 Found 276 hits with Last Name = 'qiu' and Initial = 'hy'
Found 276 hits with Last Name = 'qiu' and Initial = 'hy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of autophosphorylation of recombinant his-tagged EGFR (amino acid 645-1186) (unknown origin) expressed in Sf-9 cells after 10 mins by time... |
Bioorg Med Chem Lett 24: 2324-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.072
BindingDB Entry DOI: 10.7270/Q2X63PGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492595
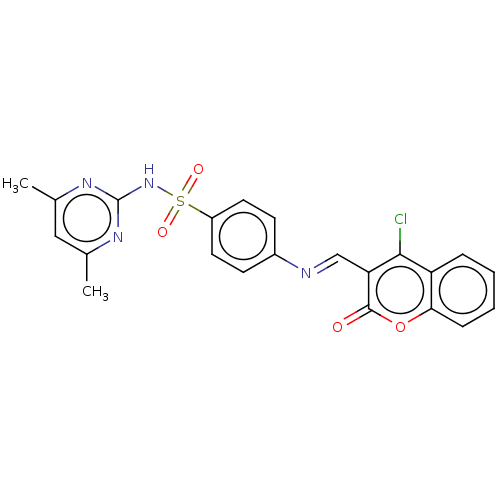
(CHEMBL2408077)Show SMILES Cc1cc(C)nc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3ccccc3oc2=O)n1 Show InChI InChI=1S/C22H17ClN4O4S/c1-13-11-14(2)26-22(25-13)27-32(29,30)16-9-7-15(8-10-16)24-12-18-20(23)17-5-3-4-6-19(17)31-21(18)28/h3-12H,1-2H3,(H,25,26,27)/b24-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492590
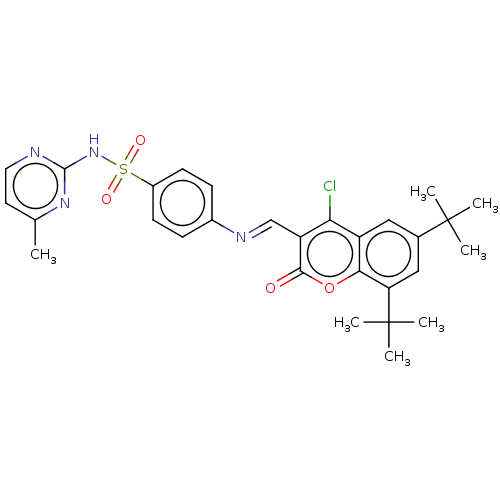
(CHEMBL2408085)Show SMILES Cc1ccnc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3cc(cc(c3oc2=O)C(C)(C)C)C(C)(C)C)n1 Show InChI InChI=1S/C29H31ClN4O4S/c1-17-12-13-31-27(33-17)34-39(36,37)20-10-8-19(9-11-20)32-16-22-24(30)21-14-18(28(2,3)4)15-23(29(5,6)7)25(21)38-26(22)35/h8-16H,1-7H3,(H,31,33,34)/b32-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492592
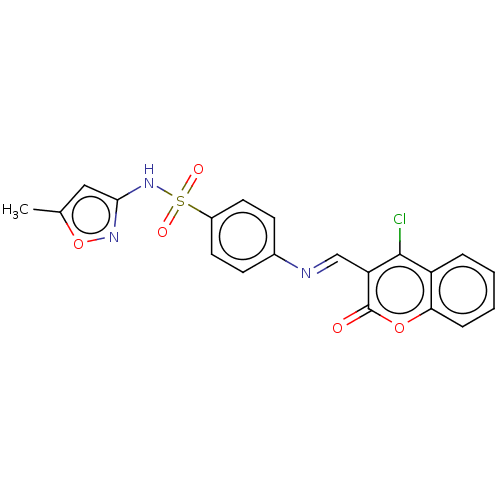
(CHEMBL2408078)Show SMILES Cc1cc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3ccccc3oc2=O)no1 Show InChI InChI=1S/C20H14ClN3O5S/c1-12-10-18(23-29-12)24-30(26,27)14-8-6-13(7-9-14)22-11-16-19(21)15-4-2-3-5-17(15)28-20(16)25/h2-11H,1H3,(H,23,24)/b22-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM50440135
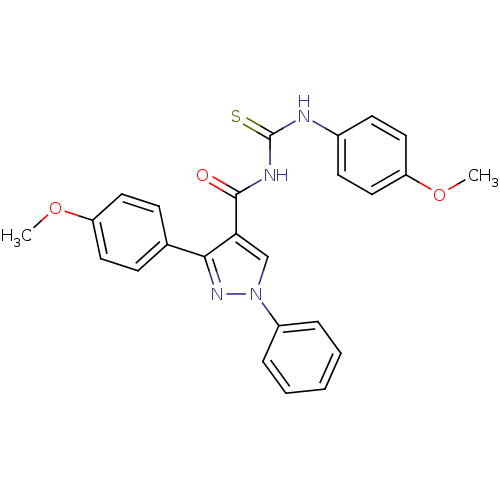
(CHEMBL2426395)Show SMILES COc1ccc(NC(=S)NC(=O)c2cn(nc2-c2ccc(OC)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C25H22N4O3S/c1-31-20-12-8-17(9-13-20)23-22(16-29(28-23)19-6-4-3-5-7-19)24(30)27-25(33)26-18-10-14-21(32-2)15-11-18/h3-16H,1-2H3,(H2,26,27,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of CDK6 (unknown origin) using histone H1 as substrate after 10 mins in presence of [gamma-32P]-ATP |
Eur J Med Chem 68: 1-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.003
BindingDB Entry DOI: 10.7270/Q2PK0HKH |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492599
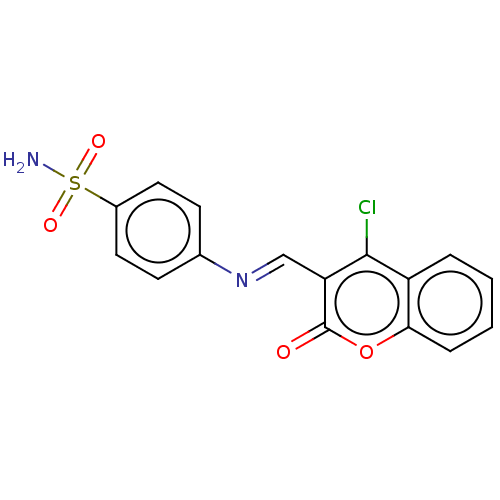
(CHEMBL2408074)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=C\c1c(Cl)c2ccccc2oc1=O Show InChI InChI=1S/C16H11ClN2O4S/c17-15-12-3-1-2-4-14(12)23-16(20)13(15)9-19-10-5-7-11(8-6-10)24(18,21)22/h1-9H,(H2,18,21,22)/b19-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM50440136
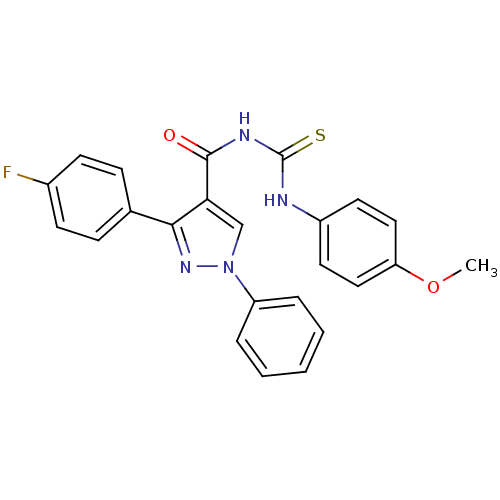
(CHEMBL2426391)Show SMILES COc1ccc(NC(=S)NC(=O)c2cn(nc2-c2ccc(F)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H19FN4O2S/c1-31-20-13-11-18(12-14-20)26-24(32)27-23(30)21-15-29(19-5-3-2-4-6-19)28-22(21)16-7-9-17(25)10-8-16/h2-15H,1H3,(H2,26,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of CDK6 (unknown origin) using histone H1 as substrate after 10 mins in presence of [gamma-32P]-ATP |
Eur J Med Chem 68: 1-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.003
BindingDB Entry DOI: 10.7270/Q2PK0HKH |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492593
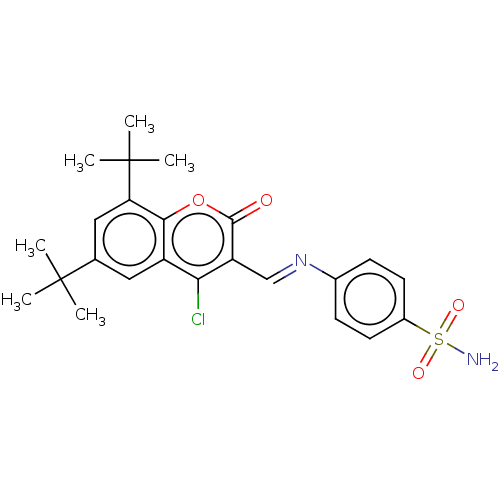
(CHEMBL2408080)Show SMILES CC(C)(C)c1cc(c2oc(=O)c(\C=N\c3ccc(cc3)S(N)(=O)=O)c(Cl)c2c1)C(C)(C)C Show InChI InChI=1S/C24H27ClN2O4S/c1-23(2,3)14-11-17-20(25)18(22(28)31-21(17)19(12-14)24(4,5)6)13-27-15-7-9-16(10-8-15)32(26,29)30/h7-13H,1-6H3,(H2,26,29,30)/b27-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM50440137
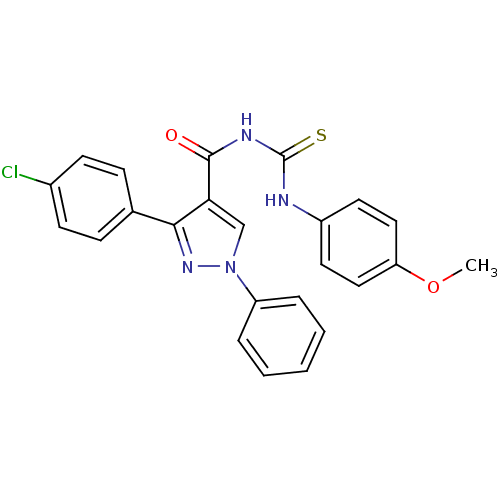
(CHEMBL2426387)Show SMILES COc1ccc(NC(=S)NC(=O)c2cn(nc2-c2ccc(Cl)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H19ClN4O2S/c1-31-20-13-11-18(12-14-20)26-24(32)27-23(30)21-15-29(19-5-3-2-4-6-19)28-22(21)16-7-9-17(25)10-8-16/h2-15H,1H3,(H2,26,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of CDK6 (unknown origin) using histone H1 as substrate after 10 mins in presence of [gamma-32P]-ATP |
Eur J Med Chem 68: 1-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.003
BindingDB Entry DOI: 10.7270/Q2PK0HKH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM50440138
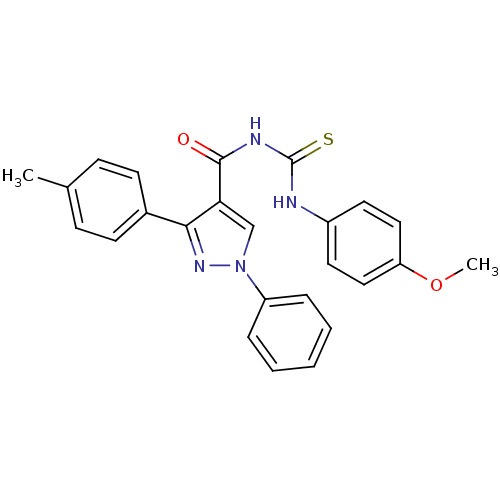
(CHEMBL2426383)Show SMILES COc1ccc(NC(=S)NC(=O)c2cn(nc2-c2ccc(C)cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C25H22N4O2S/c1-17-8-10-18(11-9-17)23-22(16-29(28-23)20-6-4-3-5-7-20)24(30)27-25(32)26-19-12-14-21(31-2)15-13-19/h3-16H,1-2H3,(H2,26,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of CDK6 (unknown origin) using histone H1 as substrate after 10 mins in presence of [gamma-32P]-ATP |
Eur J Med Chem 68: 1-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.003
BindingDB Entry DOI: 10.7270/Q2PK0HKH |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492588
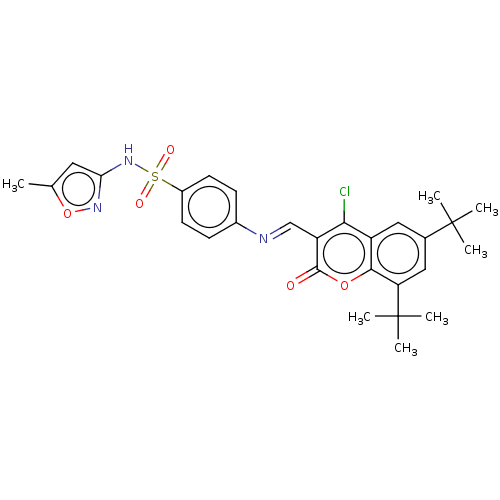
(CHEMBL2408084)Show SMILES Cc1cc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3cc(cc(c3oc2=O)C(C)(C)C)C(C)(C)C)no1 Show InChI InChI=1S/C28H30ClN3O5S/c1-16-12-23(31-37-16)32-38(34,35)19-10-8-18(9-11-19)30-15-21-24(29)20-13-17(27(2,3)4)14-22(28(5,6)7)25(20)36-26(21)33/h8-15H,1-7H3,(H,31,32)/b30-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492600

(CHEMBL2408073)Show SMILES Cc1ccc2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)Nc3nccc(C)n3)c(Cl)c2c1 Show InChI InChI=1S/C22H17ClN4O4S/c1-13-3-8-19-17(11-13)20(23)18(21(28)31-19)12-25-15-4-6-16(7-5-15)32(29,30)27-22-24-10-9-14(2)26-22/h3-12H,1-2H3,(H,24,26,27)/b25-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM50440139
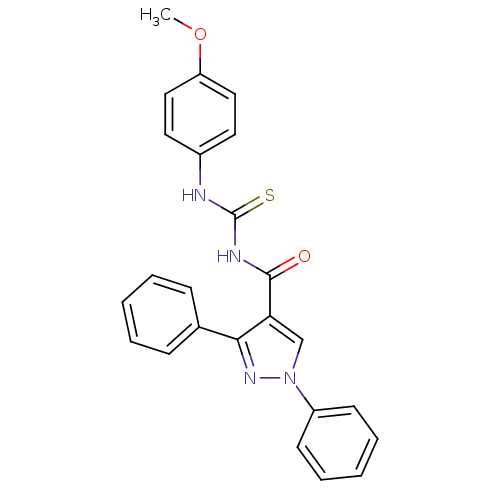
(CHEMBL2426399)Show SMILES COc1ccc(NC(=S)NC(=O)c2cn(nc2-c2ccccc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H20N4O2S/c1-30-20-14-12-18(13-15-20)25-24(31)26-23(29)21-16-28(19-10-6-3-7-11-19)27-22(21)17-8-4-2-5-9-17/h2-16H,1H3,(H2,25,26,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of CDK6 (unknown origin) using histone H1 as substrate after 10 mins in presence of [gamma-32P]-ATP |
Eur J Med Chem 68: 1-9 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.003
BindingDB Entry DOI: 10.7270/Q2PK0HKH |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492598

(CHEMBL2408088)Show SMILES Cc1ccc2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)NC(N)=N)c(Cl)c2c1 Show InChI InChI=1S/C18H15ClN4O4S/c1-10-2-7-15-13(8-10)16(19)14(17(24)27-15)9-22-11-3-5-12(6-4-11)28(25,26)23-18(20)21/h2-9H,1H3,(H4,20,21,23)/b22-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492594

(CHEMBL2408079)Show SMILES Cc1ccnc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3ccccc3oc2=O)n1 Show InChI InChI=1S/C21H15ClN4O4S/c1-13-10-11-23-21(25-13)26-31(28,29)15-8-6-14(7-9-15)24-12-17-19(22)16-4-2-3-5-18(16)30-20(17)27/h2-12H,1H3,(H,23,25,26)/b24-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492597
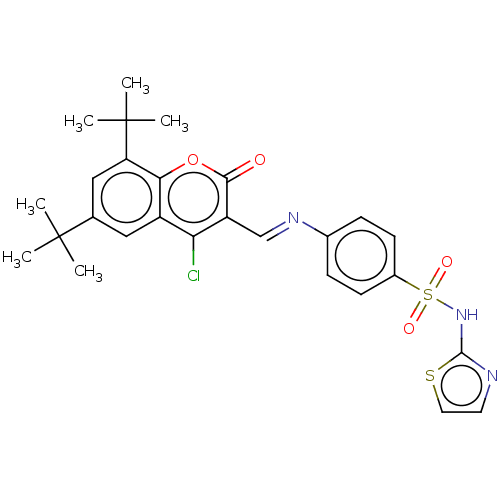
(CHEMBL2408081)Show SMILES CC(C)(C)c1cc(c2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)Nc3nccs3)c(Cl)c2c1)C(C)(C)C Show InChI InChI=1S/C27H28ClN3O4S2/c1-26(2,3)16-13-19-22(28)20(24(32)35-23(19)21(14-16)27(4,5)6)15-30-17-7-9-18(10-8-17)37(33,34)31-25-29-11-12-36-25/h7-15H,1-6H3,(H,29,31)/b30-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492589

(CHEMBL2408090)Show SMILES Cc1cc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3cc(C)ccc3oc2=O)no1 Show InChI InChI=1S/C21H16ClN3O5S/c1-12-3-8-18-16(9-12)20(22)17(21(26)29-18)11-23-14-4-6-15(7-5-14)31(27,28)25-19-10-13(2)30-24-19/h3-11H,1-2H3,(H,24,25)/b23-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492596
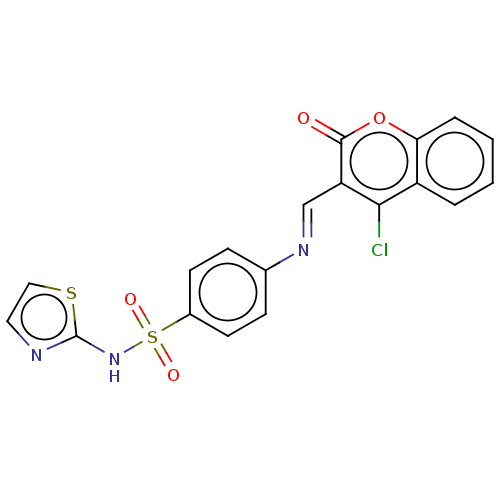
(CHEMBL2408075)Show SMILES Clc1c(\C=N\c2ccc(cc2)S(=O)(=O)Nc2nccs2)c(=O)oc2ccccc12 Show InChI InChI=1S/C19H12ClN3O4S2/c20-17-14-3-1-2-4-16(14)27-18(24)15(17)11-22-12-5-7-13(8-6-12)29(25,26)23-19-21-9-10-28-19/h1-11H,(H,21,23)/b22-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492600

(CHEMBL2408073)Show SMILES Cc1ccc2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)Nc3nccc(C)n3)c(Cl)c2c1 Show InChI InChI=1S/C22H17ClN4O4S/c1-13-3-8-19-17(11-13)20(23)18(21(28)31-19)12-25-15-4-6-16(7-5-15)32(29,30)27-22-24-10-9-14(2)26-22/h3-12H,1-2H3,(H,24,26,27)/b25-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492590

(CHEMBL2408085)Show SMILES Cc1ccnc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3cc(cc(c3oc2=O)C(C)(C)C)C(C)(C)C)n1 Show InChI InChI=1S/C29H31ClN4O4S/c1-17-12-13-31-27(33-17)34-39(36,37)20-10-8-19(9-11-20)32-16-22-24(30)21-14-18(28(2,3)4)15-23(29(5,6)7)25(21)38-26(22)35/h8-16H,1-7H3,(H,31,33,34)/b32-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492601

(CHEMBL2408089)Show SMILES Cc1ccc2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)Nc3nc(C)cc(C)n3)c(Cl)c2c1 Show InChI InChI=1S/C23H19ClN4O4S/c1-13-4-9-20-18(10-13)21(24)19(22(29)32-20)12-25-16-5-7-17(8-6-16)33(30,31)28-23-26-14(2)11-15(3)27-23/h4-12H,1-3H3,(H,26,27,28)/b25-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492602
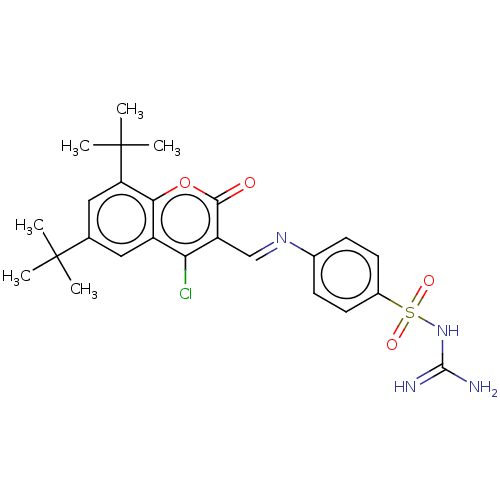
(CHEMBL2408082)Show SMILES CC(C)(C)c1cc(c2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)NC(N)=N)c(Cl)c2c1)C(C)(C)C Show InChI InChI=1S/C25H29ClN4O4S/c1-24(2,3)14-11-17-20(26)18(22(31)34-21(17)19(12-14)25(4,5)6)13-29-15-7-9-16(10-8-15)35(32,33)30-23(27)28/h7-13H,1-6H3,(H4,27,28,30)/b29-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492594

(CHEMBL2408079)Show SMILES Cc1ccnc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3ccccc3oc2=O)n1 Show InChI InChI=1S/C21H15ClN4O4S/c1-13-10-11-23-21(25-13)26-31(28,29)15-8-6-14(7-9-15)24-12-17-19(22)16-4-2-3-5-18(16)30-20(17)27/h2-12H,1H3,(H,23,25,26)/b24-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492587
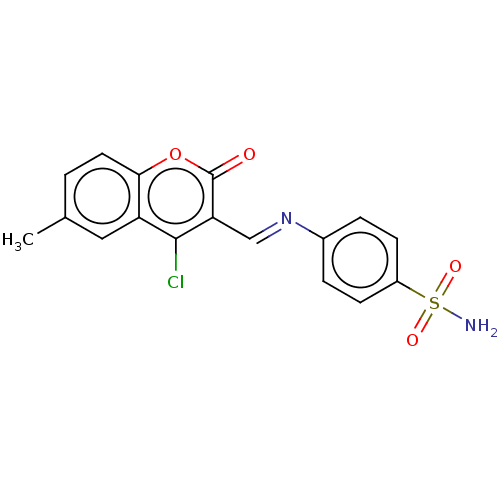
(CHEMBL2408086)Show SMILES Cc1ccc2oc(=O)c(\C=N\c3ccc(cc3)S(N)(=O)=O)c(Cl)c2c1 Show InChI InChI=1S/C17H13ClN2O4S/c1-10-2-7-15-13(8-10)16(18)14(17(21)24-15)9-20-11-3-5-12(6-4-11)25(19,22)23/h2-9H,1H3,(H2,19,22,23)/b20-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492603
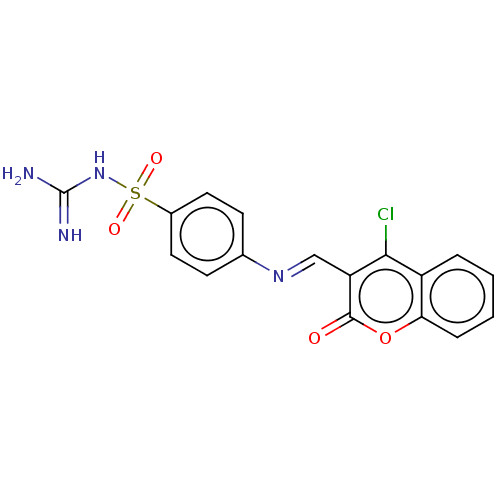
(CHEMBL2408076)Show SMILES NC(=N)NS(=O)(=O)c1ccc(cc1)\N=C\c1c(Cl)c2ccccc2oc1=O Show InChI InChI=1S/C17H13ClN4O4S/c18-15-12-3-1-2-4-14(12)26-16(23)13(15)9-21-10-5-7-11(8-6-10)27(24,25)22-17(19)20/h1-9H,(H4,19,20,22)/b21-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492586

(CHEMBL2408087)Show SMILES Cc1ccc2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)Nc3nccs3)c(Cl)c2c1 Show InChI InChI=1S/C20H14ClN3O4S2/c1-12-2-7-17-15(10-12)18(21)16(19(25)28-17)11-23-13-3-5-14(6-4-13)30(26,27)24-20-22-8-9-29-20/h2-11H,1H3,(H,22,24)/b23-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492587

(CHEMBL2408086)Show SMILES Cc1ccc2oc(=O)c(\C=N\c3ccc(cc3)S(N)(=O)=O)c(Cl)c2c1 Show InChI InChI=1S/C17H13ClN2O4S/c1-10-2-7-15-13(8-10)16(18)14(17(21)24-15)9-20-11-3-5-12(6-4-11)25(19,22)23/h2-9H,1H3,(H2,19,22,23)/b20-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492591
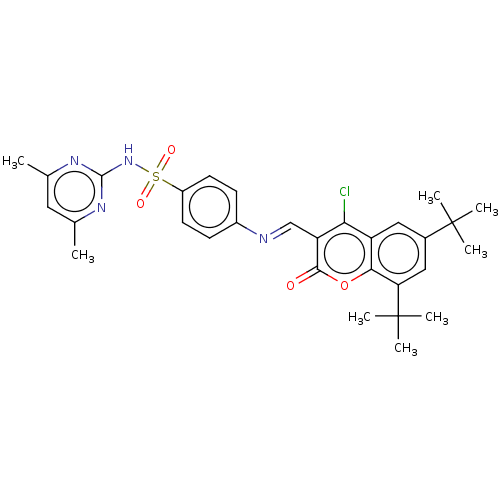
(CHEMBL2408083)Show SMILES Cc1cc(C)nc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3cc(cc(c3oc2=O)C(C)(C)C)C(C)(C)C)n1 Show InChI InChI=1S/C30H33ClN4O4S/c1-17-13-18(2)34-28(33-17)35-40(37,38)21-11-9-20(10-12-21)32-16-23-25(31)22-14-19(29(3,4)5)15-24(30(6,7)8)26(22)39-27(23)36/h9-16H,1-8H3,(H,33,34,35)/b32-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492599

(CHEMBL2408074)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=C\c1c(Cl)c2ccccc2oc1=O Show InChI InChI=1S/C16H11ClN2O4S/c17-15-12-3-1-2-4-14(12)23-16(20)13(15)9-19-10-5-7-11(8-6-10)24(18,21)22/h1-9H,(H2,18,21,22)/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492598

(CHEMBL2408088)Show SMILES Cc1ccc2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)NC(N)=N)c(Cl)c2c1 Show InChI InChI=1S/C18H15ClN4O4S/c1-10-2-7-15-13(8-10)16(19)14(17(24)27-15)9-22-11-3-5-12(6-4-11)28(25,26)23-18(20)21/h2-9H,1H3,(H4,20,21,23)/b22-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492602

(CHEMBL2408082)Show SMILES CC(C)(C)c1cc(c2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)NC(N)=N)c(Cl)c2c1)C(C)(C)C Show InChI InChI=1S/C25H29ClN4O4S/c1-24(2,3)14-11-17-20(26)18(22(31)34-21(17)19(12-14)25(4,5)6)13-29-15-7-9-16(10-8-15)35(32,33)30-23(27)28/h7-13H,1-6H3,(H4,27,28,30)/b29-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM21
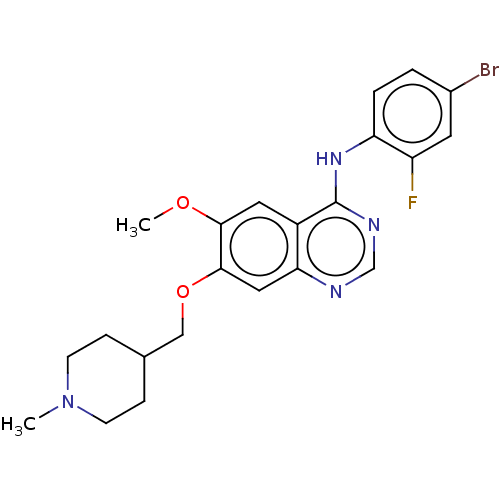
(CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou Children's Hospital
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) incubated for 30 mins by enzyme immunoassay |
Bioorg Med Chem 27: 3813-3824 (2019)
Article DOI: 10.1016/j.bmc.2019.07.007
BindingDB Entry DOI: 10.7270/Q2FT8QFG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492593

(CHEMBL2408080)Show SMILES CC(C)(C)c1cc(c2oc(=O)c(\C=N\c3ccc(cc3)S(N)(=O)=O)c(Cl)c2c1)C(C)(C)C Show InChI InChI=1S/C24H27ClN2O4S/c1-23(2,3)14-11-17-20(25)18(22(28)31-21(17)19(12-14)24(4,5)6)13-27-15-7-9-16(10-8-15)32(26,29)30/h7-13H,1-6H3,(H2,26,29,30)/b27-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50013397
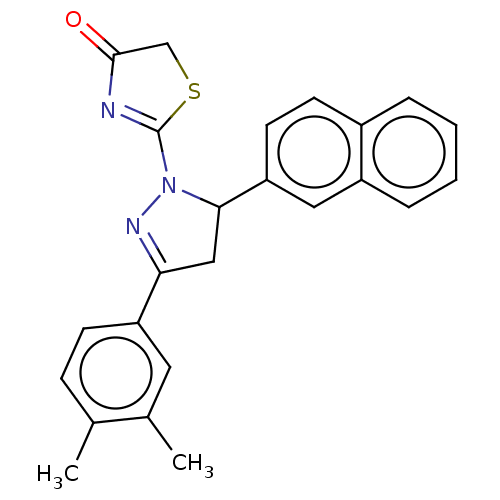
(CHEMBL3263380)Show SMILES Cc1ccc(cc1C)C1=NN(C(C1)c1ccc2ccccc2c1)C1=NC(=O)CS1 |t:9,27| Show InChI InChI=1S/C24H21N3OS/c1-15-7-8-19(11-16(15)2)21-13-22(27(26-21)24-25-23(28)14-29-24)20-10-9-17-5-3-4-6-18(17)12-20/h3-12,22H,13-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of autophosphorylation of recombinant his-tagged EGFR (amino acid 645-1186) (unknown origin) expressed in Sf-9 cells after 10 mins by time... |
Bioorg Med Chem Lett 24: 2324-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.072
BindingDB Entry DOI: 10.7270/Q2X63PGR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492586

(CHEMBL2408087)Show SMILES Cc1ccc2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)Nc3nccs3)c(Cl)c2c1 Show InChI InChI=1S/C20H14ClN3O4S2/c1-12-2-7-17-15(10-12)18(21)16(19(25)28-17)11-23-13-3-5-14(6-4-13)30(26,27)24-20-22-8-9-29-20/h2-11H,1H3,(H,22,24)/b23-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492597

(CHEMBL2408081)Show SMILES CC(C)(C)c1cc(c2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)Nc3nccs3)c(Cl)c2c1)C(C)(C)C Show InChI InChI=1S/C27H28ClN3O4S2/c1-26(2,3)16-13-19-22(28)20(24(32)35-23(19)21(14-16)27(4,5)6)15-30-17-7-9-18(10-8-17)37(33,34)31-25-29-11-12-36-25/h7-15H,1-6H3,(H,29,31)/b30-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492595

(CHEMBL2408077)Show SMILES Cc1cc(C)nc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3ccccc3oc2=O)n1 Show InChI InChI=1S/C22H17ClN4O4S/c1-13-11-14(2)26-22(25-13)27-32(29,30)16-9-7-15(8-10-16)24-12-18-20(23)17-5-3-4-6-19(17)31-21(18)28/h3-12H,1-2H3,(H,25,26,27)/b24-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Mus musculus) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of mouse full length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... |
Bioorg Med Chem 26: 2372-2380 (2018)
Article DOI: 10.1016/j.bmc.2018.03.038
BindingDB Entry DOI: 10.7270/Q27M0BKR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492596

(CHEMBL2408075)Show SMILES Clc1c(\C=N\c2ccc(cc2)S(=O)(=O)Nc2nccs2)c(=O)oc2ccccc12 Show InChI InChI=1S/C19H12ClN3O4S2/c20-17-14-3-1-2-4-16(14)27-18(24)15(17)11-22-12-5-7-13(8-6-12)29(25,26)23-19-21-9-10-28-19/h1-11H,(H,21,23)/b22-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492592

(CHEMBL2408078)Show SMILES Cc1cc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3ccccc3oc2=O)no1 Show InChI InChI=1S/C20H14ClN3O5S/c1-12-10-18(23-29-12)24-30(26,27)14-8-6-13(7-9-14)22-11-16-19(21)15-4-2-3-5-17(15)28-20(16)25/h2-11H,1H3,(H,23,24)/b22-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492601

(CHEMBL2408089)Show SMILES Cc1ccc2oc(=O)c(\C=N\c3ccc(cc3)S(=O)(=O)Nc3nc(C)cc(C)n3)c(Cl)c2c1 Show InChI InChI=1S/C23H19ClN4O4S/c1-13-4-9-20-18(10-13)21(24)19(22(29)32-20)12-25-16-5-7-17(8-6-16)33(30,31)28-23-26-14(2)11-15(3)27-23/h4-12H,1-3H3,(H,26,27,28)/b25-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492591

(CHEMBL2408083)Show SMILES Cc1cc(C)nc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3cc(cc(c3oc2=O)C(C)(C)C)C(C)(C)C)n1 Show InChI InChI=1S/C30H33ClN4O4S/c1-17-13-18(2)34-28(33-17)35-40(37,38)21-11-9-20(10-12-21)32-16-23-25(31)22-14-19(29(3,4)5)15-24(30(6,7)8)26(22)39-27(23)36/h9-16H,1-8H3,(H,33,34,35)/b32-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492588

(CHEMBL2408084)Show SMILES Cc1cc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3cc(cc(c3oc2=O)C(C)(C)C)C(C)(C)C)no1 Show InChI InChI=1S/C28H30ClN3O5S/c1-16-12-23(31-37-16)32-38(34,35)19-10-8-18(9-11-19)30-15-21-24(29)20-13-17(27(2,3)4)14-22(28(5,6)7)25(20)36-26(21)33/h8-15H,1-7H3,(H,31,32)/b30-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Mus musculus) | BDBM50461196
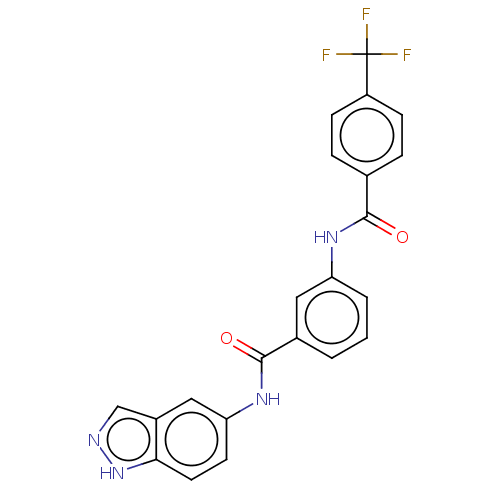
(CHEMBL4228405)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)Nc1cccc(c1)C(=O)Nc1ccc2[nH]ncc2c1 Show InChI InChI=1S/C22H15F3N4O2/c23-22(24,25)16-6-4-13(5-7-16)20(30)27-17-3-1-2-14(10-17)21(31)28-18-8-9-19-15(11-18)12-26-29-19/h1-12H,(H,26,29)(H,27,30)(H,28,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of mouse full length GST-tagged BRAF V600E mutant using recombinant human full length N-terminal His-tagged MEK1 as substrate preincubated... |
Bioorg Med Chem 26: 2372-2380 (2018)
Article DOI: 10.1016/j.bmc.2018.03.038
BindingDB Entry DOI: 10.7270/Q27M0BKR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492603

(CHEMBL2408076)Show SMILES NC(=N)NS(=O)(=O)c1ccc(cc1)\N=C\c1c(Cl)c2ccccc2oc1=O Show InChI InChI=1S/C17H13ClN4O4S/c18-15-12-3-1-2-4-14(12)26-16(23)13(15)9-21-10-5-7-11(8-6-10)27(24,25)22-17(19)20/h1-9H,(H4,19,20,22)/b21-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50123932
(CHEMBL3622226)Show SMILES Cc1ccc(C2=NN(C(C2)c2cccc3ccccc23)c2ccc(cc2)S(N)(=O)=O)c(C)c1 |t:5| Show InChI InChI=1S/C27H25N3O2S/c1-18-10-15-23(19(2)16-18)26-17-27(25-9-5-7-20-6-3-4-8-24(20)25)30(29-26)21-11-13-22(14-12-21)33(28,31)32/h3-16,27H,17H2,1-2H3,(H2,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of APMA-activated human recombinant MMP2 incubated for 5 mins using 4-nitrophenylacetate substrate by esterase assay |
Bioorg Med Chem Lett 25: 4664-71 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.026
BindingDB Entry DOI: 10.7270/Q2RN39NC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50123932

(CHEMBL3622226)Show SMILES Cc1ccc(C2=NN(C(C2)c2cccc3ccccc23)c2ccc(cc2)S(N)(=O)=O)c(C)c1 |t:5| Show InChI InChI=1S/C27H25N3O2S/c1-18-10-15-23(19(2)16-18)26-17-27(25-9-5-7-20-6-3-4-8-24(20)25)30(29-26)21-11-13-22(14-12-21)33(28,31)32/h3-16,27H,17H2,1-2H3,(H2,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of APMA-activated human recombinant MMP2 incubated for 5 mins using 4-nitrophenylacetate substrate by esterase assay |
Bioorg Med Chem Lett 25: 4664-71 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.026
BindingDB Entry DOI: 10.7270/Q2RN39NC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50492589

(CHEMBL2408090)Show SMILES Cc1cc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3cc(C)ccc3oc2=O)no1 Show InChI InChI=1S/C21H16ClN3O5S/c1-12-3-8-18-16(9-12)20(22)17(21(26)29-18)11-23-14-4-6-15(7-5-14)31(27,28)25-19-10-13(2)30-24-19/h3-11H,1-2H3,(H,24,25)/b23-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA2 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data