

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090023 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090018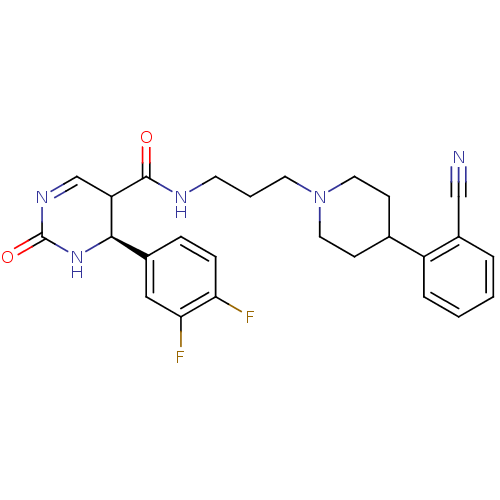 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090027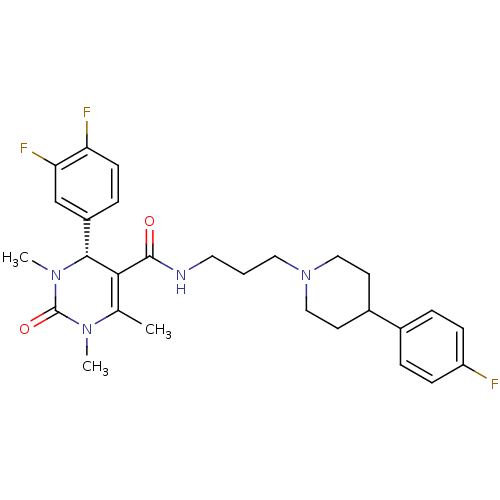 ((R)-4-(3,4-Difluoro-phenyl)-1,3,6-trimethyl-2-oxo-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50245653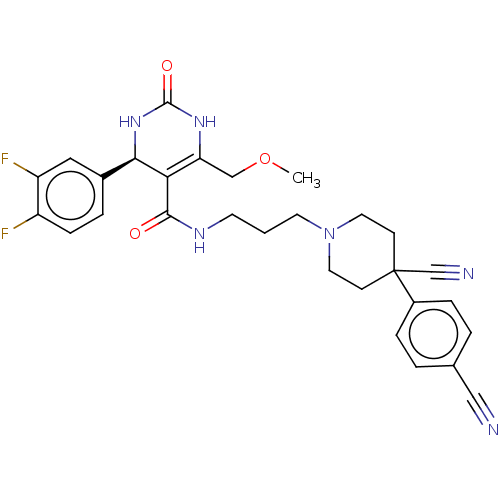 (CHEMBL4100620) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50245650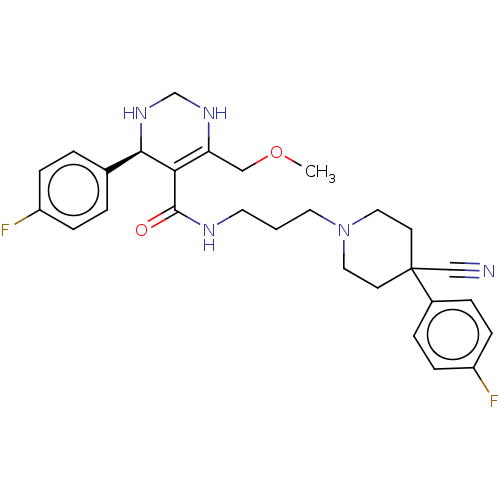 (CHEMBL4062896) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090031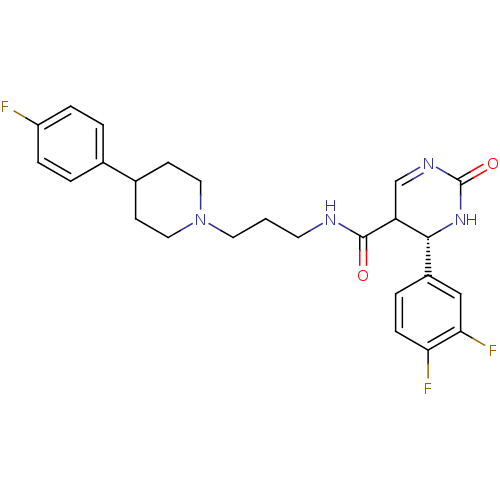 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50245652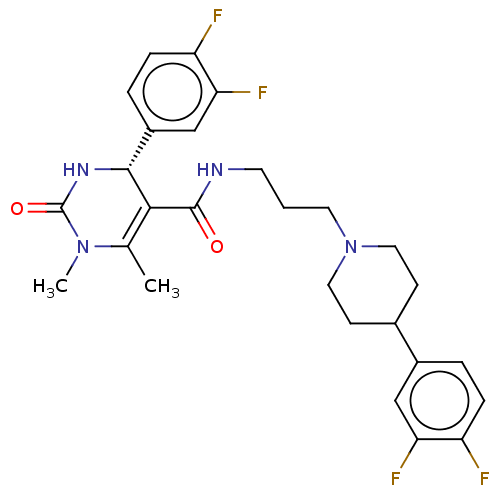 (CHEMBL4081581) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090043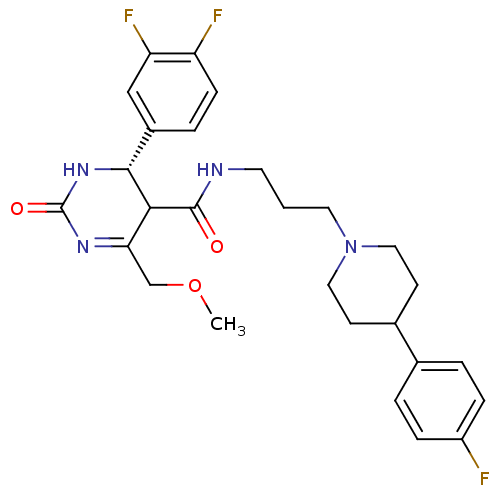 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-2-oxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090013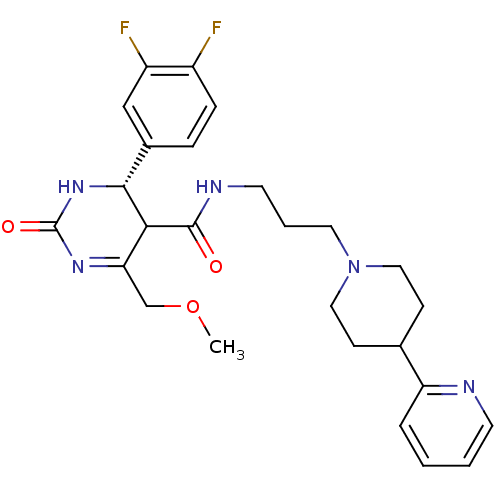 (4-(3,4-Difluoro-phenyl)-6-methoxymethyl-2-oxo-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090041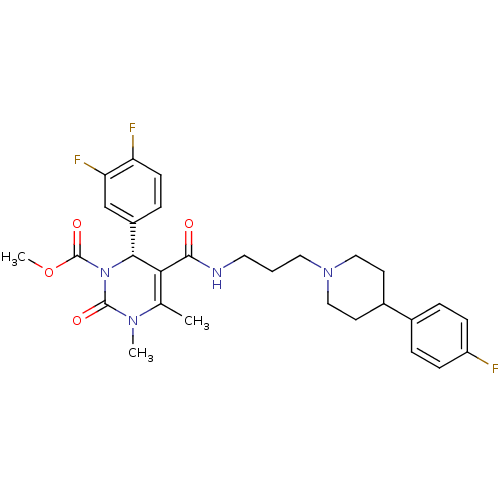 (6-(3,4-Difluoro-phenyl)-5-{3-[4-(4-fluoro-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090021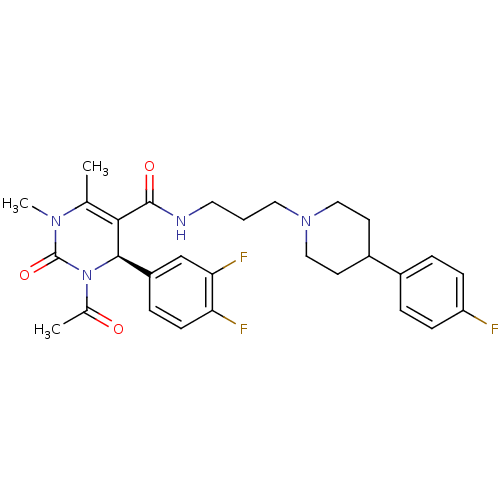 (3-Acetyl-4-(3,4-difluoro-phenyl)-1,6-dimethyl-2-ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [125I]L-762,459 from recombinant human alpha1a adrenergic receptor expressed in mammalian cells measured after 1 hr | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50245651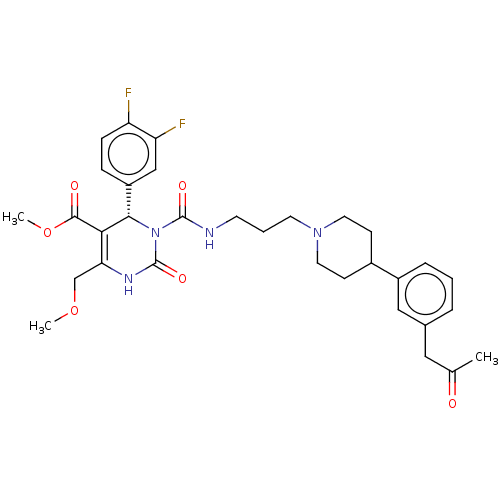 (CHEMBL4071004) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Displacement of [3H]SNAP-7941 from recombinant human MCHR1 expressed in African green monkey COS7 cell membranes | Eur J Med Chem 132: 108-134 (2017) Article DOI: 10.1016/j.ejmech.2017.03.025 BindingDB Entry DOI: 10.7270/Q2X350V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316806 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50240561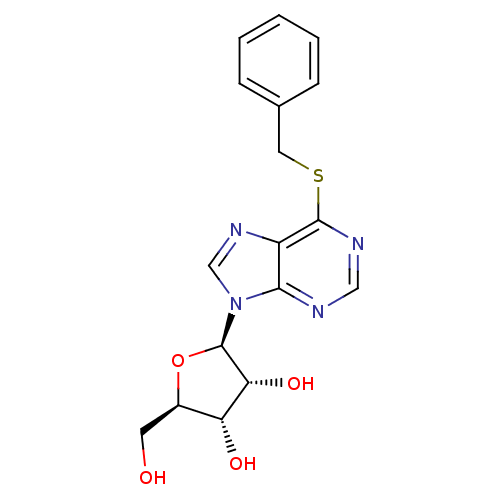 ((2R,3R,4S,5R)-2-(6-(benzylthio)-9H-purin-9-yl)-5-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316801 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316811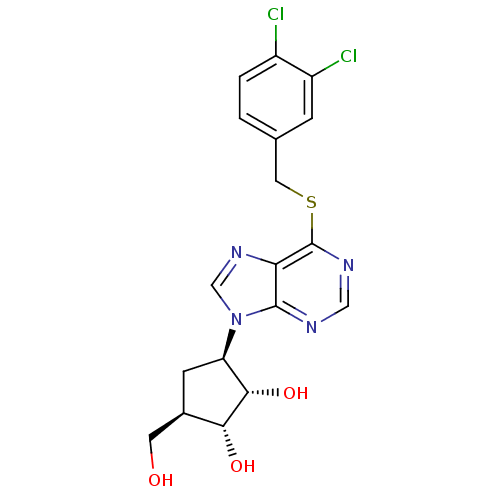 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316802 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316798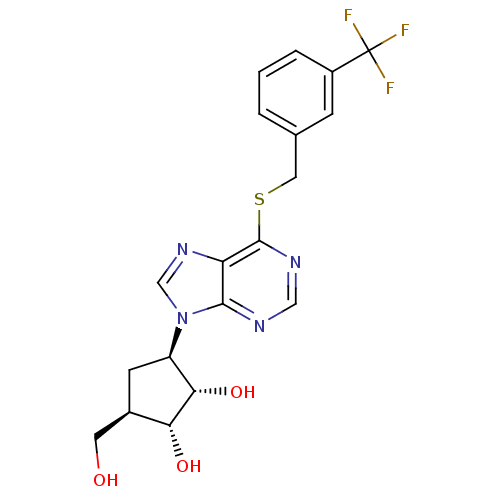 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316795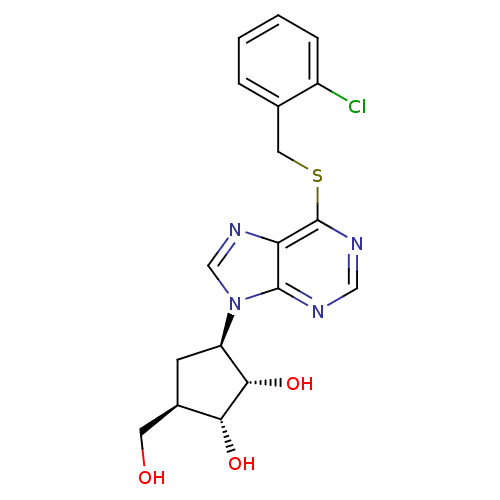 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316796 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50403048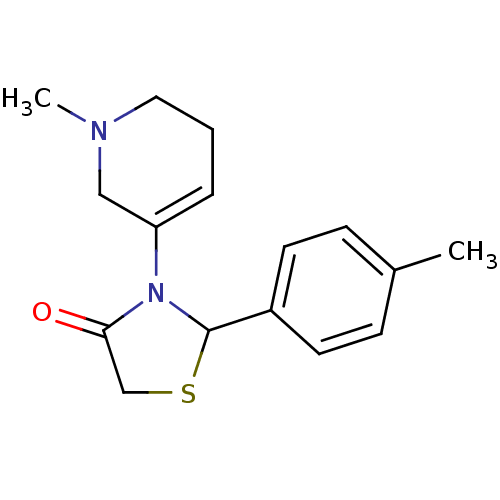 (CHEMBL2216805) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Hari Singh Gour University Curated by ChEMBL | Assay Description Displacement of [3H]QNB from M1 receptor in Wistar rat cerebral cortex homogenate | Bioorg Med Chem 20: 3378-95 (2012) Article DOI: 10.1016/j.bmc.2012.03.069 BindingDB Entry DOI: 10.7270/Q2222VX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316797 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316799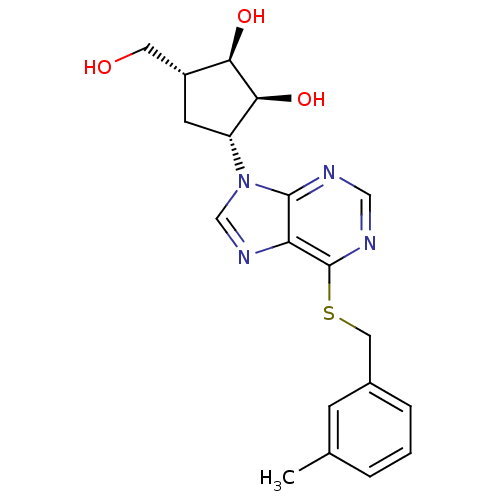 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316812 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316800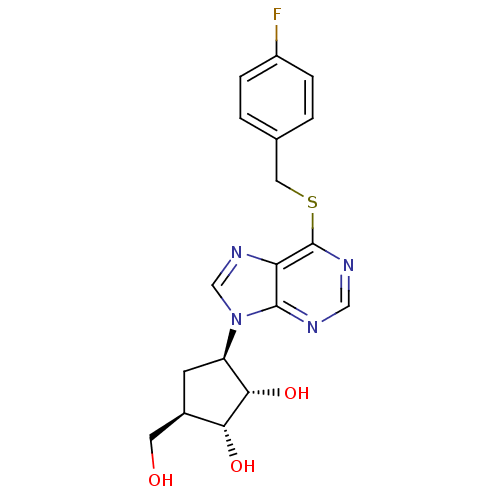 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316805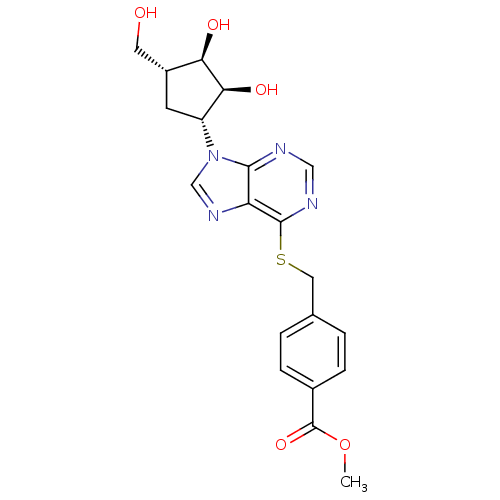 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316794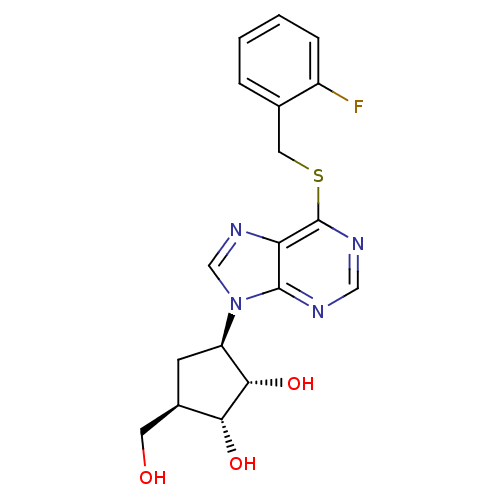 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316810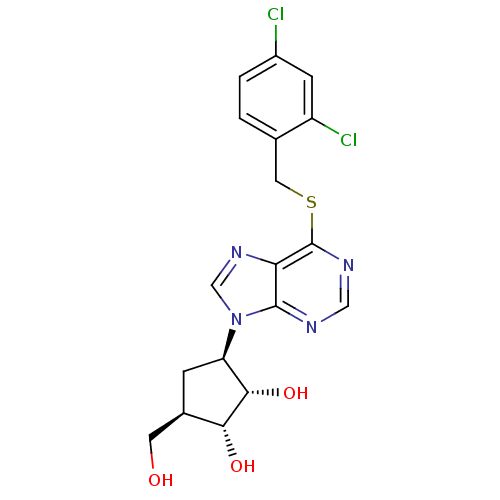 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316809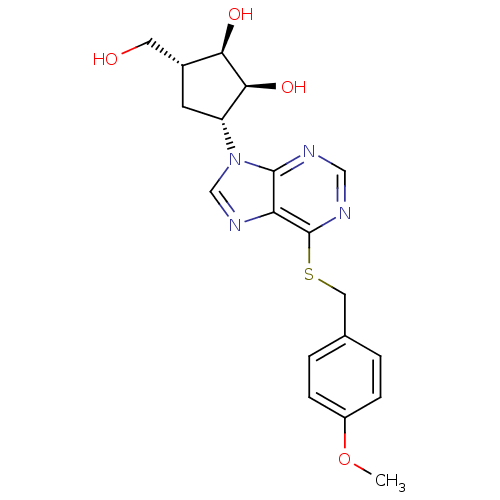 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316813 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316808 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316807 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316803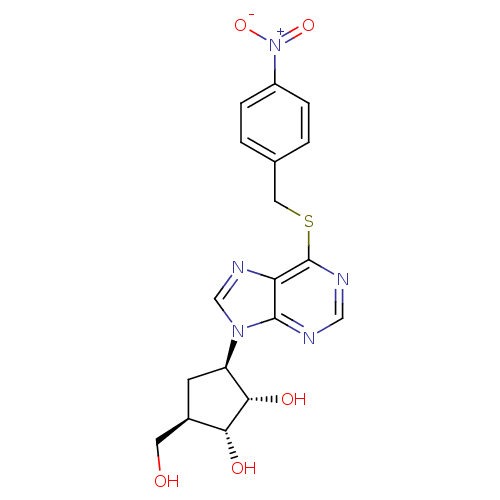 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316793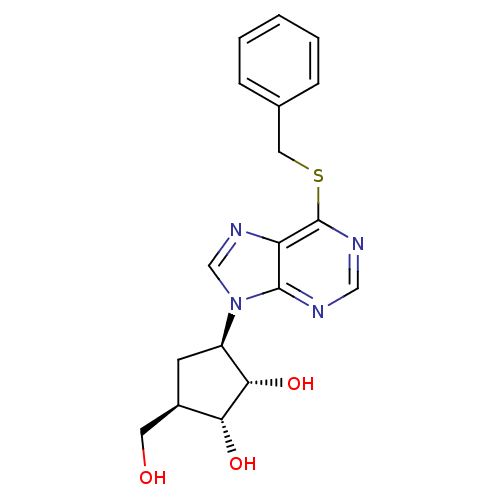 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Toxoplasma gondii) | BDBM50316804 ((1'R,2'S,3'R,4'R)-1-[2,3-Dihydroxy-4-(hydroxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Georgia Curated by ChEMBL | Assay Description Binding affinity to Toxoplasma gondii adenosine kinase after 20 mins by radioactivity method | Bioorg Med Chem 18: 3403-12 (2010) Article DOI: 10.1016/j.bmc.2010.04.003 BindingDB Entry DOI: 10.7270/Q2WH2Q43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM8793 (7-[4-(4-methylpiperazin-1-yl)cyclohexyl]-5-(4-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Inhibition of Fyn (unknown origin) using Src-family kinase bisamide rhodamine 110 peptide substrate after 1 hr by fluorescence assay | Eur J Med Chem 157: 503-526 (2018) Article DOI: 10.1016/j.ejmech.2018.08.023 BindingDB Entry DOI: 10.7270/Q2DF6TX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM50413999 (CGP77675 | CHEMBL475584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Inhibition of Lyn (unknown origin) using Src-family kinase bisamide rhodamine 110 peptide substrate after 1 hr by fluorescence assay | Eur J Med Chem 157: 503-526 (2018) Article DOI: 10.1016/j.ejmech.2018.08.023 BindingDB Entry DOI: 10.7270/Q2DF6TX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50142887 (1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Inhibition of Src (unknown origin) using Src-family kinase bisamide rhodamine 110 peptide substrate after 1 hr by fluorescence assay | Eur J Med Chem 157: 503-526 (2018) Article DOI: 10.1016/j.ejmech.2018.08.023 BindingDB Entry DOI: 10.7270/Q2DF6TX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50403053 (CHEMBL2216810) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Hari Singh Gour University Curated by ChEMBL | Assay Description Antagonist activity at CCR4 | Bioorg Med Chem 20: 3378-95 (2012) Article DOI: 10.1016/j.bmc.2012.03.069 BindingDB Entry DOI: 10.7270/Q2222VX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50142347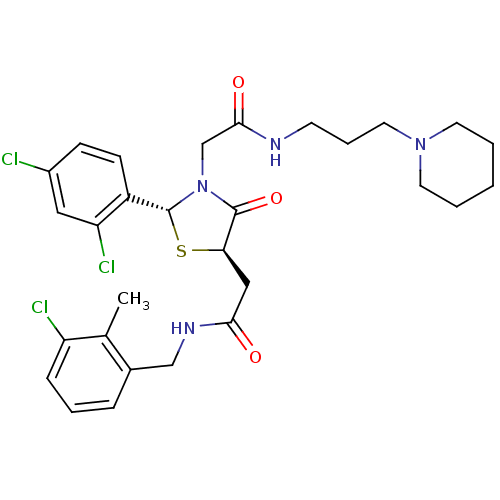 (2-[(2R,5R)-5-[(3-Chloro-2-methyl-benzylcarbamoyl)-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Hari Singh Gour University Curated by ChEMBL | Assay Description Antagonist activity at CCR4 | Bioorg Med Chem 20: 3378-95 (2012) Article DOI: 10.1016/j.bmc.2012.03.069 BindingDB Entry DOI: 10.7270/Q2222VX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50403049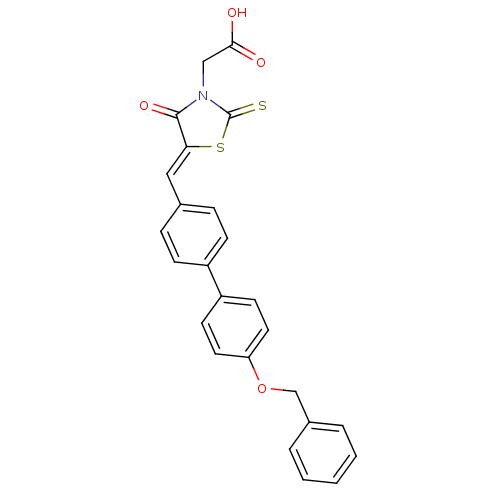 (CHEMBL2216806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Hari Singh Gour University Curated by ChEMBL | Assay Description Inhibition of GST-tagged PTP1B using pNPP as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 3378-95 (2012) Article DOI: 10.1016/j.bmc.2012.03.069 BindingDB Entry DOI: 10.7270/Q2222VX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50528546 (CHEMBL4516243) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University Curated by ChEMBL | Assay Description Inhibition of MMP2 catalytic domain (unknown origin) using Ac-PLG-[2-mercapto-4-methylpentanoyl]-LG-OC2H5 as substrate preincubated for 45 to 120 min... | Eur J Med Chem 180: 486-508 (2019) Article DOI: 10.1016/j.ejmech.2019.07.043 BindingDB Entry DOI: 10.7270/Q2959N1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) by EIA | Eur J Med Chem 180: 486-508 (2019) Article DOI: 10.1016/j.ejmech.2019.07.043 BindingDB Entry DOI: 10.7270/Q2959N1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50528543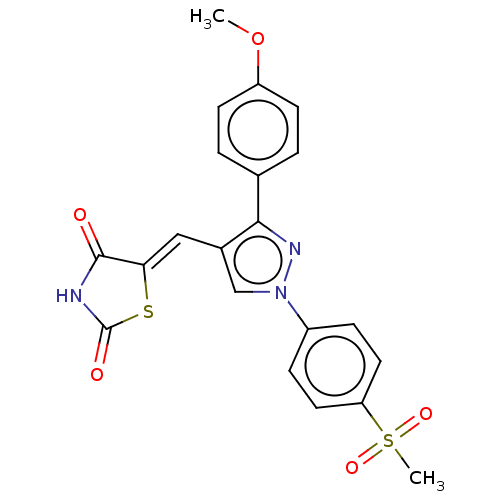 (CHEMBL4540611) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) by EIA | Eur J Med Chem 180: 486-508 (2019) Article DOI: 10.1016/j.ejmech.2019.07.043 BindingDB Entry DOI: 10.7270/Q2959N1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Large neutral amino acids transporter small subunit 1 (Homo sapiens (Human)) | BDBM50528545 (CHEMBL4526588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University Curated by ChEMBL | Assay Description Inhibition of recombinant human LAT1 | Eur J Med Chem 180: 486-508 (2019) Article DOI: 10.1016/j.ejmech.2019.07.043 BindingDB Entry DOI: 10.7270/Q2959N1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM50403052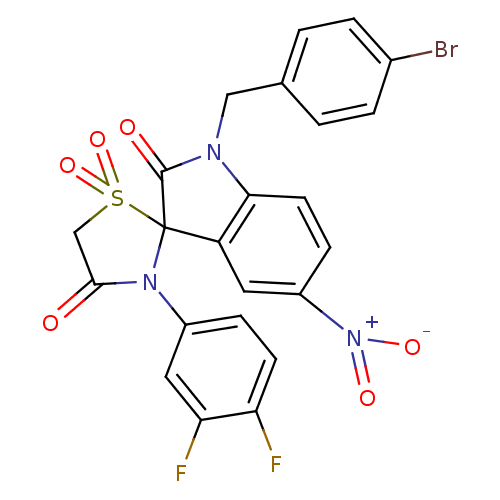 (CHEMBL2216811) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Hari Singh Gour University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis protein tyrosine phosphatase B | Bioorg Med Chem 20: 3378-95 (2012) Article DOI: 10.1016/j.bmc.2012.03.069 BindingDB Entry DOI: 10.7270/Q2222VX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50502004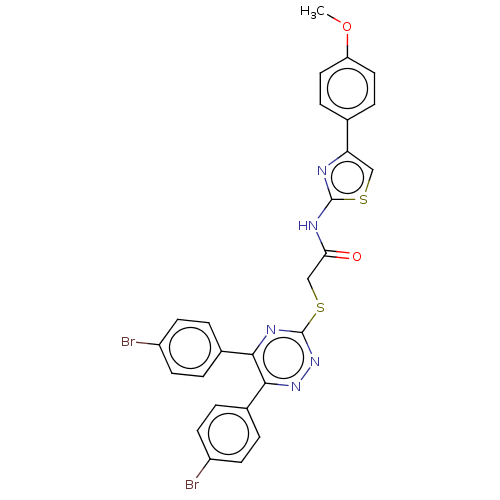 (CHEMBL4453256) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) using p-nitrophenyl alpha-d-glucopyranoside as substrate preincubated for 15 mins followed by substr... | Eur J Med Chem 180: 486-508 (2019) Article DOI: 10.1016/j.ejmech.2019.07.043 BindingDB Entry DOI: 10.7270/Q2959N1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University Curated by ChEMBL | Assay Description Inhibition of 5-LOX (unknown origin) | Eur J Med Chem 180: 486-508 (2019) Article DOI: 10.1016/j.ejmech.2019.07.043 BindingDB Entry DOI: 10.7270/Q2959N1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM152734 (11-bromo-5-[4-(2-fluorophenyl)piperazine-1- carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maharaja Ranjit Singh Punjab Technical University Curated by ChEMBL | Assay Description Inhibition of 5-LOX (unknown origin) | Eur J Med Chem 180: 486-508 (2019) Article DOI: 10.1016/j.ejmech.2019.07.043 BindingDB Entry DOI: 10.7270/Q2959N1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indo-Soviet Friendship College of Pharmacy (ISFCP) Curated by ChEMBL | Assay Description Inhibition of human cytomegalovirus DNA polymerase (95 uL) activity in a solution containing 6.4 mM HEPES (pH 7.5), incubation for 12 minutes at 26 d... | Bioorg Med Chem 25: 4533-4552 (2017) Article DOI: 10.1016/j.bmc.2017.07.003 BindingDB Entry DOI: 10.7270/Q2125W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |