 Found 125 hits with Last Name = 'poole' and Initial = 'jc'
Found 125 hits with Last Name = 'poole' and Initial = 'jc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-K receptor
(Mus musculus) | BDBM50290298

((1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimet...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(=O)N2CCOCC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C38H45Cl2N3O6/c1-46-32-23-27(24-33(47-2)34(32)48-3)35(44)43-18-12-37(26-43,29-9-10-30(39)31(40)25-29)11-15-41-16-13-38(14-17-41,28-7-5-4-6-8-28)36(45)42-19-21-49-22-20-42/h4-10,23-25H,11-22,26H2,1-3H3/t37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290306
((1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimet...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(=O)N2CCN(C)CC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C39H48Cl2N4O5/c1-42-20-22-44(23-21-42)37(47)39(29-8-6-5-7-9-29)14-17-43(18-15-39)16-12-38(30-10-11-31(40)32(41)26-30)13-19-45(27-38)36(46)28-24-33(48-2)35(50-4)34(25-28)49-3/h5-11,24-26H,12-23,27H2,1-4H3/t38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290302
(1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2ccncc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C33H38Cl2N4O5/c1-42-27-18-22(19-28(43-2)29(27)44-3)30(40)39-17-9-32(21-39,24-4-5-25(34)26(35)20-24)8-14-38-15-10-33(11-16-38,31(36)41)23-6-12-37-13-7-23/h4-7,12-13,18-20H,8-11,14-17,21H2,1-3H3,(H2,36,41)/t32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290313

((1'-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trime...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(=O)N2CCN(C)CC2)c2cccnc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C38H47Cl2N5O5/c1-42-18-20-44(21-19-42)36(47)38(29-6-5-13-41-25-29)11-15-43(16-12-38)14-9-37(28-7-8-30(39)31(40)24-28)10-17-45(26-37)35(46)27-22-32(48-2)34(50-4)33(23-27)49-3/h5-8,13,22-25H,9-12,14-21,26H2,1-4H3/t37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290303
((1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(=O)N2CCN(C)CC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C39H48Cl2N4O5/c1-42-20-22-44(23-21-42)37(47)39(29-8-6-5-7-9-29)14-17-43(18-15-39)16-12-38(30-10-11-31(40)32(41)26-30)13-19-45(27-38)36(46)28-24-33(48-2)35(50-4)34(25-28)49-3/h5-11,24-26H,12-23,27H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290297
(1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C34H39Cl2N3O5/c1-42-28-19-23(20-29(43-2)30(28)44-3)31(40)39-18-12-33(22-39,25-9-10-26(35)27(36)21-25)11-15-38-16-13-34(14-17-38,32(37)41)24-7-5-4-6-8-24/h4-10,19-21H,11-18,22H2,1-3H3,(H2,37,41)/t33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290312
((1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(=O)N2CCOCC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C38H45Cl2N3O6/c1-46-32-23-27(24-33(47-2)34(32)48-3)35(44)43-18-12-37(26-43,29-9-10-30(39)31(40)25-29)11-15-41-16-13-38(14-17-41,28-7-5-4-6-8-28)36(45)42-19-21-49-22-20-42/h4-10,23-25H,11-22,26H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290306

((1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimet...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(=O)N2CCN(C)CC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C39H48Cl2N4O5/c1-42-20-22-44(23-21-42)37(47)39(29-8-6-5-7-9-29)14-17-43(18-15-39)16-12-38(30-10-11-31(40)32(41)26-30)13-19-45(27-38)36(46)28-24-33(48-2)35(50-4)34(25-28)49-3/h5-11,24-26H,12-23,27H2,1-4H3/t38-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290307
(1'-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimet...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2cccnc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C33H38Cl2N4O5/c1-42-27-17-22(18-28(43-2)29(27)44-3)30(40)39-16-9-32(21-39,23-6-7-25(34)26(35)19-23)8-13-38-14-10-33(11-15-38,31(36)41)24-5-4-12-37-20-24/h4-7,12,17-20H,8-11,13-16,21H2,1-3H3,(H2,36,41)/t32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290298

((1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimet...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(=O)N2CCOCC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C38H45Cl2N3O6/c1-46-32-23-27(24-33(47-2)34(32)48-3)35(44)43-18-12-37(26-43,29-9-10-30(39)31(40)25-29)11-15-41-16-13-38(14-17-41,28-7-5-4-6-8-28)36(45)42-19-21-49-22-20-42/h4-10,23-25H,11-22,26H2,1-3H3/t37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290303

((1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(=O)N2CCN(C)CC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C39H48Cl2N4O5/c1-42-20-22-44(23-21-42)37(47)39(29-8-6-5-7-9-29)14-17-43(18-15-39)16-12-38(30-10-11-31(40)32(41)26-30)13-19-45(27-38)36(46)28-24-33(48-2)35(50-4)34(25-28)49-3/h5-11,24-26H,12-23,27H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290313

((1'-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trime...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(=O)N2CCN(C)CC2)c2cccnc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C38H47Cl2N5O5/c1-42-18-20-44(21-19-42)36(47)38(29-6-5-13-41-25-29)11-15-43(16-12-38)14-9-37(28-7-8-30(39)31(40)24-28)10-17-45(26-37)35(46)27-22-32(48-2)34(50-4)33(23-27)49-3/h5-8,13,22-25H,9-12,14-21,26H2,1-4H3/t37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290305
(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccncc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C33H38Cl2N4O5/c1-42-27-18-22(19-28(43-2)29(27)44-3)30(40)39-17-9-32(21-39,24-4-5-25(34)26(35)20-24)8-14-38-15-10-33(11-16-38,31(36)41)23-6-12-37-13-7-23/h4-7,12-13,18-20H,8-11,14-17,21H2,1-3H3,(H2,36,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50175494
(1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H39Cl2N3O5/c1-42-28-19-23(20-29(43-2)30(28)44-3)31(40)39-18-12-33(22-39,25-9-10-26(35)27(36)21-25)11-15-38-16-13-34(14-17-38,32(37)41)24-7-5-4-6-8-24/h4-10,19-21H,11-18,22H2,1-3H3,(H2,37,41)/t33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290299
(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(=O)NCCN2CCOCC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C40H50Cl2N4O6/c1-49-34-25-29(26-35(50-2)36(34)51-3)37(47)46-19-12-39(28-46,31-9-10-32(41)33(42)27-31)11-16-44-17-13-40(14-18-44,30-7-5-4-6-8-30)38(48)43-15-20-45-21-23-52-24-22-45/h4-10,25-27H,11-24,28H2,1-3H3,(H,43,48) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290307

(1'-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimet...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2cccnc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C33H38Cl2N4O5/c1-42-27-17-22(18-28(43-2)29(27)44-3)30(40)39-16-9-32(21-39,23-6-7-25(34)26(35)19-23)8-13-38-14-10-33(11-15-38,31(36)41)24-5-4-12-37-20-24/h4-7,12,17-20H,8-11,13-16,21H2,1-3H3,(H2,36,41)/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290295
(1'-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2cccnc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C33H38Cl2N4O5/c1-42-27-17-22(18-28(43-2)29(27)44-3)30(40)39-16-9-32(21-39,23-6-7-25(34)26(35)19-23)8-13-38-14-10-33(11-15-38,31(36)41)24-5-4-12-37-20-24/h4-7,12,17-20H,8-11,13-16,21H2,1-3H3,(H2,36,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290297

(1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C34H39Cl2N3O5/c1-42-28-19-23(20-29(43-2)30(28)44-3)31(40)39-18-12-33(22-39,25-9-10-26(35)27(36)21-25)11-15-38-16-13-34(14-17-38,32(37)41)24-7-5-4-6-8-24/h4-10,19-21H,11-18,22H2,1-3H3,(H2,37,41)/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290314
(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy-...)Show SMILES COc1cccc(c1)C1(CCN(CCC2(CCN(C2)C(=O)c2cc(OC)c(OC)c(OC)c2)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C35H41Cl2N3O6/c1-43-26-7-5-6-25(20-26)35(33(38)42)12-15-39(16-13-35)14-10-34(24-8-9-27(36)28(37)21-24)11-17-40(22-34)32(41)23-18-29(44-2)31(46-4)30(19-23)45-3/h5-9,18-21H,10-17,22H2,1-4H3,(H2,38,42) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290311
(1-{2-[3-(4-Chloro-phenyl)-1-(3,4,5-trimethoxy-benz...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C34H40ClN3O5/c1-41-28-21-24(22-29(42-2)30(28)43-3)31(39)38-20-14-33(23-38,25-9-11-27(35)12-10-25)13-17-37-18-15-34(16-19-37,32(36)40)26-7-5-4-6-8-26/h4-12,21-22H,13-20,23H2,1-3H3,(H2,36,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290312

((1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(=O)N2CCOCC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C38H45Cl2N3O6/c1-46-32-23-27(24-33(47-2)34(32)48-3)35(44)43-18-12-37(26-43,29-9-10-30(39)31(40)25-29)11-15-41-16-13-38(14-17-41,28-7-5-4-6-8-28)36(45)42-19-21-49-22-20-42/h4-10,23-25H,11-22,26H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290300
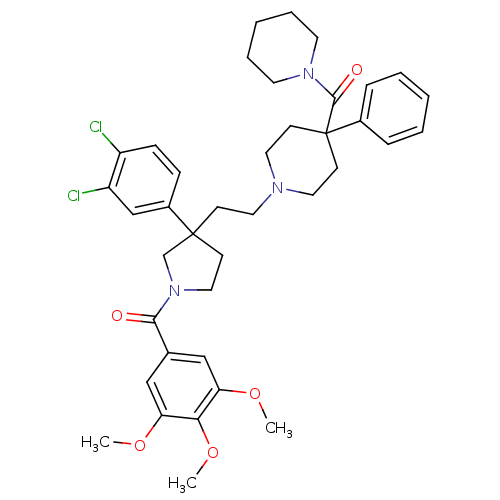
((1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(=O)N2CCCCC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C39H47Cl2N3O5/c1-47-33-24-28(25-34(48-2)35(33)49-3)36(45)44-23-15-38(27-44,30-12-13-31(40)32(41)26-30)14-20-42-21-16-39(17-22-42,29-10-6-4-7-11-29)37(46)43-18-8-5-9-19-43/h4,6-7,10-13,24-26H,5,8-9,14-23,27H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290308
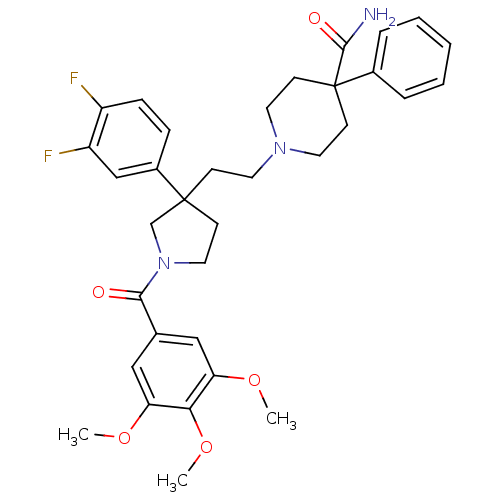
(1-{2-[3-(3,4-Difluoro-phenyl)-1-(3,4,5-trimethoxy-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(F)c(F)c1 Show InChI InChI=1S/C34H39F2N3O5/c1-42-28-19-23(20-29(43-2)30(28)44-3)31(40)39-18-12-33(22-39,25-9-10-26(35)27(36)21-25)11-15-38-16-13-34(14-17-38,32(37)41)24-7-5-4-6-8-24/h4-10,19-21H,11-18,22H2,1-3H3,(H2,37,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290302

(1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2ccncc2)(C1)c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C33H38Cl2N4O5/c1-42-27-18-22(19-28(43-2)29(27)44-3)30(40)39-17-9-32(21-39,24-4-5-25(34)26(35)20-24)8-14-38-15-10-33(11-16-38,31(36)41)23-6-12-37-13-7-23/h4-7,12-13,18-20H,8-11,14-17,21H2,1-3H3,(H2,36,41)/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290309
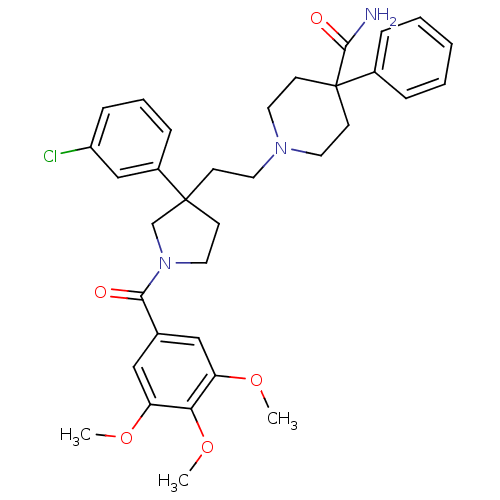
(1-{2-[3-(3-Chloro-phenyl)-1-(3,4,5-trimethoxy-benz...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1cccc(Cl)c1 Show InChI InChI=1S/C34H40ClN3O5/c1-41-28-20-24(21-29(42-2)30(28)43-3)31(39)38-19-13-33(23-38,26-10-7-11-27(35)22-26)12-16-37-17-14-34(15-18-37,32(36)40)25-8-5-4-6-9-25/h4-11,20-22H,12-19,23H2,1-3H3,(H2,36,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290299

(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(=O)NCCN2CCOCC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C40H50Cl2N4O6/c1-49-34-25-29(26-35(50-2)36(34)51-3)37(47)46-19-12-39(28-46,31-9-10-32(41)33(42)27-31)11-16-44-17-13-40(14-18-44,30-7-5-4-6-8-30)38(48)43-15-20-45-21-23-52-24-22-45/h4-10,25-27H,11-24,28H2,1-3H3,(H,43,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290310
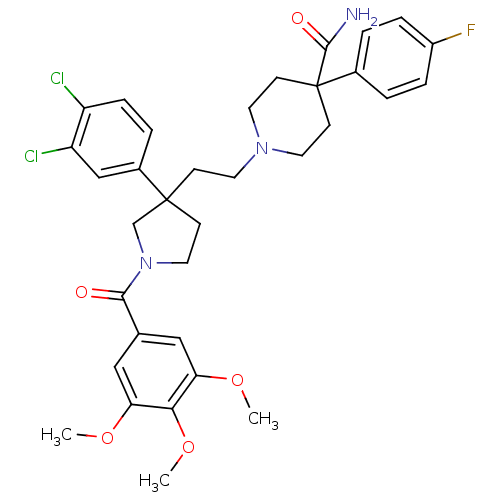
(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccc(F)cc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H38Cl2FN3O5/c1-43-28-18-22(19-29(44-2)30(28)45-3)31(41)40-17-11-33(21-40,24-6-9-26(35)27(36)20-24)10-14-39-15-12-34(13-16-39,32(38)42)23-4-7-25(37)8-5-23/h4-9,18-20H,10-17,21H2,1-3H3,(H2,38,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290310

(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccc(F)cc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H38Cl2FN3O5/c1-43-28-18-22(19-29(44-2)30(28)45-3)31(41)40-17-11-33(21-40,24-6-9-26(35)27(36)20-24)10-14-39-15-12-34(13-16-39,32(38)42)23-4-7-25(37)8-5-23/h4-9,18-20H,10-17,21H2,1-3H3,(H2,38,42) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290305

(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccncc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C33H38Cl2N4O5/c1-42-27-18-22(19-28(43-2)29(27)44-3)30(40)39-17-9-32(21-39,24-4-5-25(34)26(35)20-24)8-14-38-15-10-33(11-16-38,31(36)41)23-6-12-37-13-7-23/h4-7,12-13,18-20H,8-11,14-17,21H2,1-3H3,(H2,36,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290295

(1'-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2cccnc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C33H38Cl2N4O5/c1-42-27-17-22(18-28(43-2)29(27)44-3)30(40)39-16-9-32(21-39,23-6-7-25(34)26(35)19-23)8-13-38-14-10-33(11-15-38,31(36)41)24-5-4-12-37-20-24/h4-7,12,17-20H,8-11,13-16,21H2,1-3H3,(H2,36,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290314

(1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy-...)Show SMILES COc1cccc(c1)C1(CCN(CCC2(CCN(C2)C(=O)c2cc(OC)c(OC)c(OC)c2)c2ccc(Cl)c(Cl)c2)CC1)C(N)=O Show InChI InChI=1S/C35H41Cl2N3O6/c1-43-26-7-5-6-25(20-26)35(33(38)42)12-15-39(16-13-35)14-10-34(24-8-9-27(36)28(37)21-24)11-17-40(22-34)32(41)23-18-29(44-2)31(46-4)30(19-23)45-3/h5-9,18-21H,10-17,22H2,1-4H3,(H2,38,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50175494

(1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CC[C@@](CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H39Cl2N3O5/c1-42-28-19-23(20-29(43-2)30(28)44-3)31(40)39-18-12-33(22-39,25-9-10-26(35)27(36)21-25)11-15-38-16-13-34(14-17-38,32(37)41)24-7-5-4-6-8-24/h4-10,19-21H,11-18,22H2,1-3H3,(H2,37,41)/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290304

(1-{2-[3-(4-Fluoro-phenyl)-1-(3,4,5-trimethoxy-benz...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(F)cc1 Show InChI InChI=1S/C34H40FN3O5/c1-41-28-21-24(22-29(42-2)30(28)43-3)31(39)38-20-14-33(23-38,25-9-11-27(35)12-10-25)13-17-37-18-15-34(16-19-37,32(36)40)26-7-5-4-6-8-26/h4-12,21-22H,13-20,23H2,1-3H3,(H2,36,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290300

((1-{2-[3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimethoxy...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(=O)N2CCCCC2)c2ccccc2)(C1)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C39H47Cl2N3O5/c1-47-33-24-28(25-34(48-2)35(33)49-3)36(45)44-23-15-38(27-44,30-12-13-31(40)32(41)26-30)14-20-42-21-16-39(17-22-42,29-10-6-4-7-11-29)37(46)43-18-8-5-9-19-43/h4,6-7,10-13,24-26H,5,8-9,14-23,27H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(GUINEA PIG) | BDBM50290296
(1-{2-[3-(3,4-Dimethoxy-phenyl)-1-(3,4,5-trimethoxy...)Show SMILES COc1ccc(cc1OC)C1(CCN2CCC(CC2)(C(N)=O)c2ccccc2)CCN(C1)C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C36H45N3O7/c1-42-28-12-11-27(23-29(28)43-2)35(13-17-38-18-15-36(16-19-38,34(37)41)26-9-7-6-8-10-26)14-20-39(24-35)33(40)25-21-30(44-3)32(46-5)31(22-25)45-4/h6-12,21-23H,13-20,24H2,1-5H3,(H2,37,41) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SP |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Mus musculus) | BDBM50111203

(CHEMBL3604735)Show SMILES Cc1cc(NCc2c(Cl)ccc(Cl)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H14Cl3N3O/c1-9-7-15(10-3-2-4-11(18(22)25)17(10)24-9)23-8-12-13(19)5-6-14(20)16(12)21/h2-7H,8H2,1H3,(H2,22,25)(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse CD38 |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290311

(1-{2-[3-(4-Chloro-phenyl)-1-(3,4,5-trimethoxy-benz...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(Cl)cc1 Show InChI InChI=1S/C34H40ClN3O5/c1-41-28-21-24(22-29(42-2)30(28)43-3)31(39)38-20-14-33(23-38,25-9-11-27(35)12-10-25)13-17-37-18-15-34(16-19-37,32(36)40)26-7-5-4-6-8-26/h4-12,21-22H,13-20,23H2,1-3H3,(H2,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290308

(1-{2-[3-(3,4-Difluoro-phenyl)-1-(3,4,5-trimethoxy-...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(F)c(F)c1 Show InChI InChI=1S/C34H39F2N3O5/c1-42-28-19-23(20-29(43-2)30(28)44-3)31(40)39-18-12-33(22-39,25-9-10-26(35)27(36)21-25)11-15-38-16-13-34(14-17-38,32(37)41)24-7-5-4-6-8-24/h4-10,19-21H,11-18,22H2,1-3H3,(H2,37,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111135
(CHEMBL3604758)Show SMILES Cc1cc(NCc2c(Cl)ccc(Cl)c2Cl)c2cc(F)cc(C(N)=O)c2n1 Show InChI InChI=1S/C18H13Cl3FN3O/c1-8-4-15(24-7-12-13(19)2-3-14(20)16(12)21)10-5-9(22)6-11(18(23)26)17(10)25-8/h2-6H,7H2,1H3,(H2,23,26)(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Mus musculus) | BDBM50111218
(CHEMBL3604734)Show SMILES Cc1cc(NCc2c(F)cccc2C(F)(F)F)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C19H15F4N3O/c1-10-8-16(11-4-2-5-12(18(24)27)17(11)26-10)25-9-13-14(19(21,22)23)6-3-7-15(13)20/h2-8H,9H2,1H3,(H2,24,27)(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of mouse CD38 |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111158
(CHEMBL3604739)Show SMILES Cc1cc(NCc2c(F)ccc(Cl)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H14Cl2FN3O/c1-9-7-15(10-3-2-4-11(18(22)25)17(10)24-9)23-8-12-14(21)6-5-13(19)16(12)20/h2-7H,8H2,1H3,(H2,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111203

(CHEMBL3604735)Show SMILES Cc1cc(NCc2c(Cl)ccc(Cl)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H14Cl3N3O/c1-9-7-15(10-3-2-4-11(18(22)25)17(10)24-9)23-8-12-13(19)5-6-14(20)16(12)21/h2-7H,8H2,1H3,(H2,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111177

(CHEMBL3604737)Show SMILES Cc1cc(NCc2c(F)ccc(F)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H14ClF2N3O/c1-9-7-15(10-3-2-4-11(18(22)25)17(10)24-9)23-8-12-13(20)5-6-14(21)16(12)19/h2-7H,8H2,1H3,(H2,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290309

(1-{2-[3-(3-Chloro-phenyl)-1-(3,4,5-trimethoxy-benz...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1cccc(Cl)c1 Show InChI InChI=1S/C34H40ClN3O5/c1-41-28-20-24(21-29(42-2)30(28)43-3)31(39)38-19-13-33(23-38,26-10-7-11-27(35)22-26)12-16-37-17-14-34(15-18-37,32(36)40)25-8-5-4-6-9-25/h4-11,20-22H,12-19,23H2,1-3H3,(H2,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Mus musculus) | BDBM50290304

(1-{2-[3-(4-Fluoro-phenyl)-1-(3,4,5-trimethoxy-benz...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N1CCC(CCN2CCC(CC2)(C(N)=O)c2ccccc2)(C1)c1ccc(F)cc1 Show InChI InChI=1S/C34H40FN3O5/c1-41-28-21-24(22-29(42-2)30(28)43-3)31(39)38-20-14-33(23-38,25-9-11-27(35)12-10-25)13-17-37-18-15-34(16-19-37,32(36)40)26-7-5-4-6-8-26/h4-12,21-22H,13-20,23H2,1-3H3,(H2,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA. |
Bioorg Med Chem Lett 7: 2531-2536 (1997)
Article DOI: 10.1016/S0960-894X(97)10013-0
BindingDB Entry DOI: 10.7270/Q2154H1T |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111153
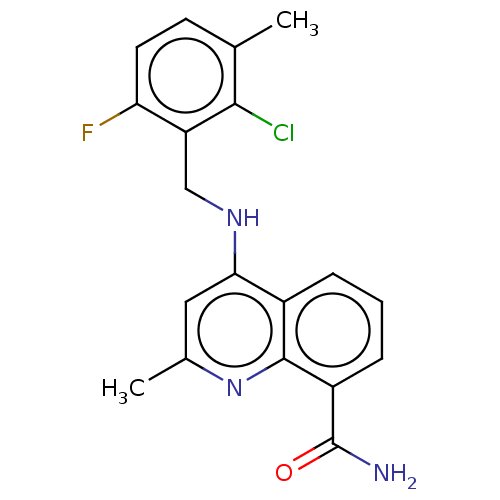
(CHEMBL3604740)Show SMILES Cc1cc(NCc2c(F)ccc(C)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C19H17ClFN3O/c1-10-6-7-15(21)14(17(10)20)9-23-16-8-11(2)24-18-12(16)4-3-5-13(18)19(22)25/h3-8H,9H2,1-2H3,(H2,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50111218

(CHEMBL3604734)Show SMILES Cc1cc(NCc2c(F)cccc2C(F)(F)F)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C19H15F4N3O/c1-10-8-16(11-4-2-5-12(18(24)27)17(11)26-10)25-9-13-14(19(21,22)23)6-3-7-15(13)20/h2-8H,9H2,1H3,(H2,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50110915
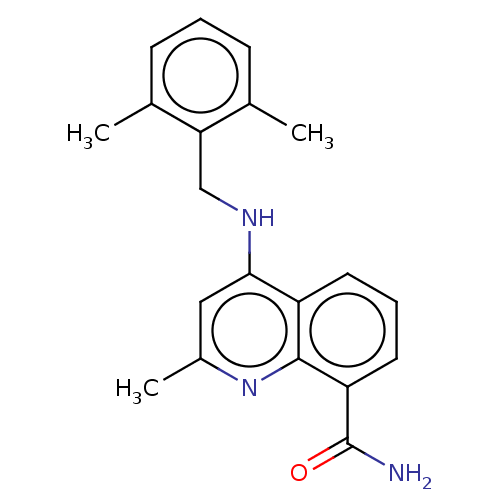
(CHEMBL3604701)Show InChI InChI=1S/C20H21N3O/c1-12-6-4-7-13(2)17(12)11-22-18-10-14(3)23-19-15(18)8-5-9-16(19)20(21)24/h4-10H,11H2,1-3H3,(H2,21,24)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50111203

(CHEMBL3604735)Show SMILES Cc1cc(NCc2c(Cl)ccc(Cl)c2Cl)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C18H14Cl3N3O/c1-9-7-15(10-3-2-4-11(18(22)25)17(10)24-9)23-8-12-13(19)5-6-14(20)16(12)21/h2-7H,8H2,1H3,(H2,22,25)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50111218

(CHEMBL3604734)Show SMILES Cc1cc(NCc2c(F)cccc2C(F)(F)F)c2cccc(C(N)=O)c2n1 Show InChI InChI=1S/C19H15F4N3O/c1-10-8-16(11-4-2-5-12(18(24)27)17(11)26-10)25-9-13-14(19(21,22)23)6-3-7-15(13)20/h2-8H,9H2,1H3,(H2,24,27)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CD38 extracellular domain expressed in Pichia pastoris using CHAPS and NAD by colorimetric-based assay |
J Med Chem 58: 7021-56 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00992
BindingDB Entry DOI: 10.7270/Q2542QCX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data