 Found 185 hits with Last Name = 'renard' and Initial = 'py'
Found 185 hits with Last Name = 'renard' and Initial = 'py' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556952

(CHEMBL4754487)Show SMILES COc1cc(CNC(=O)CCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:34,TLB:23:22:19:34.31.32,THB:16:17:19:34.31.32| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed-type inhibition of recombinant human AChE assessed as inhibition constant using acetylthiocholine iodide as substrate by Cornish-Bowden plot an... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556952

(CHEMBL4754487)Show SMILES COc1cc(CNC(=O)CCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:34,TLB:23:22:19:34.31.32,THB:16:17:19:34.31.32| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed-type inhibition of recombinant human AChE assessed as dissociation constant for protein-substrate-compound complex using acetylthiocholine iodi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556952

(CHEMBL4754487)Show SMILES COc1cc(CNC(=O)CCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:34,TLB:23:22:19:34.31.32,THB:16:17:19:34.31.32| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523281

(CHEMBL4583650)Show SMILES O=C(Nc1ccc(cn1)-c1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-16-15-22(20-37-31)29-21-43(42-41-29)19-17-36-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,36,38)(H2,37,39,40,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50369748
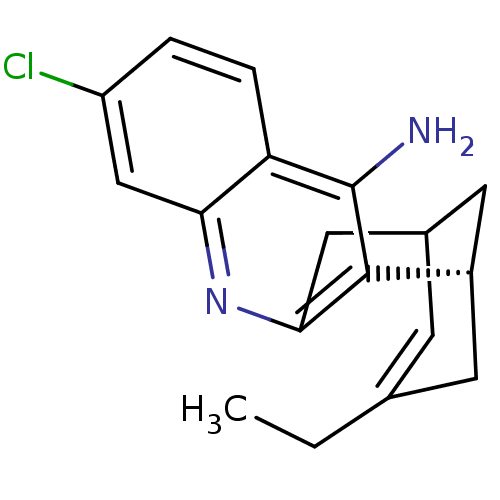
(CHEMBL208599)Show SMILES CCC1=CC2C[C@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:19:8:5:3.2.7,THB:11:9:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21)/t11?,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
COBRA-CNRS UMR 6014& FR 3038
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE in erythrocytes by Ellman's assay |
Bioorg Med Chem 17: 4523-36 (2009)
Article DOI: 10.1016/j.bmc.2009.05.005
BindingDB Entry DOI: 10.7270/Q2K938GQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10592

(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556958
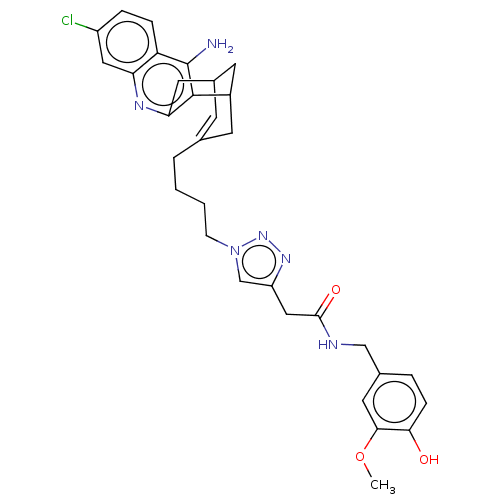
(CHEMBL4744528)Show SMILES COc1cc(CNC(=O)Cc2cn(CCCCC3=CC4CC(C3)c3c(N)c5ccc(Cl)cc5nc3C4)nn2)ccc1O |t:17,TLB:33:34:20:22.18.17,THB:24:23:20:22.18.17| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10592

(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.84 | n/a | n/a | n/a | n/a | n/a | n/a |
COBRA-CNRS UMR 6014& FR 3038
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE in erythrocytes by Ellman's assay |
Bioorg Med Chem 17: 4523-36 (2009)
Article DOI: 10.1016/j.bmc.2009.05.005
BindingDB Entry DOI: 10.7270/Q2K938GQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556953

(CHEMBL4748114)Show SMILES COc1cc(CNC(=O)CCCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:35,TLB:24:23:20:35.32.33,THB:17:18:20:35.32.33| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556954

(CHEMBL4782921)Show SMILES COc1cc(CNC(=O)CCCCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:36,TLB:25:24:21:36.33.34,THB:18:19:21:36.33.34| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298409

((+/-)-3-chloro-6,7,10,11-tetrahydro-N-(2-(4-(6-(3,...)Show SMILES COc1cc2CCN(CCCCCCc3cn(CCNc4c5C6CC(Cc5nc5cc(Cl)ccc45)C=C(C)C6)nn3)C(c3cccc(c3)[N+]([O-])=O)c2cc1OC |t:38,TLB:20:21:23:38.35.36,THB:37:36:23:25.26.21,27:26:23:38.35.36| Show InChI InChI=1S/C44H50ClN7O4/c1-28-19-29-21-32(20-28)42-39(22-29)47-38-25-33(45)12-13-36(38)43(42)46-15-18-51-27-34(48-49-51)10-6-4-5-7-16-50-17-14-30-24-40(55-2)41(56-3)26-37(30)44(50)31-9-8-11-35(23-31)52(53)54/h8-9,11-13,19,23-27,29,32,44H,4-7,10,14-18,20-22H2,1-3H3,(H,46,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.95 | n/a | n/a | n/a | n/a | n/a | n/a |
COBRA-CNRS UMR 6014& FR 3038
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE in erythrocytes by Ellman's assay |
Bioorg Med Chem 17: 4523-36 (2009)
Article DOI: 10.1016/j.bmc.2009.05.005
BindingDB Entry DOI: 10.7270/Q2K938GQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556951
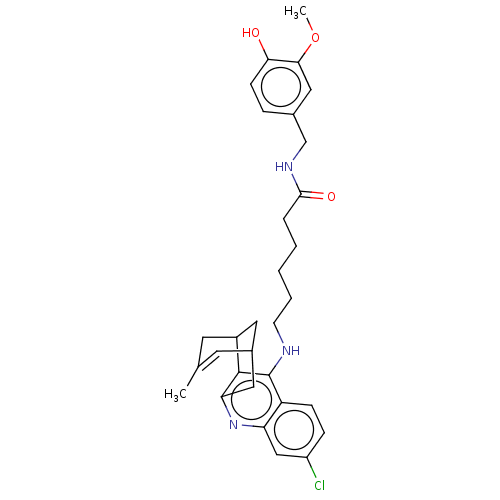
(CHEMBL4788434)Show SMILES COc1cc(CNC(=O)CCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:33,TLB:22:21:18:33.30.31,THB:15:16:18:33.30.31| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556956
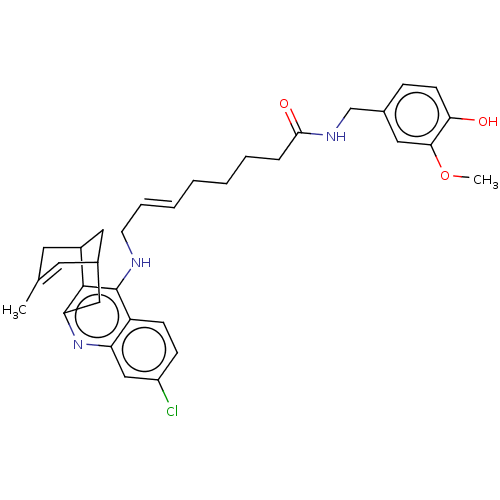
(CHEMBL4741162)Show SMILES COc1cc(CNC(=O)CCCC\C=C\CNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:35,TLB:24:23:20:35.32.33,THB:17:18:20:35.32.33| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556950
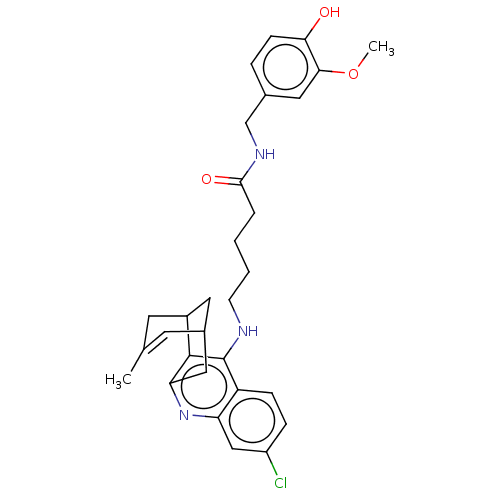
(CHEMBL4743210)Show SMILES COc1cc(CNC(=O)CCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:32,TLB:21:20:17:32.29.30,THB:14:15:17:32.29.30| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556957

(CHEMBL4794769)Show SMILES COc1cc(CNC(=O)CCCC\C=C\CCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:37,TLB:26:25:22:37.34.35,THB:19:20:22:37.34.35| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298408
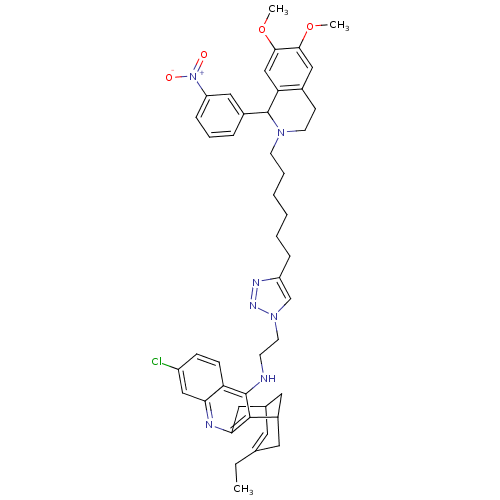
((+/-)-3-chloro-6,7,10,11-tetrahydro-N-(2-(4-(6-(3,...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCn1cc(CCCCCCN2CCc3cc(OC)c(OC)cc3C2c2cccc(c2)[N+]([O-])=O)nn1 |t:2,TLB:19:8:5:7.3.2,THB:1:2:5:10.9.8,11:9:5:7.3.2| Show InChI InChI=1S/C45H52ClN7O4/c1-4-29-20-30-22-33(21-29)43-40(23-30)48-39-26-34(46)13-14-37(39)44(43)47-16-19-52-28-35(49-50-52)11-7-5-6-8-17-51-18-15-31-25-41(56-2)42(57-3)27-38(31)45(51)32-10-9-12-36(24-32)53(54)55/h9-10,12-14,20,24-28,30,33,45H,4-8,11,15-19,21-23H2,1-3H3,(H,47,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.96 | n/a | n/a | n/a | n/a | n/a | n/a |
COBRA-CNRS UMR 6014& FR 3038
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE in erythrocytes by Ellman's assay |
Bioorg Med Chem 17: 4523-36 (2009)
Article DOI: 10.1016/j.bmc.2009.05.005
BindingDB Entry DOI: 10.7270/Q2K938GQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298403
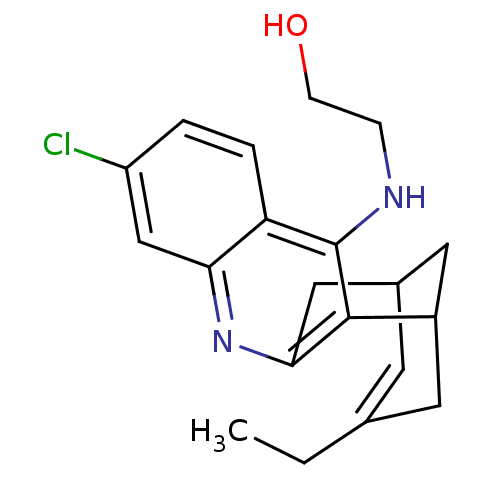
((+/-)-N-(2-Hydroxyethyl)-3-chloro-6,7,10,11-tetrah...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCO |t:2,TLB:19:8:5:7.3.2,THB:1:2:5:10.9.8,11:9:5:7.3.2| Show InChI InChI=1S/C20H23ClN2O/c1-2-12-7-13-9-14(8-12)19-18(10-13)23-17-11-15(21)3-4-16(17)20(19)22-5-6-24/h3-4,7,11,13-14,24H,2,5-6,8-10H2,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.75 | n/a | n/a | n/a | n/a | n/a | n/a |
COBRA-CNRS UMR 6014& FR 3038
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE in erythrocytes by Ellman's assay |
Bioorg Med Chem 17: 4523-36 (2009)
Article DOI: 10.1016/j.bmc.2009.05.005
BindingDB Entry DOI: 10.7270/Q2K938GQ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523276

(CHEMBL4443989)Show SMILES [H][C@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)-c3cn(CCNc4c5CCCCc5nc5ccccc45)nn3)c21 |r| Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46)/t30-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50556958

(CHEMBL4744528)Show SMILES COc1cc(CNC(=O)Cc2cn(CCCCC3=CC4CC(C3)c3c(N)c5ccc(Cl)cc5nc3C4)nn2)ccc1O |t:17,TLB:33:34:20:22.18.17,THB:24:23:20:22.18.17| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523265

(CHEMBL4531222)Show SMILES O=C(Nc1cc(COCc2cn(CCCc3ccccc3)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C31H33N7O3/c39-30-25-11-6-12-26(29(25)27-13-4-5-17-38(27)30)33-31(40)34-28-18-23(14-15-32-28)20-41-21-24-19-37(36-35-24)16-7-10-22-8-2-1-3-9-22/h1-3,6,8-9,11-12,14-15,18-19,27H,4-5,7,10,13,16-17,20-21H2,(H2,32,33,34,40) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298405

((+/-)-N-(2-azidoethyl)-3-chloro-6,7,10,11-tetrahyd...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCN=[N+]=[N-] |t:1,TLB:18:7:4:6.2.1,THB:0:1:4:9.8.7,10:8:4:6.2.1| Show InChI InChI=1S/C19H20ClN5/c1-11-6-12-8-13(7-11)18-17(9-12)24-16-10-14(20)2-3-15(16)19(18)22-4-5-23-25-21/h2-3,6,10,12-13H,4-5,7-9H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.69 | n/a | n/a | n/a | n/a | n/a | n/a |
COBRA-CNRS UMR 6014& FR 3038
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE in erythrocytes by Ellman's assay |
Bioorg Med Chem 17: 4523-36 (2009)
Article DOI: 10.1016/j.bmc.2009.05.005
BindingDB Entry DOI: 10.7270/Q2K938GQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523276

(CHEMBL4443989)Show SMILES [H][C@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)-c3cn(CCNc4c5CCCCc5nc5ccccc45)nn3)c21 |r| Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46)/t30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298402

((+/-)-N-(2-Hydroxyethyl)-3-chloro-6,7,10,11-tetrah...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCO |t:1,TLB:18:7:4:6.2.1,THB:0:1:4:9.8.7,10:8:4:6.2.1| Show InChI InChI=1S/C19H21ClN2O/c1-11-6-12-8-13(7-11)18-17(9-12)22-16-10-14(20)2-3-15(16)19(18)21-4-5-23/h2-3,6,10,12-13,23H,4-5,7-9H2,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.66 | n/a | n/a | n/a | n/a | n/a | n/a |
COBRA-CNRS UMR 6014& FR 3038
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE in erythrocytes by Ellman's assay |
Bioorg Med Chem 17: 4523-36 (2009)
Article DOI: 10.1016/j.bmc.2009.05.005
BindingDB Entry DOI: 10.7270/Q2K938GQ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523277
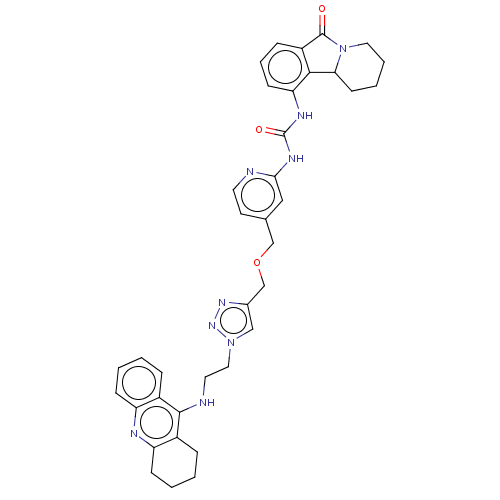
(CHEMBL4516356)Show SMILES O=C(Nc1cc(COCc2cn(CCNc3c4CCCCc4nc4ccccc34)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C37H39N9O3/c47-36-28-10-7-13-31(34(28)32-14-5-6-18-46(32)36)41-37(48)42-33-20-24(15-16-38-33)22-49-23-25-21-45(44-43-25)19-17-39-35-26-8-1-3-11-29(26)40-30-12-4-2-9-27(30)35/h1,3,7-8,10-11,13,15-16,20-21,32H,2,4-6,9,12,14,17-19,22-23H2,(H,39,40)(H2,38,41,42,48) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523269

(CHEMBL4446287)Show SMILES CN(Cc1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Cc1ccnc(NC(=O)Nc2cccc3C(=O)N4CCCCC4c23)c1 Show InChI InChI=1S/C38H42N10O2/c1-46(23-26-24-47(45-44-26)20-18-40-36-27-9-2-4-12-30(27)41-31-13-5-3-10-28(31)36)22-25-16-17-39-34(21-25)43-38(50)42-32-14-8-11-29-35(32)33-15-6-7-19-48(33)37(29)49/h2,4,8-9,11-12,14,16-17,21,24,33H,3,5-7,10,13,15,18-20,22-23H2,1H3,(H,40,41)(H2,39,42,43,50) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523267

(CHEMBL4559936)Show SMILES O=C(Nc1cc(ccn1)-c1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523266

(CHEMBL4444585)Show SMILES CN(CC#C)Cc1ccnc(NC(=O)Nc2cccc3C(=O)N4CCCCC4c23)c1 Show InChI InChI=1S/C23H25N5O2/c1-3-12-27(2)15-16-10-11-24-20(14-16)26-23(30)25-18-8-6-7-17-21(18)19-9-4-5-13-28(19)22(17)29/h1,6-8,10-11,14,19H,4-5,9,12-13,15H2,2H3,(H2,24,25,26,30) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298406
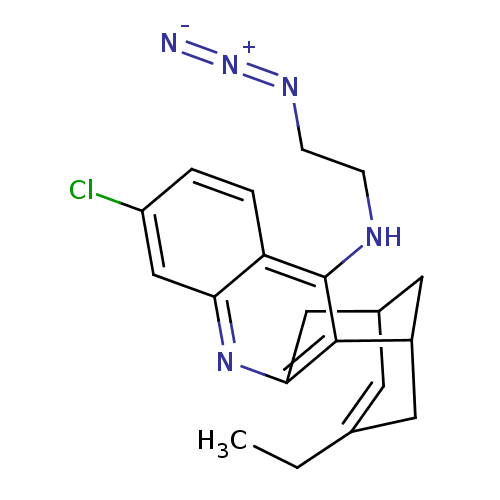
((+/-)-N-(2-azidoethyl)-3-chloro-6,7,10,11-tetrahyd...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCN=[N+]=[N-] |t:2,TLB:19:8:5:7.3.2,THB:1:2:5:10.9.8,11:9:5:7.3.2| Show InChI InChI=1S/C20H22ClN5/c1-2-12-7-13-9-14(8-12)19-18(10-13)25-17-11-15(21)3-4-16(17)20(19)23-5-6-24-26-22/h3-4,7,11,13-14H,2,5-6,8-10H2,1H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a |
COBRA-CNRS UMR 6014& FR 3038
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE in erythrocytes by Ellman's assay |
Bioorg Med Chem 17: 4523-36 (2009)
Article DOI: 10.1016/j.bmc.2009.05.005
BindingDB Entry DOI: 10.7270/Q2K938GQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523262

(CHEMBL4443468)Show SMILES [H][C@@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)-c3cn(CCNc4c5CCCCc5nc5ccccc45)nn3)c21 |r| Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523282

(CHEMBL4515295)Show SMILES O=C(Nc1cc(CCC#C)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C22H22N4O2/c1-2-3-7-15-11-12-23-19(14-15)25-22(28)24-17-9-6-8-16-20(17)18-10-4-5-13-26(18)21(16)27/h1,6,8-9,11-12,14,18H,3-5,7,10,13H2,(H2,23,24,25,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523267

(CHEMBL4559936)Show SMILES O=C(Nc1cc(ccn1)-c1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50523278
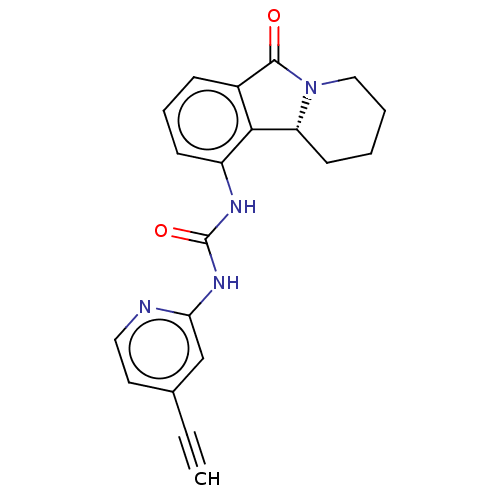
(CHEMBL4463060)Show SMILES [H][C@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)C#C)c21 |r| Show InChI InChI=1S/C20H18N4O2/c1-2-13-9-10-21-17(12-13)23-20(26)22-15-7-5-6-14-18(15)16-8-3-4-11-24(16)19(14)25/h1,5-7,9-10,12,16H,3-4,8,11H2,(H2,21,22,23,26)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523262

(CHEMBL4443468)Show SMILES [H][C@@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)-c3cn(CCNc4c5CCCCc5nc5ccccc45)nn3)c21 |r| Show InChI InChI=1S/C35H35N9O2/c45-34-25-10-7-13-28(32(25)30-14-5-6-18-44(30)34)39-35(46)40-31-20-22(15-16-36-31)29-21-43(42-41-29)19-17-37-33-23-8-1-3-11-26(23)38-27-12-4-2-9-24(27)33/h1,3,7-8,10-11,13,15-16,20-21,30H,2,4-6,9,12,14,17-19H2,(H,37,38)(H2,36,39,40,46)/t30-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50523275

(CHEMBL4537026)Show SMILES O=C(Nc1cccc(n1)-c1cn(CCNc2c3CCCCc3nc3ccccc23)nn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C35H35N9O2/c45-34-24-11-7-15-28(32(24)30-16-5-6-19-44(30)34)39-35(46)40-31-17-8-14-27(38-31)29-21-43(42-41-29)20-18-36-33-22-9-1-3-12-25(22)37-26-13-4-2-10-23(26)33/h1,3,7-9,11-12,14-15,17,21,30H,2,4-6,10,13,16,18-20H2,(H,36,37)(H2,38,39,40,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using BTCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellman's me... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523268

(CHEMBL4593316)Show SMILES O=C(Nc1cc(CCc2cn(CCNc3c4CCCCc4nc4ccccc34)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C37H39N9O2/c47-36-28-10-7-13-31(34(28)32-14-5-6-20-46(32)36)41-37(48)42-33-22-24(17-18-38-33)15-16-25-23-45(44-43-25)21-19-39-35-26-8-1-3-11-29(26)40-30-12-4-2-9-27(30)35/h1,3,7-8,10-11,13,17-18,22-23,32H,2,4-6,9,12,14-16,19-21H2,(H,39,40)(H2,38,41,42,48) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523277

(CHEMBL4516356)Show SMILES O=C(Nc1cc(COCc2cn(CCNc3c4CCCCc4nc4ccccc34)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C37H39N9O3/c47-36-28-10-7-13-31(34(28)32-14-5-6-18-46(32)36)41-37(48)42-33-20-24(15-16-38-33)22-49-23-25-21-45(44-43-25)19-17-39-35-26-8-1-3-11-29(26)40-30-12-4-2-9-27(30)35/h1,3,7-8,10-11,13,15-16,20-21,32H,2,4-6,9,12,14,17-19,22-23H2,(H,39,40)(H2,38,41,42,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50399310

(CHEMBL2180854)Show InChI InChI=1S/C18H17BrN4O2/c19-11-7-8-20-15(10-11)22-18(25)21-13-5-3-4-12-16(13)14-6-1-2-9-23(14)17(12)24/h3-5,7-8,10,14H,1-2,6,9H2,(H2,20,21,22,25) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523268

(CHEMBL4593316)Show SMILES O=C(Nc1cc(CCc2cn(CCNc3c4CCCCc4nc4ccccc34)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C37H39N9O2/c47-36-28-10-7-13-31(34(28)32-14-5-6-20-46(32)36)41-37(48)42-33-22-24(17-18-38-33)15-16-25-23-45(44-43-25)21-19-39-35-26-8-1-3-11-29(26)40-30-12-4-2-9-27(30)35/h1,3,7-8,10-11,13,17-18,22-23,32H,2,4-6,9,12,14-16,19-21H2,(H,39,40)(H2,38,41,42,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50399310

(CHEMBL2180854)Show InChI InChI=1S/C18H17BrN4O2/c19-11-7-8-20-15(10-11)22-18(25)21-13-5-3-4-12-16(13)14-6-1-2-9-23(14)17(12)24/h3-5,7-8,10,14H,1-2,6,9H2,(H2,20,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50556955
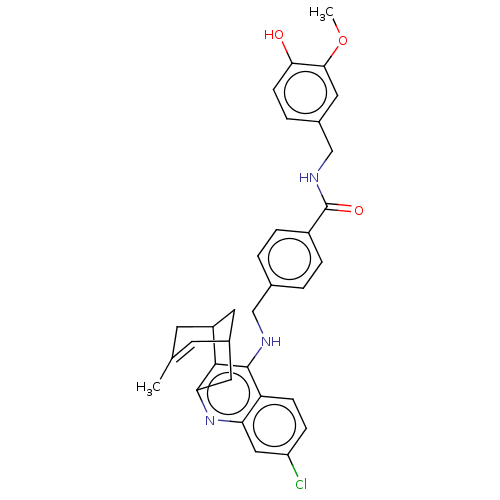
(CHEMBL4782267)Show SMILES COc1cc(CNC(=O)c2ccc(CNc3c4C5CC(Cc4nc4cc(Cl)ccc34)C=C(C)C5)cc2)ccc1O |t:33,TLB:22:21:18:33.30.31,THB:15:16:18:33.30.31| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523274

(CHEMBL4537142)Show SMILES Cn1cc(COCc2ccnc(NC(=O)Nc3cccc4C(=O)N5CCCCC5c34)c2)nn1 Show InChI InChI=1S/C23H25N7O3/c1-29-12-16(27-28-29)14-33-13-15-8-9-24-20(11-15)26-23(32)25-18-6-4-5-17-21(18)19-7-2-3-10-30(19)22(17)31/h4-6,8-9,11-12,19H,2-3,7,10,13-14H2,1H3,(H2,24,25,26,32) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523273

(CHEMBL4459540)Show SMILES O=C(Nc1cc(COCc2cn(CCNc3ccncc3)nn2)ccn1)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C29H31N9O3/c39-28-23-4-3-5-24(27(23)25-6-1-2-14-38(25)28)33-29(40)34-26-16-20(7-12-32-26)18-41-19-22-17-37(36-35-22)15-13-31-21-8-10-30-11-9-21/h3-5,7-12,16-17,25H,1-2,6,13-15,18-19H2,(H,30,31)(H2,32,33,34,40) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50556952

(CHEMBL4754487)Show SMILES COc1cc(CNC(=O)CCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:34,TLB:23:22:19:34.31.32,THB:16:17:19:34.31.32| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523278

(CHEMBL4463060)Show SMILES [H][C@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)C#C)c21 |r| Show InChI InChI=1S/C20H18N4O2/c1-2-13-9-10-21-17(12-13)23-20(26)22-15-7-5-6-14-18(15)16-8-3-4-11-24(16)19(14)25/h1,5-7,9-10,12,16H,3-4,8,11H2,(H2,21,22,23,26)/t16-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human serum BChE using BTCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellman's me... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50556954

(CHEMBL4782921)Show SMILES COc1cc(CNC(=O)CCCCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:36,TLB:25:24:21:36.33.34,THB:18:19:21:36.33.34| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta/[Tau protein] kinase
(Sus scrofa) | BDBM50523271
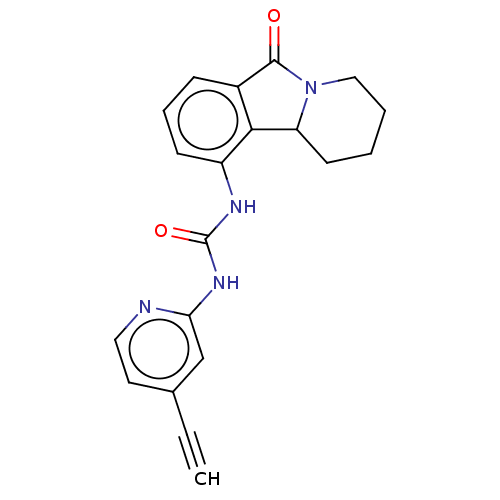
(CHEMBL4566076)Show SMILES O=C(Nc1cc(ccn1)C#C)Nc1cccc2C(=O)N3CCCCC3c12 Show InChI InChI=1S/C20H18N4O2/c1-2-13-9-10-21-17(12-13)23-20(26)22-15-7-5-6-14-18(15)16-8-3-4-11-24(16)19(14)25/h1,5-7,9-10,12,16H,3-4,8,11H2,(H2,21,22,23,26) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of native porcine brain GSK-3alpha/beta using GS-1 as substrate incubated for 30 mins in presence of [gamma-33P]ATP by liquid scintillatio... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50298404

((+/-)-N-(2-chloroethyl)-3-chloro-6,7,10,11-tetrahy...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCl |t:1,TLB:18:7:4:6.2.1,THB:0:1:4:9.8.7,10:8:4:6.2.1| Show InChI InChI=1S/C19H20Cl2N2/c1-11-6-12-8-13(7-11)18-17(9-12)23-16-10-14(21)2-3-15(16)19(18)22-5-4-20/h2-3,6,10,12-13H,4-5,7-9H2,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43.3 | n/a | n/a | n/a | n/a | n/a | n/a |
COBRA-CNRS UMR 6014& FR 3038
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE in erythrocytes by Ellman's assay |
Bioorg Med Chem 17: 4523-36 (2009)
Article DOI: 10.1016/j.bmc.2009.05.005
BindingDB Entry DOI: 10.7270/Q2K938GQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50523263
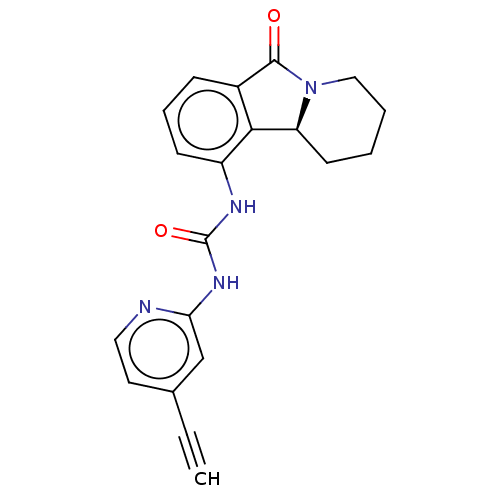
(CHEMBL4483607)Show SMILES [H][C@@]12CCCCN1C(=O)c1cccc(NC(=O)Nc3cc(ccn3)C#C)c21 |r| Show InChI InChI=1S/C20H18N4O2/c1-2-13-9-10-21-17(12-13)23-20(26)22-15-7-5-6-14-18(15)16-8-3-4-11-24(16)19(14)25/h1,5-7,9-10,12,16H,3-4,8,11H2,(H2,21,22,23,26)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CDK5/p25 using histone H1 as substrate in presence of [gamma-33P]ATP incubated for 30 mins by liquid scintillation co... |
Eur J Med Chem 168: 58-77 (2019)
Article DOI: 10.1016/j.ejmech.2018.12.063
BindingDB Entry DOI: 10.7270/Q2J969S0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50556953

(CHEMBL4748114)Show SMILES COc1cc(CNC(=O)CCCCCCCNc2c3C4CC(Cc3nc3cc(Cl)ccc23)C=C(C)C4)ccc1O |t:35,TLB:24:23:20:35.32.33,THB:17:18:20:35.32.33| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human serum BChE using butyrylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01775
BindingDB Entry DOI: 10.7270/Q2K077XJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data