21(20-8-7-19(33)15-25(20)32)22-16-34-28(37)36(26(22)14-18)27-23(30)5-4-6-24(27)31/h4-8,13-15,17H,9-12,16H2,1-3H3,(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141904
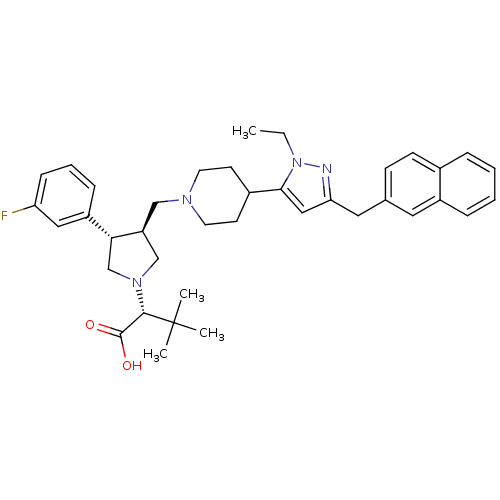
((R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-2-ylmeth...)Show SMILES CCn1nc(Cc2ccc3ccccc3c2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C38H47FN4O2/c1-5-43-35(22-33(40-43)20-26-13-14-27-9-6-7-10-29(27)19-26)28-15-17-41(18-16-28)23-31-24-42(36(37(44)45)38(2,3)4)25-34(31)30-11-8-12-32(39)21-30/h6-14,19,21-22,28,31,34,36H,5,15-18,20,23-25H2,1-4H3,(H,44,45)/t31-,34+,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141946

((2R,4R)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-1-yl...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3cccc4ccccc34)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H47FN4O2/c1-4-26(3)37(38(44)45)42-24-31(35(25-42)29-13-9-14-32(39)20-29)23-41-18-16-28(17-19-41)36-22-33(40-43(36)5-2)21-30-12-8-11-27-10-6-7-15-34(27)30/h6-15,20,22,26,28,31,35,37H,4-5,16-19,21,23-25H2,1-3H3,(H,44,45)/t26-,31+,35-,37-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222627
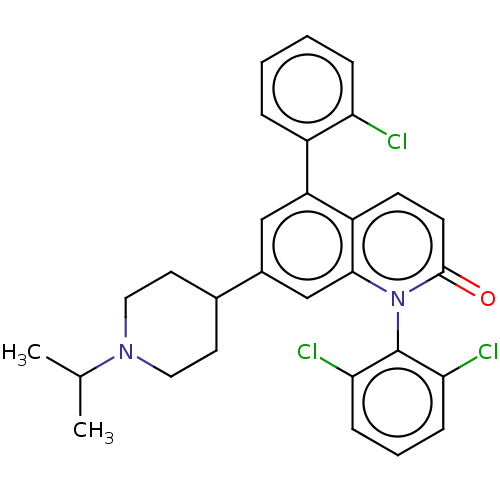
(CHEMBL356125)Show SMILES CC(C)N1CCC(CC1)c1cc(-c2ccccc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(15.97,6.5,;15.96,4.96,;17.29,4.19,;14.61,4.2,;14.61,2.66,;13.28,1.91,;11.95,2.68,;11.95,4.22,;13.28,4.97,;10.62,1.91,;10.62,.37,;9.27,-.4,;9.27,-1.94,;7.92,-2.69,;7.92,-4.23,;9.25,-5.02,;10.59,-4.25,;10.59,-2.71,;11.92,-1.92,;7.94,.37,;6.61,-.4,;5.28,.39,;5.28,1.91,;3.95,2.68,;6.63,2.68,;6.63,4.22,;7.96,4.99,;9.29,4.22,;7.96,6.53,;6.63,7.32,;5.3,6.53,;5.3,4.99,;3.95,4.24,;7.96,1.91,;9.29,2.68,)| Show InChI InChI=1S/C29H27Cl3N2O/c1-18(2)33-14-12-19(13-15-33)20-16-23(21-6-3-4-7-24(21)30)22-10-11-28(35)34(27(22)17-20)29-25(31)8-5-9-26(29)32/h3-11,16-19H,12-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222624
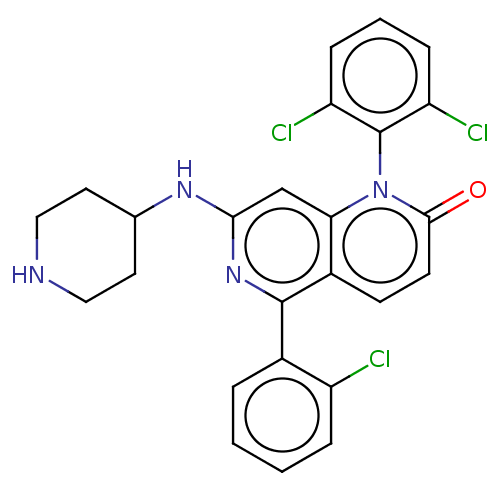
(CHEMBL357598)Show SMILES Clc1ccccc1-c1nc(NC2CCNCC2)cc2n(-c3c(Cl)cccc3Cl)c(=O)ccc12 |(10.11,-3.62,;8.78,-4.39,;8.78,-5.93,;7.43,-6.7,;6.1,-5.93,;6.11,-4.39,;7.45,-3.62,;7.47,-2.1,;8.8,-1.33,;8.8,.21,;10.13,.97,;11.46,.2,;12.79,.97,;14.12,.19,;14.12,-1.36,;12.79,-2.12,;11.46,-1.34,;7.47,.98,;6.14,.23,;4.81,.98,;4.81,2.52,;6.14,3.29,;7.47,2.52,;6.14,4.85,;4.81,5.62,;3.48,4.83,;3.48,3.29,;2.15,2.54,;3.48,.23,;2.13,1,;3.46,-1.31,;4.79,-2.08,;6.14,-1.31,)| Show InChI InChI=1S/C25H21Cl3N4O/c26-18-5-2-1-4-16(18)24-17-8-9-23(33)32(25-19(27)6-3-7-20(25)28)21(17)14-22(31-24)30-15-10-12-29-13-11-15/h1-9,14-15,29H,10-13H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAP kinase |
Bioorg Med Chem Lett 13: 467-70 (2003)
BindingDB Entry DOI: 10.7270/Q22F7QM4 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141990

((R)-Cyclohexyl-[(2S,3S)-3-{4-[5-(4-cyclopropoxy-be...)Show SMILES CCn1nc(Cc2ccc(OC3CC3)cc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C2CCCCC2)C(O)=O)CC1 Show InChI InChI=1S/C39H51FN4O3/c1-2-44-37(23-33(41-44)21-27-11-13-34(14-12-27)47-35-15-16-35)28-17-19-42(20-18-28)24-31-25-43(26-36(31)30-9-6-10-32(40)22-30)38(39(45)46)29-7-4-3-5-8-29/h6,9-14,22-23,28-29,31,35-36,38H,2-5,7-8,15-21,24-26H2,1H3,(H,45,46)/t31-,36+,38+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM17060
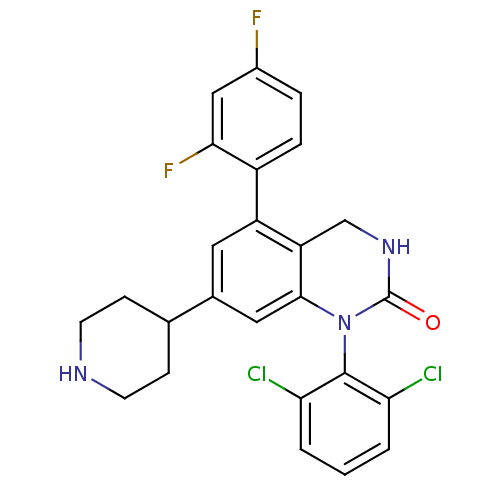
(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-(p...)Show SMILES Fc1ccc(c(F)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)C1CCNCC1 Show InChI InChI=1S/C25H21Cl2F2N3O/c26-20-2-1-3-21(27)24(20)32-23-11-15(14-6-8-30-9-7-14)10-18(19(23)13-31-25(32)33)17-5-4-16(28)12-22(17)29/h1-5,10-12,14,30H,6-9,13H2,(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175762
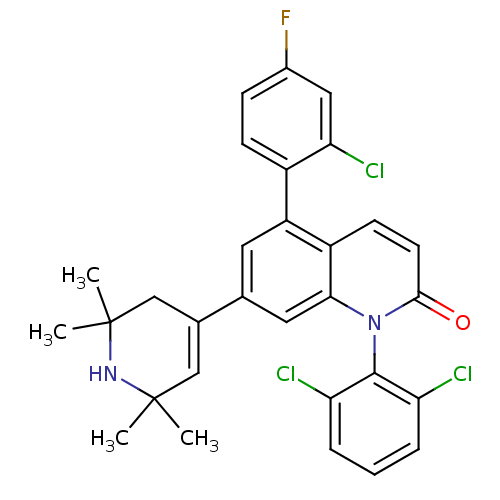
(5-(2-chloro-4-fluorophenyl)-1-(2,6-dichlorophenyl)...)Show SMILES CC1(C)CC(=CC(C)(C)N1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |c:4,(2.04,.58,;2.06,2.12,;3.39,2.89,;.73,1.35,;-.61,2.12,;-.61,3.66,;.72,4.43,;2.05,5.21,;-.61,5.2,;2.06,3.66,;-1.94,1.34,;-1.94,-.21,;-3.27,-.98,;-3.27,-2.51,;-4.61,-3.28,;-4.61,-4.82,;-3.27,-5.59,;-3.27,-7.13,;-1.94,-4.81,;-1.94,-3.28,;-.61,-2.5,;-4.61,-.21,;-5.94,-.98,;-7.28,-.21,;-7.28,1.34,;-8.61,2.11,;-5.94,2.1,;-5.94,3.64,;-7.27,4.4,;-8.6,3.63,;-7.27,5.94,;-5.93,6.72,;-4.6,5.94,;-4.6,4.41,;-3.27,3.63,;-4.61,1.34,;-3.28,2.11,)| Show InChI InChI=1S/C30H26Cl3FN2O/c1-29(2)15-18(16-30(3,4)35-29)17-12-22(20-9-8-19(34)14-25(20)33)21-10-11-27(37)36(26(21)13-17)28-23(31)6-5-7-24(28)32/h5-15,35H,16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175758
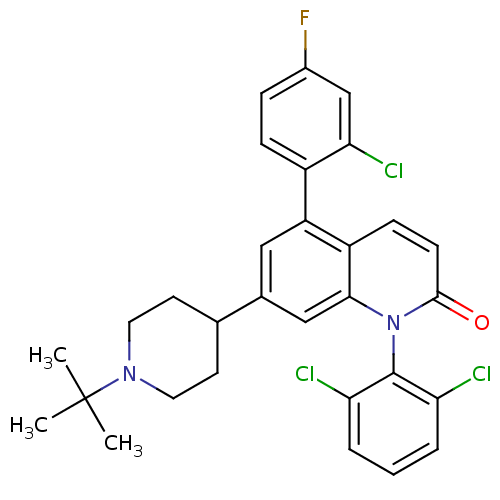
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(36.13,-13.37,;34.8,-14.15,;35.58,-15.48,;34.03,-12.81,;33.47,-14.92,;33.47,-16.46,;32.14,-17.22,;30.8,-16.46,;30.79,-14.92,;32.13,-14.15,;29.47,-17.23,;29.47,-18.78,;28.13,-19.55,;28.14,-21.09,;26.8,-21.86,;26.8,-23.4,;28.13,-24.17,;28.14,-25.71,;29.47,-23.39,;29.47,-21.85,;30.8,-21.08,;26.8,-18.78,;25.47,-19.56,;24.13,-18.79,;24.13,-17.24,;22.8,-16.47,;25.47,-16.47,;25.47,-14.94,;24.14,-14.17,;22.81,-14.95,;24.14,-12.64,;25.48,-11.86,;26.81,-12.63,;26.81,-14.17,;28.14,-14.94,;26.8,-17.24,;28.13,-16.47,)| Show InChI InChI=1S/C30H28Cl3FN2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(34)17-26(21)33)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of TNF alpha release in THP1 cells |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141942
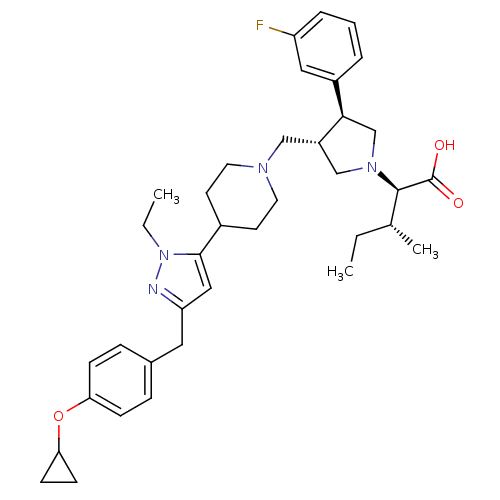
((2R,4R)-2-[(2S,3S)-3-{4-[5-(4-Cyclopropoxy-benzyl)...)Show SMILES CC[C@@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC4CC4)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C37H49FN4O3/c1-4-25(3)36(37(43)44)41-23-29(34(24-41)28-7-6-8-30(38)20-28)22-40-17-15-27(16-18-40)35-21-31(39-42(35)5-2)19-26-9-11-32(12-10-26)45-33-13-14-33/h6-12,20-21,25,27,29,33-34,36H,4-5,13-19,22-24H2,1-3H3,(H,43,44)/t25-,29+,34-,36-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141954
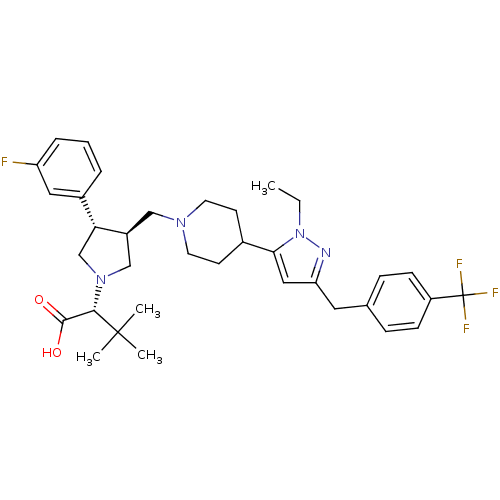
((R)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-trifluoromethyl-...)Show SMILES CCn1nc(Cc2ccc(cc2)C(F)(F)F)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1 Show InChI InChI=1S/C35H44F4N4O2/c1-5-43-31(19-29(40-43)17-23-9-11-27(12-10-23)35(37,38)39)24-13-15-41(16-14-24)20-26-21-42(32(33(44)45)34(2,3)4)22-30(26)25-7-6-8-28(36)18-25/h6-12,18-19,24,26,30,32H,5,13-17,20-22H2,1-4H3,(H,44,45)/t26-,30+,32-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50122382

(5-(2-Chloro-4-fluoro-phenyl)-7-[1-(1-cyclopropyl-e...)Show SMILES CC(C1CC1)N1CCC(CC1)c1cc2N(C(=O)NCc2c(c1)-c1ccc(F)cc1Cl)c1c(Cl)cccc1Cl Show InChI InChI=1S/C30H29Cl3FN3O/c1-17(18-5-6-18)36-11-9-19(10-12-36)20-13-23(22-8-7-21(34)15-27(22)33)24-16-35-30(38)37(28(24)14-20)29-25(31)3-2-4-26(29)32/h2-4,7-8,13-15,17-19H,5-6,9-12,16H2,1H3,(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mitogen-activated protein kinase p38 alpha |
Bioorg Med Chem Lett 13: 277-80 (2002)
BindingDB Entry DOI: 10.7270/Q25X2884 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141978

((2R,4S)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-e...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)-c3ccccc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C40H49FN4O2/c1-4-28(3)39(40(46)47)44-26-34(37(27-44)33-12-9-13-35(41)23-33)25-43-20-18-32(19-21-43)38-24-36(42-45(38)5-2)22-29-14-16-31(17-15-29)30-10-7-6-8-11-30/h6-17,23-24,28,32,34,37,39H,4-5,18-22,25-27H2,1-3H3,(H,46,47)/t28-,34-,37+,39+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141931

((R)-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl-2...)Show SMILES CCn1nc(Cc2ccc(cc2)-c2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C2CCCCC2)C(O)=O)CC1 Show InChI InChI=1S/C42H51FN4O2/c1-2-47-40(26-38(44-47)24-30-16-18-32(19-17-30)31-10-5-3-6-11-31)33-20-22-45(23-21-33)27-36-28-46(29-39(36)35-14-9-15-37(43)25-35)41(42(48)49)34-12-7-4-8-13-34/h3,5-6,9-11,14-19,25-26,33-34,36,39,41H,2,4,7-8,12-13,20-24,27-29H2,1H3,(H,48,49)/t36-,39+,41+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141925
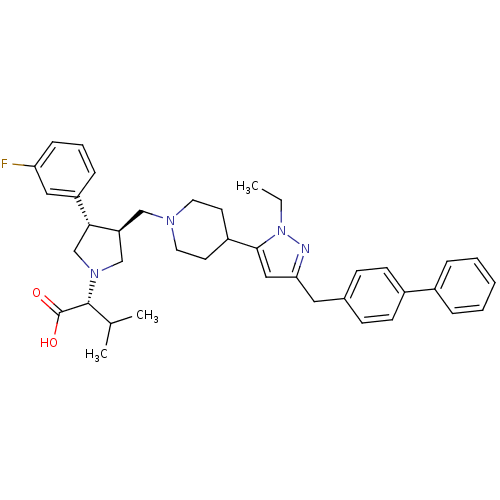
((R)-2-[(2S,3S)-3-[4-(5-Biphenyl-4-ylmethyl-2-ethyl...)Show SMILES CCn1nc(Cc2ccc(cc2)-c2ccccc2)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@H](C(C)C)C(O)=O)CC1 Show InChI InChI=1S/C39H47FN4O2/c1-4-44-37(23-35(41-44)21-28-13-15-30(16-14-28)29-9-6-5-7-10-29)31-17-19-42(20-18-31)24-33-25-43(38(27(2)3)39(45)46)26-36(33)32-11-8-12-34(40)22-32/h5-16,22-23,27,31,33,36,38H,4,17-21,24-26H2,1-3H3,(H,45,46)/t33-,36+,38+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141915
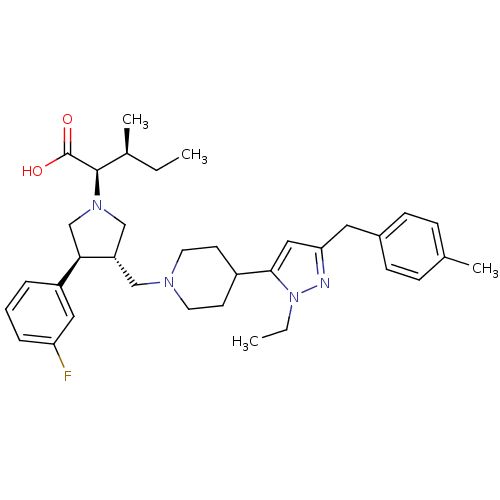
((2R,4S)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-methyl-benzy...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(C)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C35H47FN4O2/c1-5-25(4)34(35(41)42)39-22-29(32(23-39)28-8-7-9-30(36)19-28)21-38-16-14-27(15-17-38)33-20-31(37-40(33)6-2)18-26-12-10-24(3)11-13-26/h7-13,19-20,25,27,29,32,34H,5-6,14-18,21-23H2,1-4H3,(H,41,42)/t25-,29-,32+,34+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141912
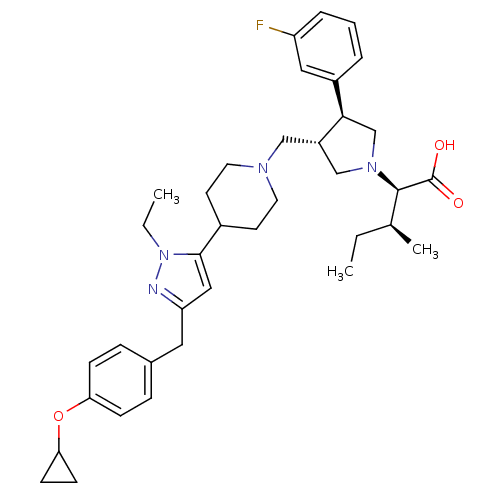
((2R,4S)-2-[(2S,3S)-3-{4-[5-(4-Cyclopropoxy-benzyl)...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(OC4CC4)cc3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C37H49FN4O3/c1-4-25(3)36(37(43)44)41-23-29(34(24-41)28-7-6-8-30(38)20-28)22-40-17-15-27(16-18-40)35-21-31(39-42(35)5-2)19-26-9-11-32(12-10-26)45-33-13-14-33/h6-12,20-21,25,27,29,33-34,36H,4-5,13-19,22-24H2,1-3H3,(H,43,44)/t25-,29-,34+,36+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141910

((2R,4S)-2-[(2S,3S)-3-{4-[2-Ethyl-5-(4-isopropyl-be...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc(cc3)C(C)C)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C37H51FN4O2/c1-6-26(5)36(37(43)44)41-23-31(34(24-41)30-9-8-10-32(38)20-30)22-40-17-15-29(16-18-40)35-21-33(39-42(35)7-2)19-27-11-13-28(14-12-27)25(3)4/h8-14,20-21,25-26,29,31,34,36H,6-7,15-19,22-24H2,1-5H3,(H,43,44)/t26-,31-,34+,36+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50175760
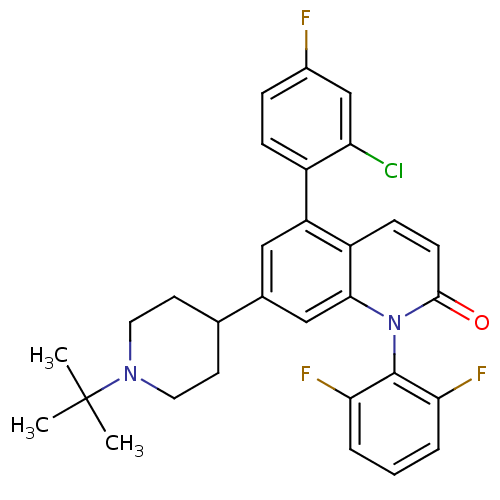
(7-(1-tert-butylpiperidin-4-yl)-5-(2-chloro-4-fluor...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2Cl)c2ccc(=O)n(-c3c(F)cccc3F)c2c1 |(36.23,-35.35,;34.9,-36.13,;35.68,-37.46,;34.13,-34.8,;33.57,-36.9,;33.57,-38.44,;32.24,-39.21,;30.9,-38.44,;30.9,-36.9,;32.23,-36.13,;29.57,-39.22,;29.57,-40.77,;28.24,-41.54,;28.24,-43.08,;26.9,-43.84,;26.9,-45.38,;28.24,-46.15,;28.24,-47.69,;29.57,-45.38,;29.57,-43.84,;30.9,-43.07,;26.9,-40.77,;25.57,-41.55,;24.23,-40.77,;24.23,-39.23,;22.9,-38.46,;25.57,-38.46,;25.57,-36.92,;24.24,-36.16,;22.91,-36.93,;24.24,-34.62,;25.58,-33.85,;26.91,-34.62,;26.91,-36.16,;28.24,-36.93,;26.9,-39.23,;28.23,-38.46,)| Show InChI InChI=1S/C30H28ClF3N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(32)17-24(21)31)22-9-10-28(37)36(27(22)16-19)29-25(33)5-4-6-26(29)34/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of P38 alpha MAPK |
Bioorg Med Chem Lett 16: 64-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.065
BindingDB Entry DOI: 10.7270/Q2TT4QH8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50141939
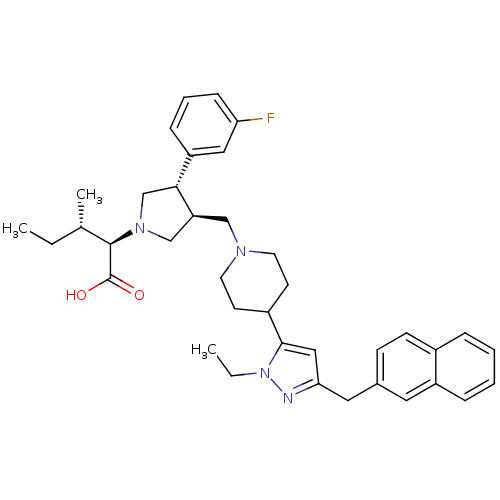
((2R,4S)-2-[(2S,3S)-3-[4-(2-Ethyl-5-naphthalen-2-yl...)Show SMILES CC[C@H](C)[C@@H](N1C[C@H](CN2CCC(CC2)c2cc(Cc3ccc4ccccc4c3)nn2CC)[C@H](C1)c1cccc(F)c1)C(O)=O Show InChI InChI=1S/C38H47FN4O2/c1-4-26(3)37(38(44)45)42-24-32(35(25-42)31-11-8-12-33(39)21-31)23-41-17-15-29(16-18-41)36-22-34(40-43(36)5-2)20-27-13-14-28-9-6-7-10-30(28)19-27/h6-14,19,21-22,26,29,32,35,37H,4-5,15-18,20,23-25H2,1-3H3,(H,44,45)/t26-,32-,35+,37+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration for displacement of [125I]-MIP-1 alpha from recombinant human CC chemokine receptor 5 (CCR5) expressed in CHO cell |
Bioorg Med Chem Lett 14: 947-52 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.006
BindingDB Entry DOI: 10.7270/Q2F18Z5V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data