 Found 815 hits with Last Name = 'roh' and Initial = 'e'
Found 815 hits with Last Name = 'roh' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50355500
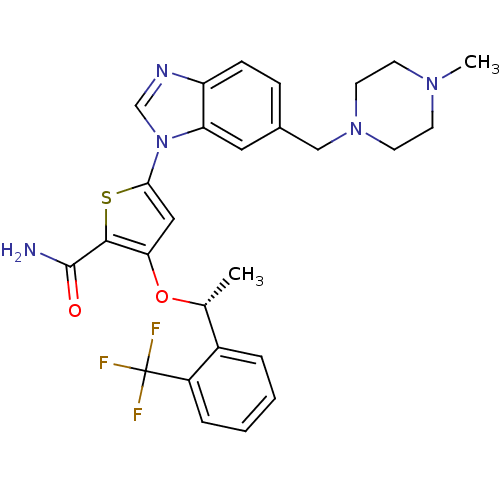
(CHEMBL1908394 | US9695172, GSK461364)Show SMILES C[C@@H](Oc1cc(sc1C(N)=O)-n1cnc2ccc(CN3CCN(C)CC3)cc12)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C27H28F3N5O2S/c1-17(19-5-3-4-6-20(19)27(28,29)30)37-23-14-24(38-25(23)26(31)36)35-16-32-21-8-7-18(13-22(21)35)15-34-11-9-33(2)10-12-34/h3-8,13-14,16-17H,9-12,15H2,1-2H3,(H2,31,36)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00533
BindingDB Entry DOI: 10.7270/Q23X8BM3 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215404
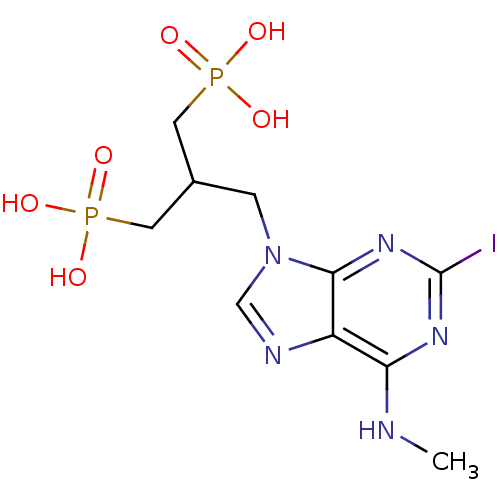
(CHEMBL227235 | [3-(2-iodo-6-methylaminopurin-9-yl)...)Show SMILES CNc1nc(I)nc2n(CC(CP(O)(O)=O)CP(O)(O)=O)cnc12 Show InChI InChI=1S/C10H16IN5O6P2/c1-12-8-7-9(15-10(11)14-8)16(5-13-7)2-6(3-23(17,18)19)4-24(20,21)22/h5-6H,2-4H2,1H3,(H,12,14,15)(H2,17,18,19)(H2,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50121987
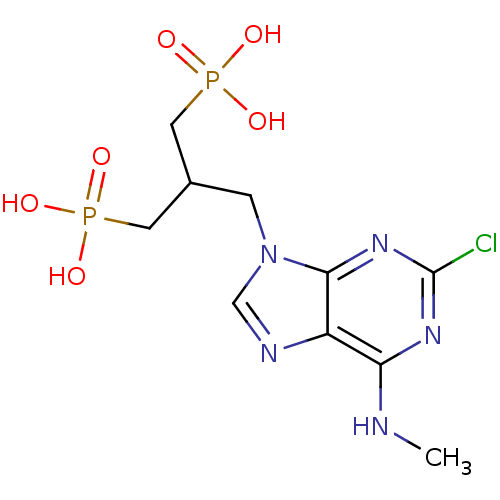
(CHEMBL153254 | [3-(2-Chloro-6-methylamino-purin-9-...)Show SMILES CNc1nc(Cl)nc2n(CC(CP(O)(O)=O)CP(O)(O)=O)cnc12 Show InChI InChI=1S/C10H16ClN5O6P2/c1-12-8-7-9(15-10(11)14-8)16(5-13-7)2-6(3-23(17,18)19)4-24(20,21)22/h5-6H,2-4H2,1H3,(H,12,14,15)(H2,17,18,19)(H2,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215406
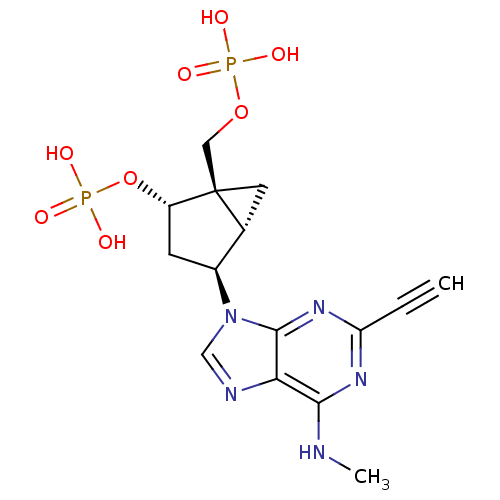
((1'R,2'S,4'S,5'S)-4-(2-ethynyl-6-methylaminopurin-...)Show SMILES CNc1nc(nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12)C#C Show InChI InChI=1S/C15H19N5O8P2/c1-3-11-18-13(16-2)12-14(19-11)20(7-17-12)9-4-10(28-30(24,25)26)15(5-8(9)15)6-27-29(21,22)23/h1,7-10H,4-6H2,2H3,(H,16,18,19)(H2,21,22,23)(H2,24,25,26)/t8-,9+,10+,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50520846

(CHEMBL4461911)Show SMILES COc1ccc(Oc2nc(Nc3ccc(nc3)N3CCOCC3)ncc2NC(=O)c2cc(OC)cc(OC)c2)cc1 Show InChI InChI=1S/C29H30N6O6/c1-37-21-5-7-22(8-6-21)41-28-25(33-27(36)19-14-23(38-2)16-24(15-19)39-3)18-31-29(34-28)32-20-4-9-26(30-17-20)35-10-12-40-13-11-35/h4-9,14-18H,10-13H2,1-3H3,(H,33,36)(H,31,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate after 120 mins in presence of [gamma-33P]-ATP by filtration method |
Eur J Med Chem 162: 161-175 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.057
BindingDB Entry DOI: 10.7270/Q29K4FM7 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215400

((1'R,2'S,4'R,5'S)-phosphoric acid mono-[4-(6-methy...)Show SMILES CNc1nc(nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12)-c1ccccc1 Show InChI InChI=1S/C19H23N5O8P2/c1-20-17-15-18(23-16(22-17)11-5-3-2-4-6-11)24(10-21-15)13-7-14(32-34(28,29)30)19(8-12(13)19)9-31-33(25,26)27/h2-6,10,12-14H,7-9H2,1H3,(H,20,22,23)(H2,25,26,27)(H2,28,29,30)/t12-,13+,14+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215402
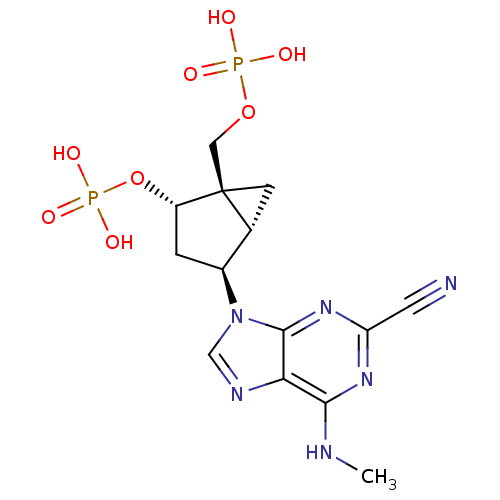
((1'R,2'S,4'R,5'S)-phosphoric acid mono-[4-(2-cyano...)Show SMILES CNc1nc(nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12)C#N Show InChI InChI=1S/C14H18N6O8P2/c1-16-12-11-13(19-10(4-15)18-12)20(6-17-11)8-2-9(28-30(24,25)26)14(3-7(8)14)5-27-29(21,22)23/h6-9H,2-3,5H2,1H3,(H,16,18,19)(H2,21,22,23)(H2,24,25,26)/t7-,8+,9+,14+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
Death-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50520846

(CHEMBL4461911)Show SMILES COc1ccc(Oc2nc(Nc3ccc(nc3)N3CCOCC3)ncc2NC(=O)c2cc(OC)cc(OC)c2)cc1 Show InChI InChI=1S/C29H30N6O6/c1-37-21-5-7-22(8-6-21)41-28-25(33-27(36)19-14-23(38-2)16-24(15-19)39-3)18-31-29(34-28)32-20-4-9-26(30-17-20)35-10-12-40-13-11-35/h4-9,14-18H,10-13H2,1-3H3,(H,33,36)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 581 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of human DAPK1 (1 to 363 residues) using KKLNRTLSFAEPG as substrate after 120 mins [gamma-33P]-ATP by filtration method |
Eur J Med Chem 162: 161-175 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.057
BindingDB Entry DOI: 10.7270/Q29K4FM7 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215403
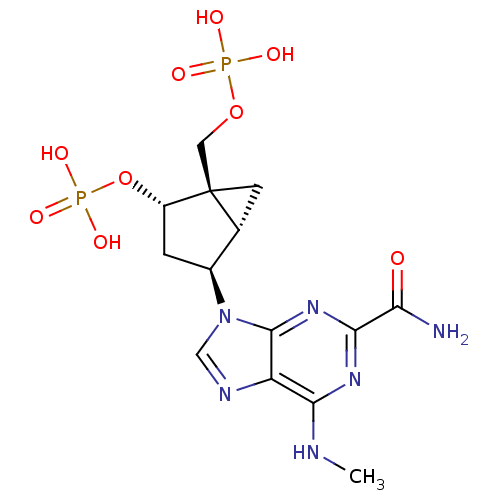
((1'R,2'S,4'R,5'S)-phosphoric acid mono-[4-(2-carba...)Show SMILES CNc1nc(nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12)C(N)=O Show InChI InChI=1S/C14H20N6O9P2/c1-16-11-9-13(19-12(18-11)10(15)21)20(5-17-9)7-2-8(29-31(25,26)27)14(3-6(7)14)4-28-30(22,23)24/h5-8H,2-4H2,1H3,(H2,15,21)(H,16,18,19)(H2,22,23,24)(H2,25,26,27)/t6-,7+,8+,14+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215401
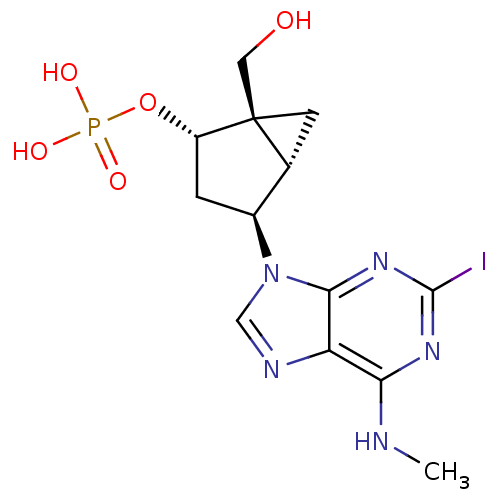
((1'R,2'S,4'S,5'S)-phosphoric acid mono-[1-hydroxym...)Show SMILES CNc1nc(I)nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(CO)C[C@H]12 Show InChI InChI=1S/C13H17IN5O5P/c1-15-10-9-11(18-12(14)17-10)19(5-16-9)7-2-8(24-25(21,22)23)13(4-20)3-6(7)13/h5-8,20H,2-4H2,1H3,(H,15,17,18)(H2,21,22,23)/t6-,7+,8+,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 706 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50215405
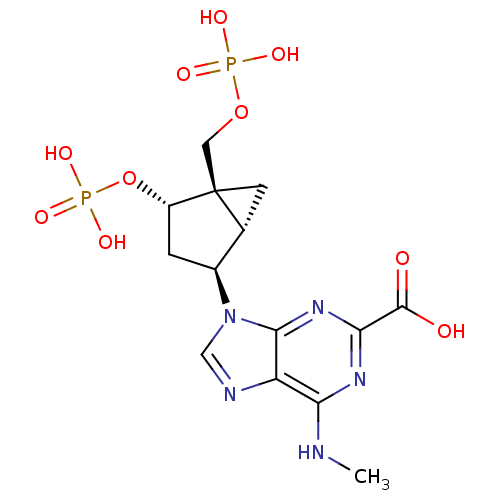
((1'R,2'S,4'R,5'S)-6-methylamino-9-(4-phosphonooxy-...)Show SMILES CNc1nc(nc2n(cnc12)[C@H]1C[C@H](OP(O)(O)=O)[C@]2(COP(O)(O)=O)C[C@H]12)C(O)=O Show InChI InChI=1S/C14H19N5O10P2/c1-15-10-9-12(18-11(17-10)13(20)21)19(5-16-9)7-2-8(29-31(25,26)27)14(3-6(7)14)4-28-30(22,23)24/h5-8H,2-4H2,1H3,(H,20,21)(H,15,17,18)(H2,22,23,24)(H2,25,26,27)/t6-,7+,8+,14+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Displacement of [3H]MRS2270 from human P2Y1 receptor expressed in Sf9 cells |
J Med Chem 50: 3229-41 (2007)
Article DOI: 10.1021/jm0700971
BindingDB Entry DOI: 10.7270/Q2BK1C2D |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50275734
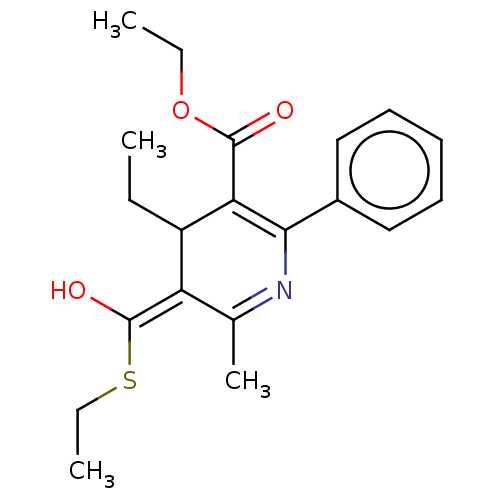
(CHEMBL108801 | ethyl 4-ethyl-5-(ethylthiocarbonyl)...)Show SMILES CCOC(=O)C1=C(N=C(C)\C(C1CC)=C(\O)SCC)c1ccccc1 |t:5,7| Show InChI InChI=1S/C20H25NO3S/c1-5-15-16(20(23)25-7-3)13(4)21-18(14-11-9-8-10-12-14)17(15)19(22)24-6-2/h8-12,15-16H,5-7H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem 16: 9349-58 (2008)
Article DOI: 10.1016/j.bmc.2008.08.048
BindingDB Entry DOI: 10.7270/Q2FJ2GMS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50275838
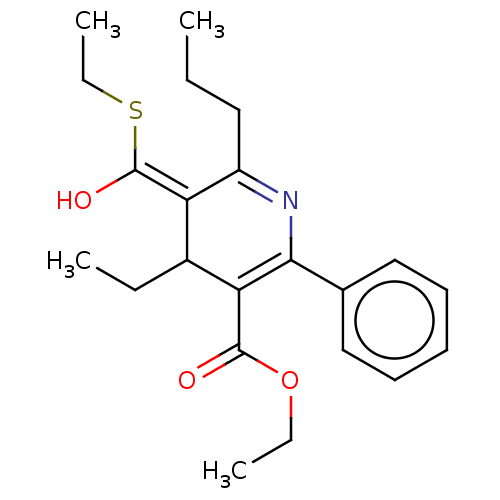
(CHEMBL111239 | ethyl 4-ethyl-5-(ethylthiocarbonyl)...)Show SMILES CCCC1=NC(=C(C(CC)\C1=C(/O)SCC)C(=O)OCC)c1ccccc1 |c:5,t:3| Show InChI InChI=1S/C22H29NO3S/c1-5-12-17-18(22(25)27-8-4)16(6-2)19(21(24)26-7-3)20(23-17)15-13-10-9-11-14-15/h9-11,13-14,16,18H,5-8,12H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem 16: 9349-58 (2008)
Article DOI: 10.1016/j.bmc.2008.08.048
BindingDB Entry DOI: 10.7270/Q2FJ2GMS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50275836
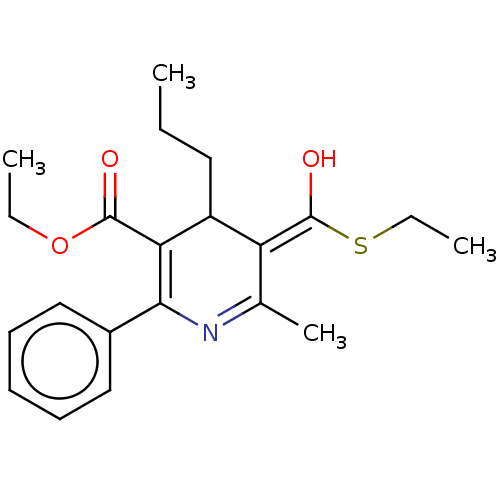
(3-ethylthio-5-ethyl-2-methyl-4-propyl-6-(3-chlorop...)Show SMILES CCCC1\C(=C(/O)SCC)C(C)=NC(=C1C(=O)OCC)c1ccccc1 |c:11,13| Show InChI InChI=1S/C21H27NO3S/c1-5-11-16-17(21(24)26-7-3)14(4)22-19(15-12-9-8-10-13-15)18(16)20(23)25-6-2/h8-10,12-13,16-17H,5-7,11H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem 16: 9349-58 (2008)
Article DOI: 10.1016/j.bmc.2008.08.048
BindingDB Entry DOI: 10.7270/Q2FJ2GMS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50025067

(CHEMBL105764)Show SMILES CCO\C(O)=C1\C(CC)C(C(=O)OCC)=C(N=C1C)c1ccccc1 |c:14,16| Show InChI InChI=1S/C20H25NO4/c1-5-15-16(19(22)24-6-2)13(4)21-18(14-11-9-8-10-12-14)17(15)20(23)25-7-3/h8-12,15,22H,5-7H2,1-4H3/b19-16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem 16: 9349-58 (2008)
Article DOI: 10.1016/j.bmc.2008.08.048
BindingDB Entry DOI: 10.7270/Q2FJ2GMS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50275899

(3,5-diethyl-2-methyl-4-ethyl-6-phenyl-1,4-(+)-dihy...)Show SMILES CCOC(=O)C1=C(N=C(C)\C(C1CC)=C(\O)SCCOC)c1ccccc1 |t:5,7| Show InChI InChI=1S/C21H27NO4S/c1-5-16-17(21(24)27-13-12-25-4)14(3)22-19(15-10-8-7-9-11-15)18(16)20(23)26-6-2/h7-11,16-17H,5-6,12-13H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem 16: 9349-58 (2008)
Article DOI: 10.1016/j.bmc.2008.08.048
BindingDB Entry DOI: 10.7270/Q2FJ2GMS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50275730

(CHEMBL108374 | diethyl 4-(dimethoxymethyl)-2-methy...)Show SMILES CCO\C(O)=C1\C(C(OC)OC)C(C(=O)OCC)=C(N=C1C)c1ccccc1 |c:17,19| Show InChI InChI=1S/C21H27NO6/c1-6-27-19(23)15-13(3)22-18(14-11-9-8-10-12-14)17(20(24)28-7-2)16(15)21(25-4)26-5/h8-12,15-16,21H,6-7H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem 16: 9349-58 (2008)
Article DOI: 10.1016/j.bmc.2008.08.048
BindingDB Entry DOI: 10.7270/Q2FJ2GMS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50275837
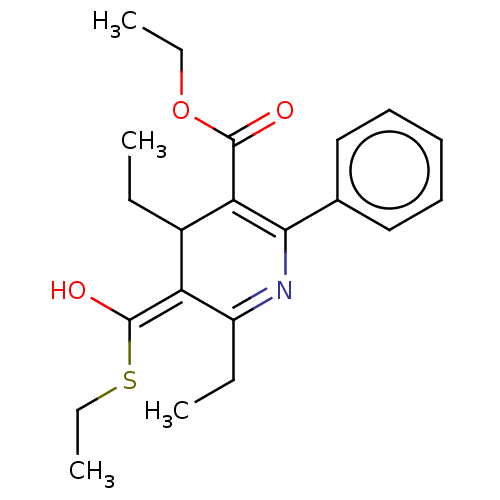
(CHEMBL110635 | ethyl 4,6-diethyl-5-(ethylthiocarbo...)Show SMILES CCOC(=O)C1=C(N=C(CC)\C(C1CC)=C(\O)SCC)c1ccccc1 |t:5,7| Show InChI InChI=1S/C21H27NO3S/c1-5-15-17(21(24)26-8-4)16(6-2)22-19(14-12-10-9-11-13-14)18(15)20(23)25-7-3/h9-13,15,17H,5-8H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Bioorg Med Chem 16: 9349-58 (2008)
Article DOI: 10.1016/j.bmc.2008.08.048
BindingDB Entry DOI: 10.7270/Q2FJ2GMS |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CAMK2b (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of RSK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Homeodomain-interacting protein kinase 1
(Homo sapiens (Human)) | BDBM50400734
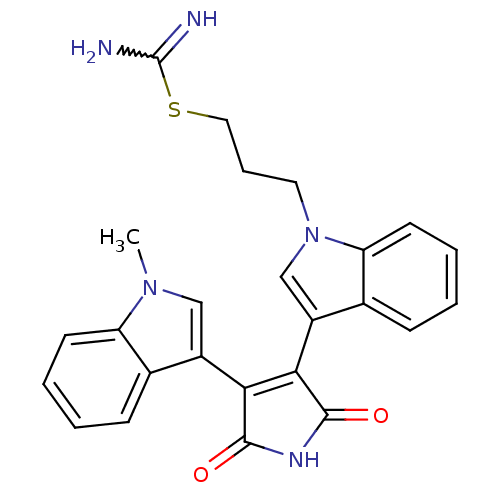
(CHEMBL1591531)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(CCCSC(N)=N)c3ccccc23)c2ccccc12 |w:18.19,t:4| Show InChI InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.367 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of HIPK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Misshapen-like kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of MINK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.473 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LYN (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Phosphorylase b kinase gamma catalytic chain, liver/testis isoform
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of PHKg2 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human AXL using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506637

(CHEMBL4476226)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCN(CC2)C(C)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C27H30F3N7O3S/c1-18(38)35-11-13-36(14-12-35)21-9-7-20(8-10-21)32-26-31-15-23(27(28,29)30)25(33-26)37-16-19-5-4-6-24(22(19)17-37)34(2)41(3,39)40/h4-10,15H,11-14,16-17H2,1-3H3,(H,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin A (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50506648
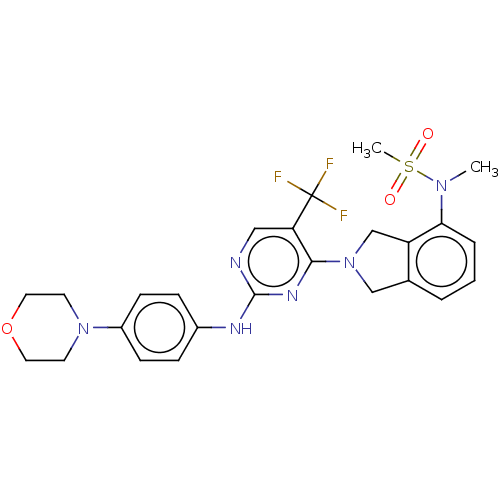
(CHEMBL4445940)Show SMILES CN(c1cccc2CN(Cc12)c1nc(Nc2ccc(cc2)N2CCOCC2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C25H27F3N6O3S/c1-32(38(2,35)36)22-5-3-4-17-15-34(16-20(17)22)23-21(25(26,27)28)14-29-24(31-23)30-18-6-8-19(9-7-18)33-10-12-37-13-11-33/h3-9,14H,10-13,15-16H2,1-2H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... |
Bioorg Med Chem Lett 28: 3761-3765 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.013
BindingDB Entry DOI: 10.7270/Q23T9MHD |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50587677
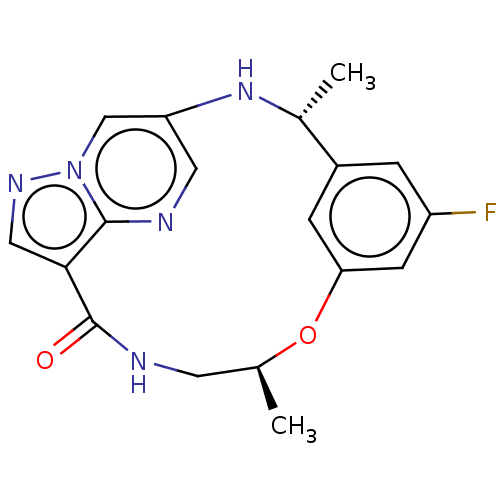
(CHEMBL5173501)Show SMILES C[C@H]1CNC(=O)c2cnn3cc(N[C@H](C)c4cc(F)cc(O1)c4)cnc23 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00533
BindingDB Entry DOI: 10.7270/Q23X8BM3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) after 120 mins in presence of 33P-ATP |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin receptor
(Homo sapiens (Human)) | BDBM50587680

(CHEMBL5182271)Show SMILES COCCN1CCN(CCn2ccc(Nc3ncc4-c5c(CC(C)c4n3)nn(C)c5-c3ccccc3)n2)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00533
BindingDB Entry DOI: 10.7270/Q23X8BM3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50358430
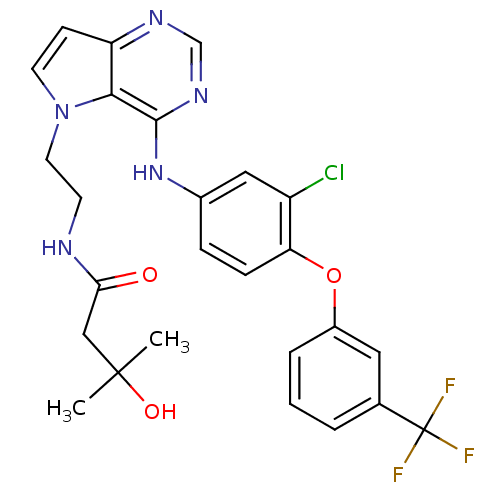
(CHEMBL1614725)Show SMILES CC(C)(O)CC(=O)NCCn1ccc2ncnc(Nc3ccc(Oc4cccc(c4)C(F)(F)F)c(Cl)c3)c12 Show InChI InChI=1S/C26H25ClF3N5O3/c1-25(2,37)14-22(36)31-9-11-35-10-8-20-23(35)24(33-15-32-20)34-17-6-7-21(19(27)13-17)38-18-5-3-4-16(12-18)26(28,29)30/h3-8,10,12-13,15,37H,9,11,14H2,1-2H3,(H,31,36)(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University of Science and Technology (UST)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-diprenorphine binding to kappa opioid receptor expressed in CHO cells |
Bioorg Med Chem Lett 25: 5147-54 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.003
BindingDB Entry DOI: 10.7270/Q27W6F0D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50587680

(CHEMBL5182271)Show SMILES COCCN1CCN(CCn2ccc(Nc3ncc4-c5c(CC(C)c4n3)nn(C)c5-c3ccccc3)n2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00533
BindingDB Entry DOI: 10.7270/Q23X8BM3 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50587677

(CHEMBL5173501)Show SMILES C[C@H]1CNC(=O)c2cnn3cc(N[C@H](C)c4cc(F)cc(O1)c4)cnc23 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00533
BindingDB Entry DOI: 10.7270/Q23X8BM3 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of TRKA (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50587677

(CHEMBL5173501)Show SMILES C[C@H]1CNC(=O)c2cnn3cc(N[C@H](C)c4cc(F)cc(O1)c4)cnc23 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00533
BindingDB Entry DOI: 10.7270/Q23X8BM3 |
More data for this
Ligand-Target Pair | |
NUAK family SNF1-like kinase 1
(Homo sapiens (Human)) | BDBM50587681
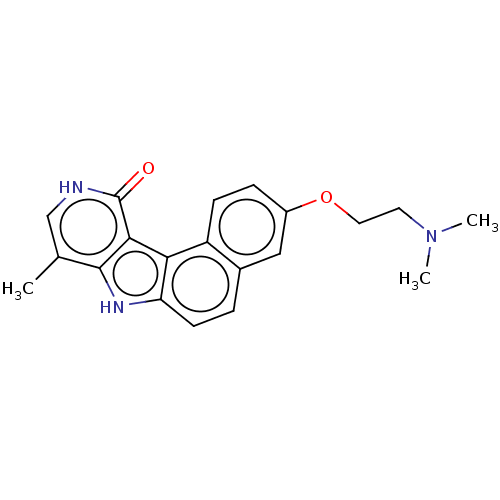
(CHEMBL5176650)Show SMILES CN(C)CCOc1ccc2c3c(ccc2c1)[nH]c1c(C)c[nH]c(=O)c31 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00533
BindingDB Entry DOI: 10.7270/Q23X8BM3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of LCK (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NUAK family SNF1-like kinase 1
(Homo sapiens (Human)) | BDBM50587675
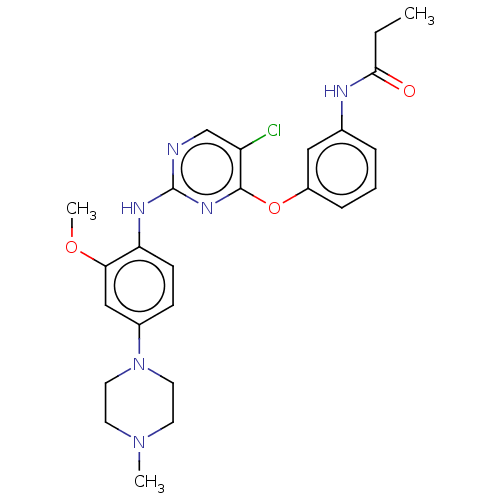
(CHEMBL4303523)Show SMILES CCC(=O)Nc1cccc(Oc2nc(Nc3ccc(cc3OC)N3CCN(C)CC3)ncc2Cl)c1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00533
BindingDB Entry DOI: 10.7270/Q23X8BM3 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sogang University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nisoxetine from human recombinant NET over-expressed in dog MDCK cells |
Bioorg Med Chem Lett 23: 5515-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.08.062
BindingDB Entry DOI: 10.7270/Q2B85C2T |
More data for this
Ligand-Target Pair | |
NUAK family SNF1-like kinase 1
(Homo sapiens (Human)) | BDBM50587675

(CHEMBL4303523)Show SMILES CCC(=O)Nc1cccc(Oc2nc(Nc3ccc(cc3OC)N3CCN(C)CC3)ncc2Cl)c1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00533
BindingDB Entry DOI: 10.7270/Q23X8BM3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data