

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50335519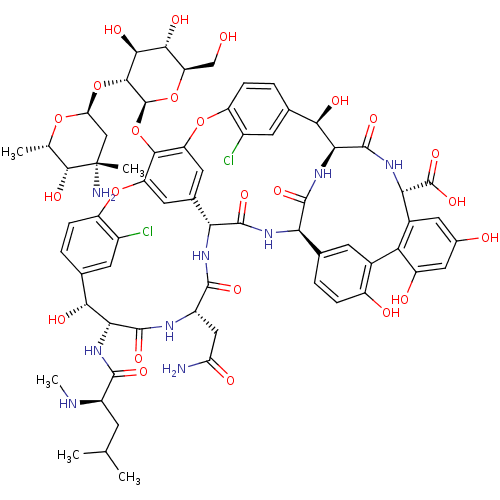 ((S)-3,6-Diamino-hexanoic acid {(3S,9S,12S,15S)-3-(...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 41.4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory Curated by ChEMBL | Assay Description Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay | Bioorg Med Chem Lett 14: 773-7 (2004) BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50103513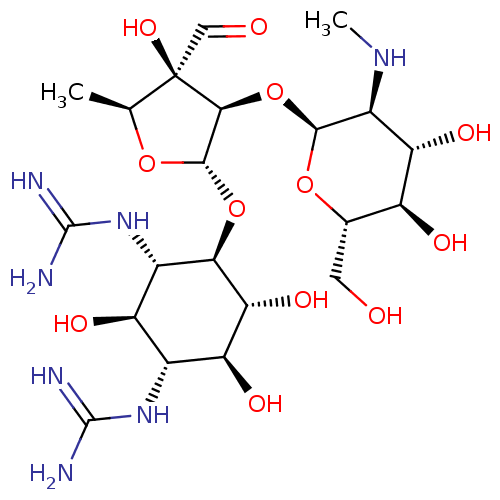 (CHEBI:17076 | Chemform | Gerox | NSC-14083 | Strep...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory Curated by ChEMBL | Assay Description Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay | Bioorg Med Chem Lett 14: 773-7 (2004) BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50221769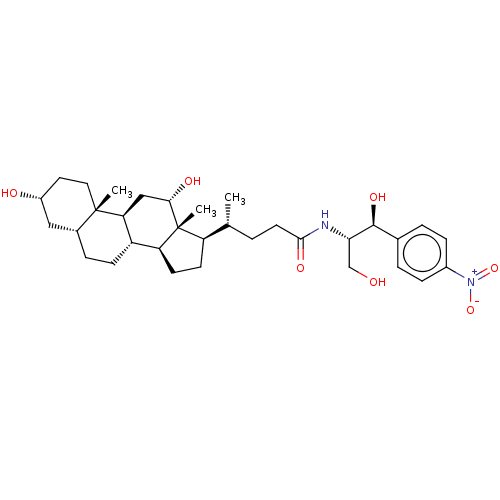 (CHEMBL166184) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory Curated by ChEMBL | Assay Description Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay | Bioorg Med Chem Lett 14: 773-7 (2004) BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50221777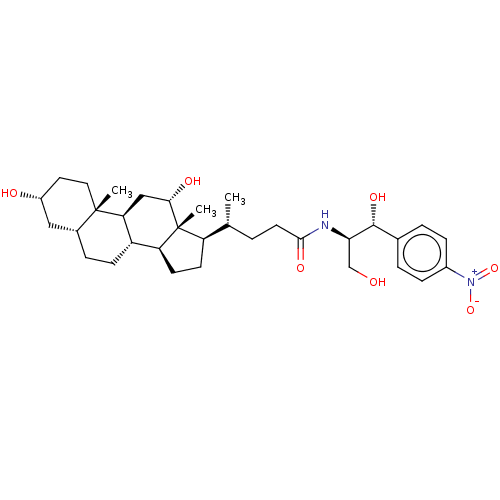 (CHEMBL352932) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory Curated by ChEMBL | Assay Description Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay | Bioorg Med Chem Lett 14: 773-7 (2004) BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50221774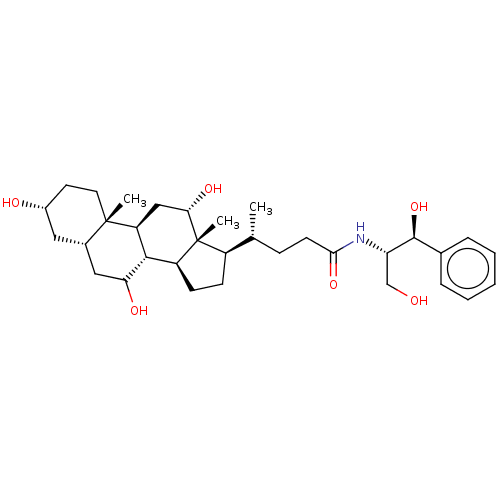 (CHEMBL353406) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory Curated by ChEMBL | Assay Description Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay | Bioorg Med Chem Lett 14: 773-7 (2004) BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50221746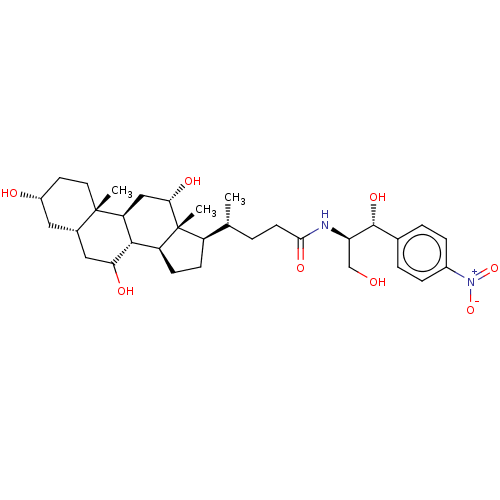 (CHEMBL354064) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory Curated by ChEMBL | Assay Description Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay | Bioorg Med Chem Lett 14: 773-7 (2004) BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50221744 (CHEMBL349656) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory Curated by ChEMBL | Assay Description Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay | Bioorg Med Chem Lett 14: 773-7 (2004) BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50221745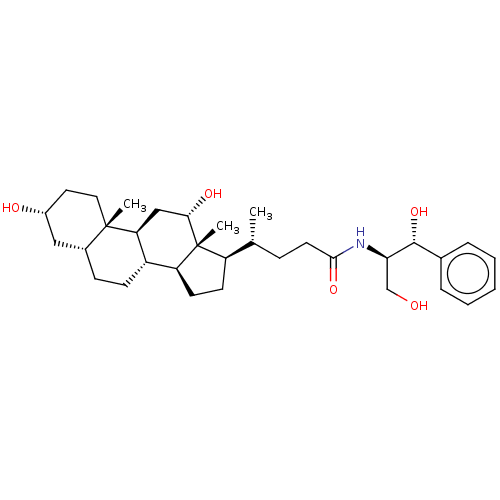 (CHEMBL350726) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory Curated by ChEMBL | Assay Description Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay | Bioorg Med Chem Lett 14: 773-7 (2004) BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50221743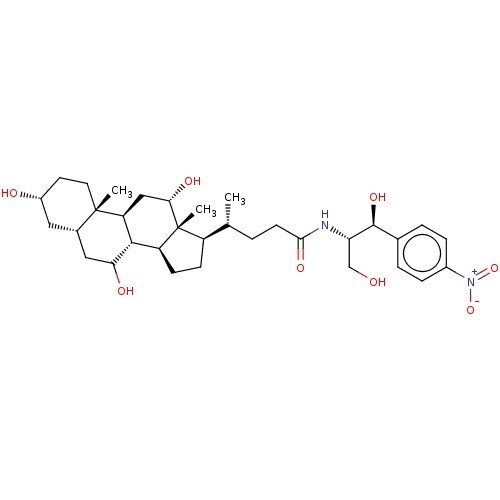 (CHEMBL352152) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory Curated by ChEMBL | Assay Description Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay | Bioorg Med Chem Lett 14: 773-7 (2004) BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50221775 (CHEMBL164621) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory Curated by ChEMBL | Assay Description Antibacterial activity against gram positive bacteria Enterococcus faecalis was determined by twofold Micro-broth dilution assay | Bioorg Med Chem Lett 14: 773-7 (2004) BindingDB Entry DOI: 10.7270/Q2FJ2JZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||