

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Bioorg Med Chem 18: 2173-7 (2010) Article DOI: 10.1016/j.bmc.2010.01.074 BindingDB Entry DOI: 10.7270/Q2R78G5X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50536193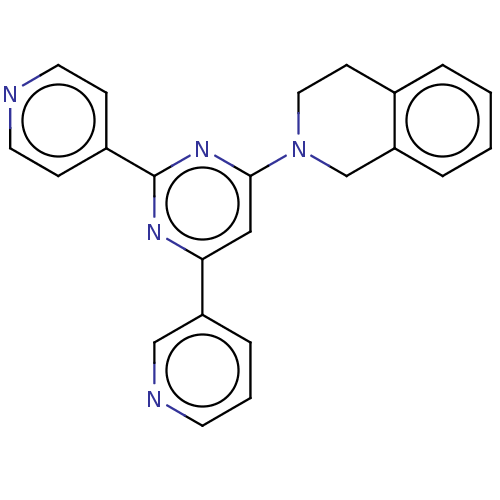 (CHEMBL548646 | GNF-Pf-1447 | TCMDC-125419) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50536191 (CHEMBL4584780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50536194 (CHEMBL4569641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50536191 (CHEMBL4584780) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(a... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50536194 (CHEMBL4569641) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(a... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry Curated by ChEMBL | Assay Description Inhibition of bovine AChE by Elman's method | Bioorg Med Chem 18: 2173-7 (2010) Article DOI: 10.1016/j.bmc.2010.01.074 BindingDB Entry DOI: 10.7270/Q2R78G5X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Bioorg Med Chem 18: 2173-7 (2010) Article DOI: 10.1016/j.bmc.2010.01.074 BindingDB Entry DOI: 10.7270/Q2R78G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50536191 (CHEMBL4584780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(t... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry Curated by ChEMBL | Assay Description Inhibition of bovine AChE by Elman's method | Bioorg Med Chem 18: 2173-7 (2010) Article DOI: 10.1016/j.bmc.2010.01.074 BindingDB Entry DOI: 10.7270/Q2R78G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50536194 (CHEMBL4569641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(t... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50222010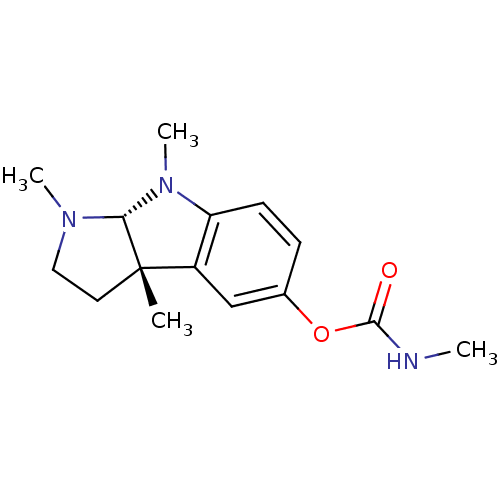 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry Curated by ChEMBL | Assay Description Inhibition of equine BChE at 100 uM by Ellman's method | J Nat Prod 70: 1529-31 (2007) Article DOI: 10.1021/np070259w BindingDB Entry DOI: 10.7270/Q2GT5P1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50536191 (CHEMBL4584780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 3-Cyano-7-Eth... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry Curated by ChEMBL | Assay Description Inhibition of bovine AChE by Elman's method | Bioorg Med Chem 18: 2173-7 (2010) Article DOI: 10.1016/j.bmc.2010.01.074 BindingDB Entry DOI: 10.7270/Q2R78G5X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50308337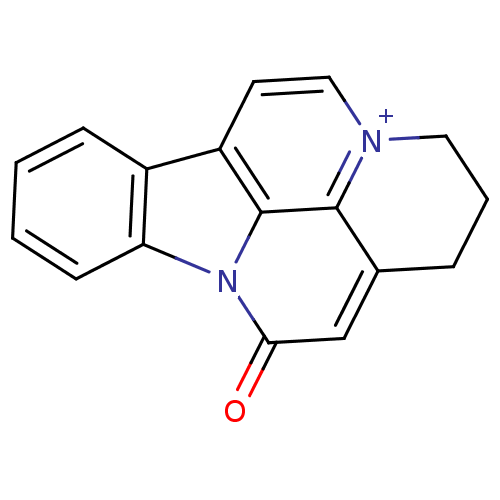 (CHEMBL589070 | Infractopicrin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry Curated by ChEMBL | Assay Description Inhibition of bovine AChE by Elman's method | Bioorg Med Chem 18: 2173-7 (2010) Article DOI: 10.1016/j.bmc.2010.01.074 BindingDB Entry DOI: 10.7270/Q2R78G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50550150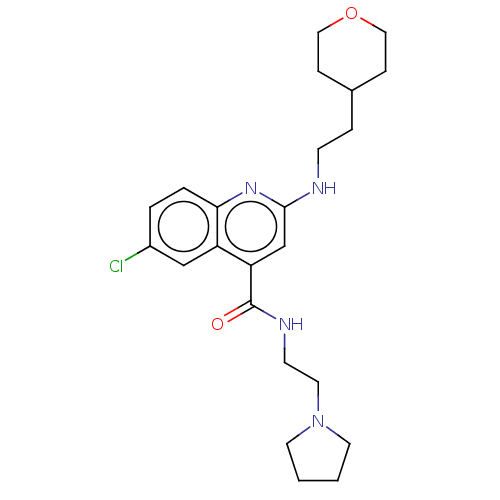 (CHEMBL4764214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by IonWorks patch-clamp electrophysiology method | Citation and Details BindingDB Entry DOI: 10.7270/Q2J67MKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50550151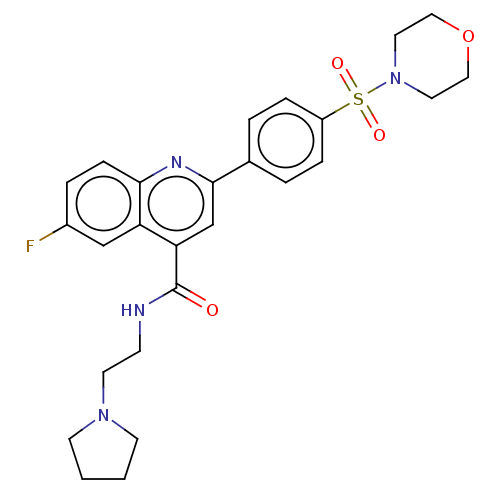 (CHEMBL4783407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by IonWorks patch-clamp electrophysiology method | Citation and Details BindingDB Entry DOI: 10.7270/Q2J67MKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50550149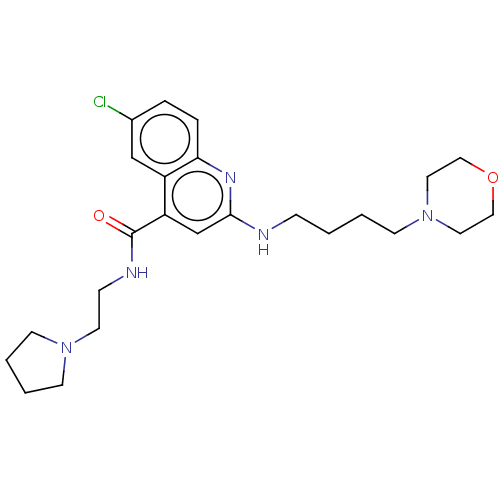 (CHEMBL4781713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by IonWorks patch-clamp electrophysiology method | Citation and Details BindingDB Entry DOI: 10.7270/Q2J67MKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50550148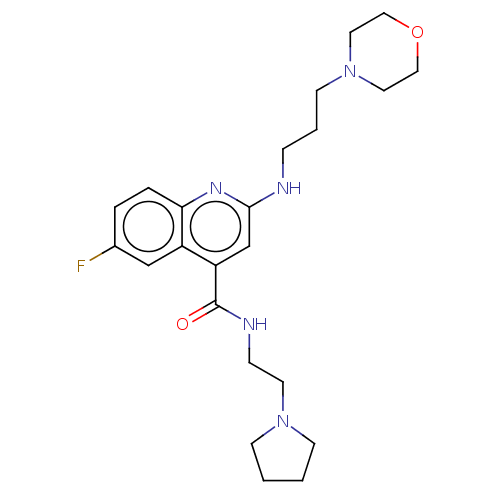 (CHEMBL4756468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by IonWorks patch-clamp electrophysiology method | Citation and Details BindingDB Entry DOI: 10.7270/Q2J67MKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50308338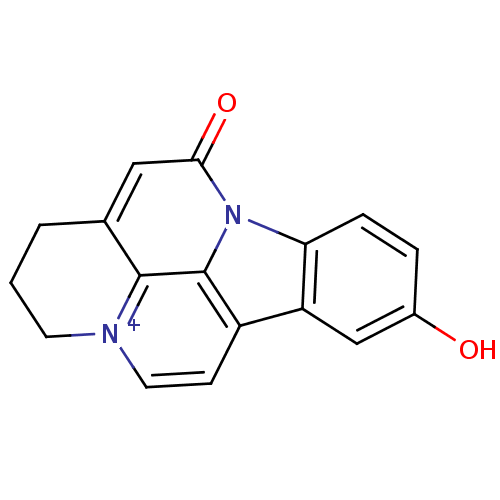 (10-Hydroxy-infractopicrin | CHEMBL589071) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry Curated by ChEMBL | Assay Description Inhibition of bovine AChE by Elman's method | Bioorg Med Chem 18: 2173-7 (2010) Article DOI: 10.1016/j.bmc.2010.01.074 BindingDB Entry DOI: 10.7270/Q2R78G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50550147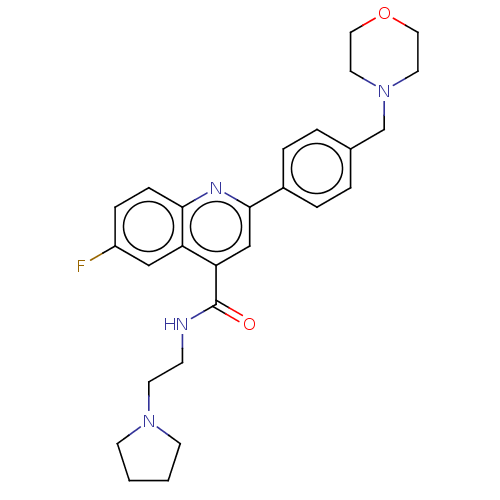 (DDD-107498 | DDD107498 | DDD498 | M-5717 | M5717 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by IonWorks patch-clamp electrophysiology method | Citation and Details BindingDB Entry DOI: 10.7270/Q2J67MKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by Ellman's method | Bioorg Med Chem 18: 2173-7 (2010) Article DOI: 10.1016/j.bmc.2010.01.074 BindingDB Entry DOI: 10.7270/Q2R78G5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50536194 (CHEMBL4569641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 3-Cyano-7-Eth... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50536192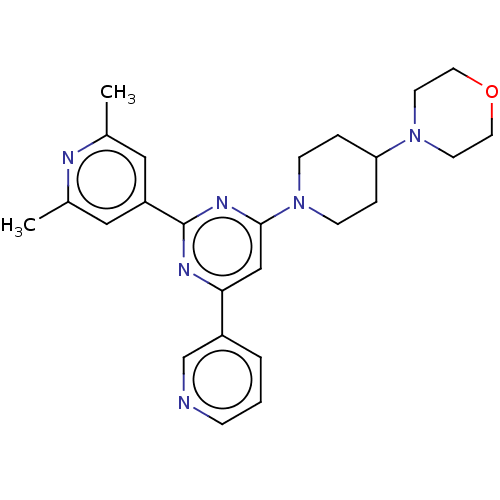 (CHEMBL4570567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Diethoxyfluore... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50536192 (CHEMBL4570567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 3-Cyano-7-Eth... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50536192 (CHEMBL4570567) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(a... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50222010 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Plant Biochemistry Curated by ChEMBL | Assay Description Inhibition of bovine AChE at 100 uM by Ellman's method | J Nat Prod 70: 1529-31 (2007) Article DOI: 10.1021/np070259w BindingDB Entry DOI: 10.7270/Q2GT5P1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50536192 (CHEMBL4570567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using 7-methoxy-4-(t... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50536191 (CHEMBL4584780) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Ethoxyresorufi... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50536194 (CHEMBL4569641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Ethoxyresorufi... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50536192 (CHEMBL4570567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 expressed in Escherichia coli pre-incubated for 5 mins before regenerating cofactor solution addition using Ethoxyresorufi... | J Med Chem 59: 6101-20 (2016) Article DOI: 10.1021/acs.jmedchem.6b00028 BindingDB Entry DOI: 10.7270/Q2H41VZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||