 Found 204 hits with Last Name = 'rollema' and Initial = 'h'
Found 204 hits with Last Name = 'rollema' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174152
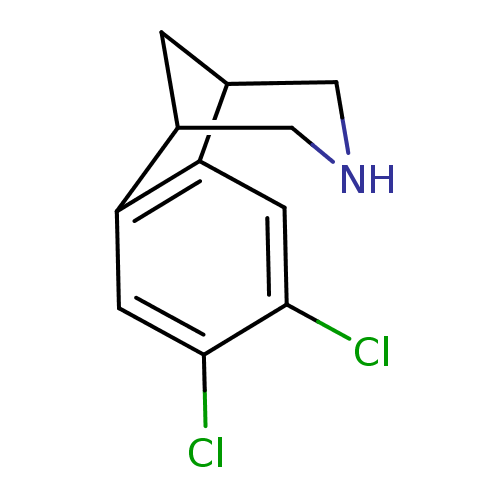
(4,5-Dichloro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2...)Show InChI InChI=1S/C11H11Cl2N/c12-10-2-8-6-1-7(5-14-4-6)9(8)3-11(10)13/h2-3,6-7,14H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174139
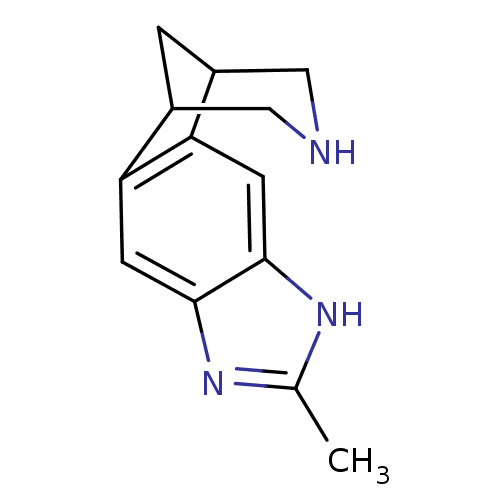
(6-methyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]...)Show InChI InChI=1S/C13H15N3/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174145
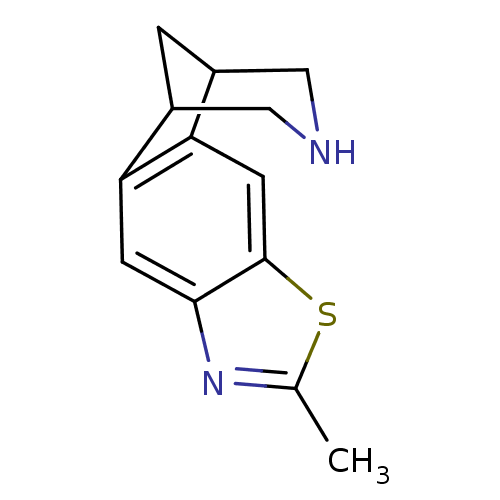
(6-methyl-7-thia-5,13-diazatetracyclo[9.3.1.02,10.0...)Show InChI InChI=1S/C13H14N2S/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174148
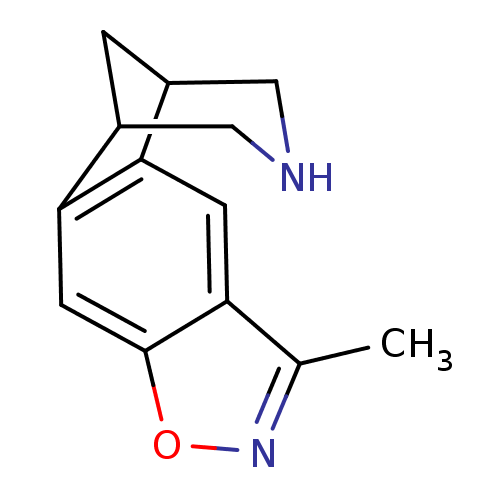
(5-methyl-7-oxa-6,13-diazatetracyclo[9.3.1.02,10.04...)Show InChI InChI=1S/C13H14N2O/c1-7-10-3-11-8-2-9(6-14-5-8)12(11)4-13(10)16-15-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174154
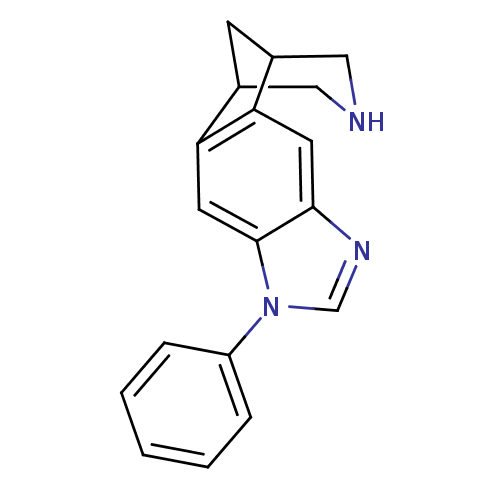
(7-phenyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]...)Show InChI InChI=1S/C18H17N3/c1-2-4-14(5-3-1)21-11-20-17-7-15-12-6-13(10-19-9-12)16(15)8-18(17)21/h1-5,7-8,11-13,19H,6,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174156
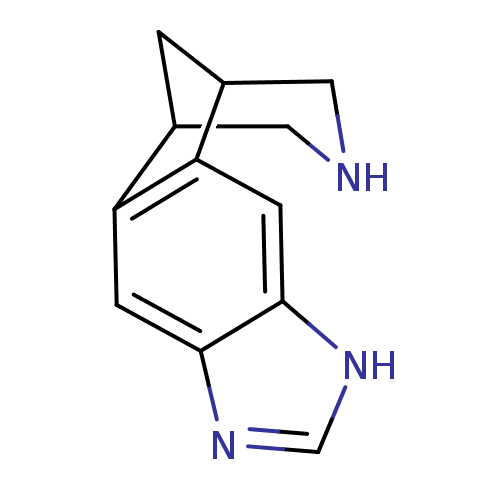
(5,7,13-triazatetracyclo[9.3.1.02,10.04,8]pentadeca...)Show InChI InChI=1S/C12H13N3/c1-7-4-13-5-8(1)10-3-12-11(2-9(7)10)14-6-15-12/h2-3,6-8,13H,1,4-5H2,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174190
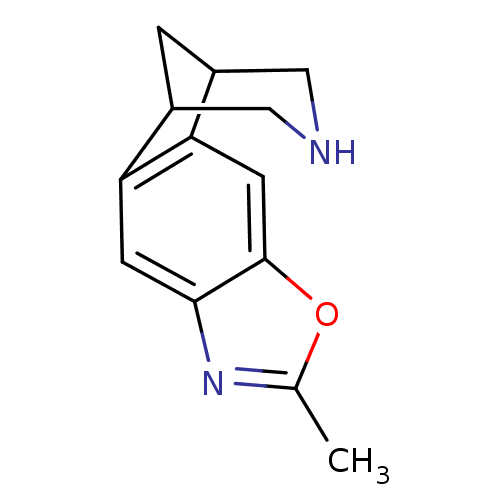
(6-methyl-7-oxa-5,13-diazatetracyclo[9.3.1.02,10.04...)Show InChI InChI=1S/C13H14N2O/c1-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16-7/h3-4,8-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174170
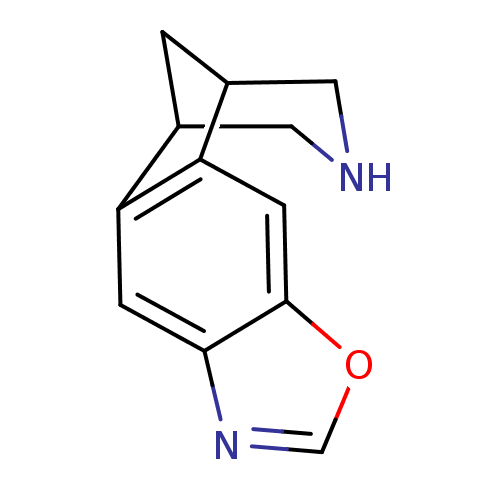
(7-oxa-5,13-diazatetracyclo[9.3.1.02,10.04,8]pentad...)Show InChI InChI=1S/C12H12N2O/c1-7-4-13-5-8(1)10-3-12-11(2-9(7)10)14-6-15-12/h2-3,6-8,13H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174141
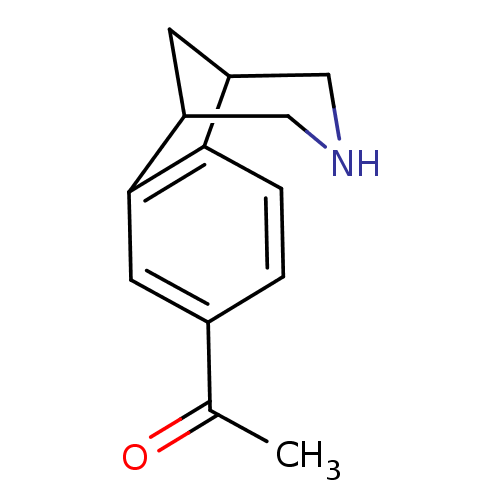
(1-(10-Aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),3,5-tr...)Show InChI InChI=1S/C13H15NO/c1-8(15)9-2-3-12-10-4-11(7-14-6-10)13(12)5-9/h2-3,5,10-11,14H,4,6-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174163
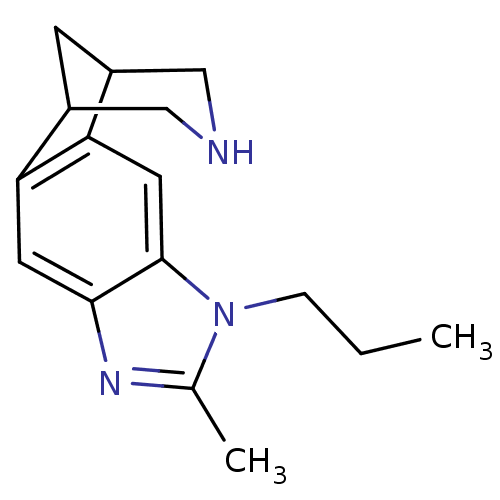
(6-methyl-7-propyl-5,7,13-triazatetracyclo[9.3.1.02...)Show InChI InChI=1S/C16H21N3/c1-3-4-19-10(2)18-15-6-13-11-5-12(9-17-8-11)14(13)7-16(15)19/h6-7,11-12,17H,3-5,8-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174147
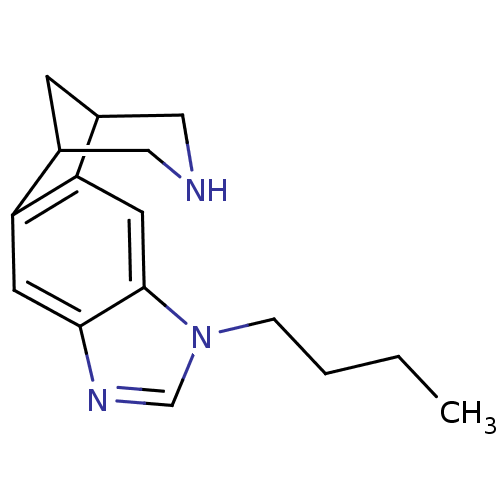
(7-butyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]p...)Show InChI InChI=1S/C16H21N3/c1-2-3-4-19-10-18-15-6-13-11-5-12(9-17-8-11)14(13)7-16(15)19/h6-7,10-12,17H,2-5,8-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174144
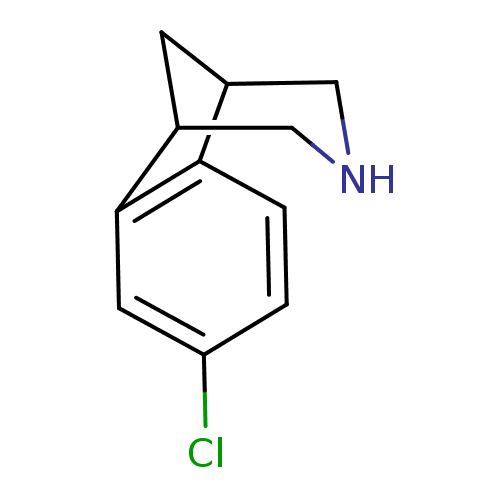
(4-Chloro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),...)Show InChI InChI=1S/C11H12ClN/c12-9-1-2-10-7-3-8(6-13-5-7)11(10)4-9/h1-2,4,7-8,13H,3,5-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50143282
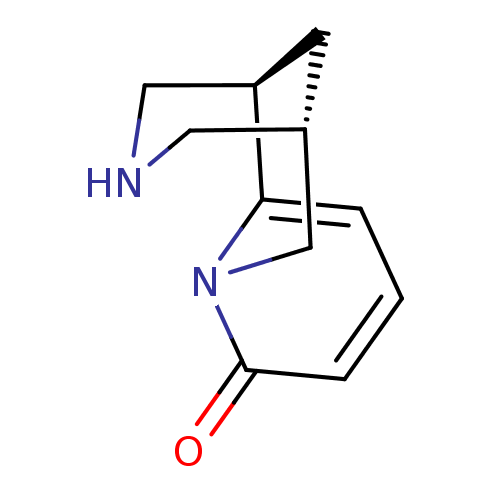
((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2/t8?,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 in human HEK293 cells using [3H]- nicotine as radioligand |
Bioorg Med Chem Lett 15: 2974-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.036
BindingDB Entry DOI: 10.7270/Q25Q4WWB |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50143282

((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2/t8?,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174142
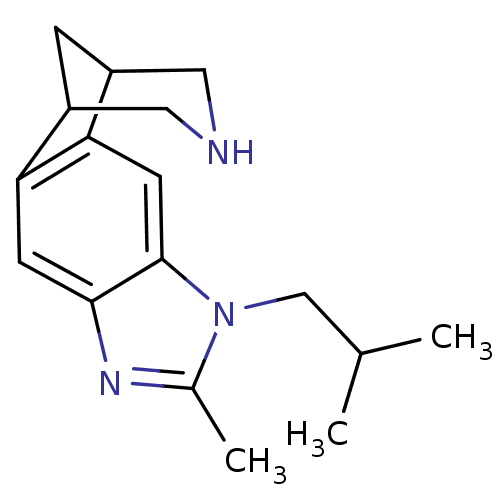
(7-isobutyl-6-methyl-5,7,13-triazatetracyclo[9.3.1....)Show InChI InChI=1S/C17H23N3/c1-10(2)9-20-11(3)19-16-5-14-12-4-13(8-18-7-12)15(14)6-17(16)20/h5-6,10,12-13,18H,4,7-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174158
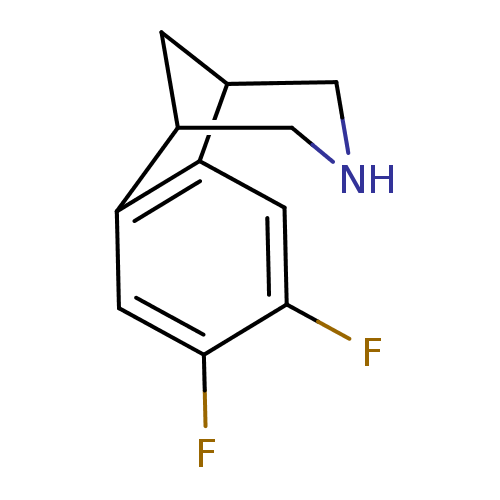
(4,5-Difluoro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2...)Show InChI InChI=1S/C11H11F2N/c12-10-2-8-6-1-7(5-14-4-6)9(8)3-11(10)13/h2-3,6-7,14H,1,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50166908

(5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...)Show InChI InChI=1S/C13H13N3/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1/h1-2,4-5,8-9,14H,3,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from alpha4beta2 nicotinic receptor expressed in human HEK293 cells |
Bioorg Med Chem Lett 20: 4749-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.142
BindingDB Entry DOI: 10.7270/Q2QC04GV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174176
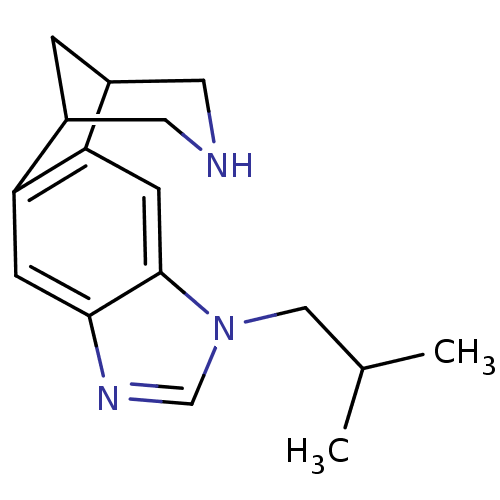
(7-isobutyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,...)Show InChI InChI=1S/C16H21N3/c1-10(2)8-19-9-18-15-4-13-11-3-12(7-17-6-11)14(13)5-16(15)19/h4-5,9-12,17H,3,6-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174180
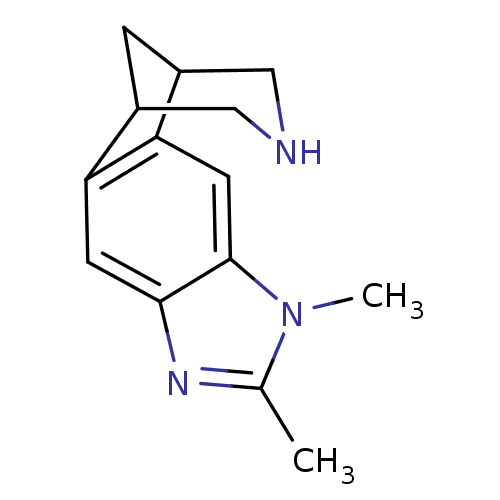
(6,7-dimethyl-5,7,13-triazatetracyclo[9.3.1.02,10.0...)Show InChI InChI=1S/C14H17N3/c1-8-16-13-4-11-9-3-10(7-15-6-9)12(11)5-14(13)17(8)2/h4-5,9-10,15H,3,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174183
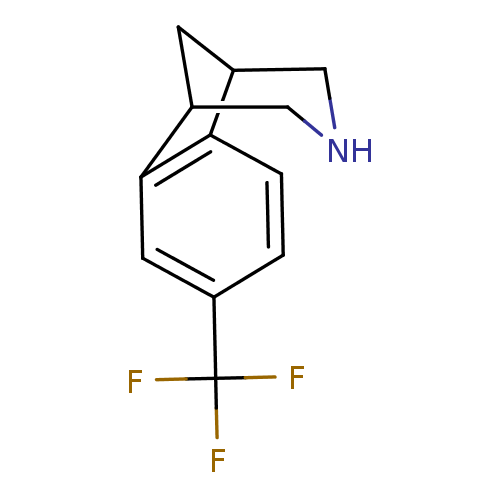
(4-Trifluoromethyl-10-aza-tricyclo[6.3.1.0*2,7*]dod...)Show InChI InChI=1S/C12H12F3N/c13-12(14,15)9-1-2-10-7-3-8(6-16-5-7)11(10)4-9/h1-2,4,7-8,16H,3,5-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174177
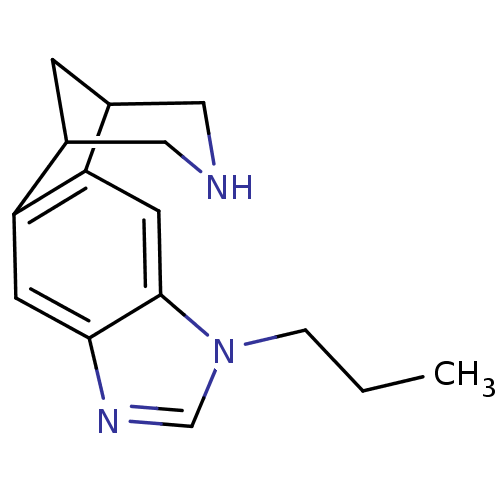
(7-propyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]...)Show InChI InChI=1S/C15H19N3/c1-2-3-18-9-17-14-5-12-10-4-11(8-16-7-10)13(12)6-15(14)18/h5-6,9-11,16H,2-4,7-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50167457
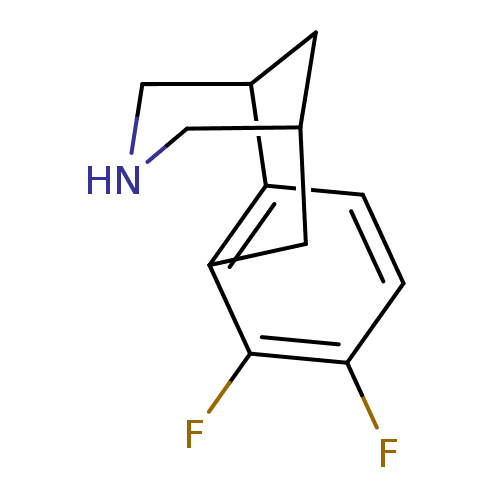
(5,6-Difluoro-11-aza-tricyclo[7.3.1.0*2,7*]trideca-...)Show InChI InChI=1S/C12H13F2N/c13-11-2-1-9-8-3-7(5-15-6-8)4-10(9)12(11)14/h1-2,7-8,15H,3-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 in human HEK293 cells using [3H]- nicotine as radioligand |
Bioorg Med Chem Lett 15: 2974-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.036
BindingDB Entry DOI: 10.7270/Q25Q4WWB |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174143
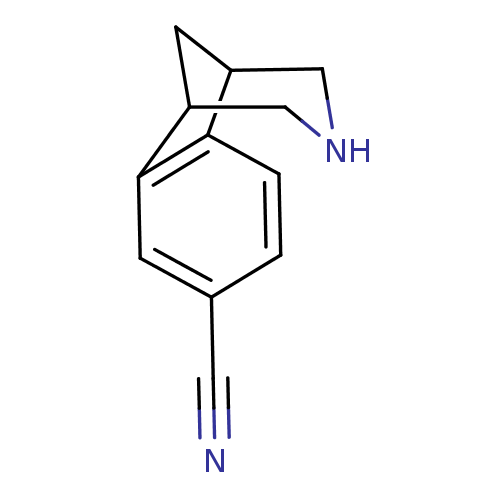
(10-Aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),3,5-trien...)Show InChI InChI=1S/C12H12N2/c13-5-8-1-2-11-9-4-10(7-14-6-9)12(11)3-8/h1-3,9-10,14H,4,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174174
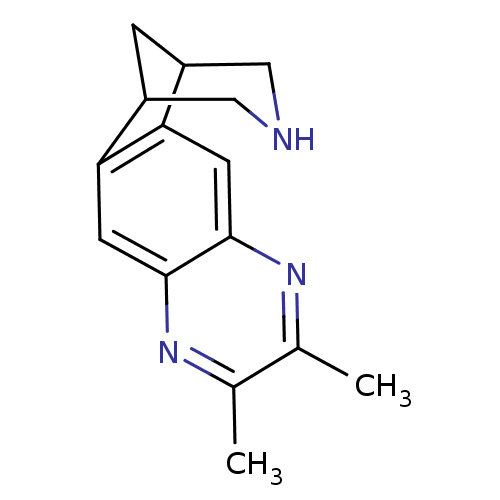
(6,7-dimethyl-5,8,14-triazatetracyclo[10.3.1.02,11....)Show InChI InChI=1S/C15H17N3/c1-8-9(2)18-15-5-13-11-3-10(6-16-7-11)12(13)4-14(15)17-8/h4-5,10-11,16H,3,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174164
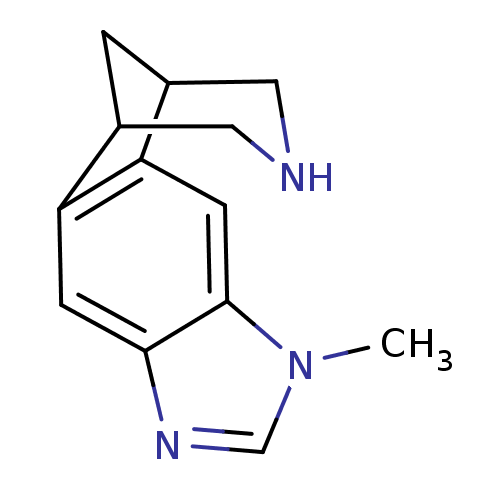
(7-methyl-5,7,13-triazatetracyclo[9.3.1.02,10.04,8]...)Show InChI InChI=1S/C13H15N3/c1-16-7-15-12-3-10-8-2-9(6-14-5-8)11(10)4-13(12)16/h3-4,7-9,14H,2,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174155
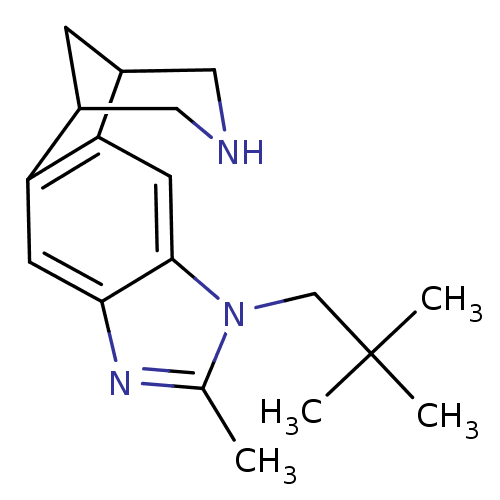
(6-methyl-7-neopentyl-5,7,13-triazatetracyclo[9.3.1...)Show InChI InChI=1S/C18H25N3/c1-11-20-16-6-14-12-5-13(9-19-8-12)15(14)7-17(16)21(11)10-18(2,3)4/h6-7,12-13,19H,5,8-10H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401001

(CHEMBL2206288)Show SMILES C([C@H]1C[C@H](C1)c1ccc(CN2CCCC2)cc1)N1CCOCC1 |r,wU:3.5,1.0,(12.44,-17.63,;13.93,-17.23,;14.7,-15.89,;16.03,-16.66,;15.26,-18,;17.52,-16.26,;18.61,-17.35,;20.1,-16.95,;20.5,-15.47,;21.98,-15.07,;23.07,-16.16,;22.83,-17.68,;24.2,-18.38,;25.29,-17.29,;24.59,-15.92,;19.41,-14.38,;17.92,-14.78,;11.35,-16.54,;11.75,-15.05,;10.66,-13.96,;9.18,-14.36,;8.78,-15.85,;9.87,-16.93,)| Show InChI InChI=1S/C20H30N2O/c1-2-8-21(7-1)15-17-3-5-19(6-4-17)20-13-18(14-20)16-22-9-11-23-12-10-22/h3-6,18,20H,1-2,7-16H2/t18-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401001

(CHEMBL2206288)Show SMILES C([C@H]1C[C@H](C1)c1ccc(CN2CCCC2)cc1)N1CCOCC1 |r,wU:3.5,1.0,(12.44,-17.63,;13.93,-17.23,;14.7,-15.89,;16.03,-16.66,;15.26,-18,;17.52,-16.26,;18.61,-17.35,;20.1,-16.95,;20.5,-15.47,;21.98,-15.07,;23.07,-16.16,;22.83,-17.68,;24.2,-18.38,;25.29,-17.29,;24.59,-15.92,;19.41,-14.38,;17.92,-14.78,;11.35,-16.54,;11.75,-15.05,;10.66,-13.96,;9.18,-14.36,;8.78,-15.85,;9.87,-16.93,)| Show InChI InChI=1S/C20H30N2O/c1-2-8-21(7-1)15-17-3-5-19(6-4-17)20-13-18(14-20)16-22-9-11-23-12-10-22/h3-6,18,20H,1-2,7-16H2/t18-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174173
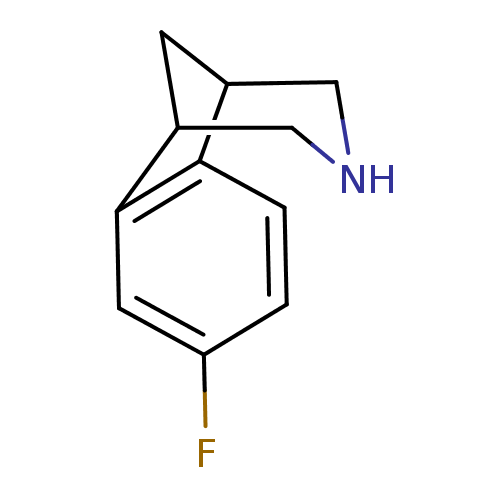
(4-Fluoro-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),...)Show InChI InChI=1S/C11H12FN/c12-9-1-2-10-7-3-8(6-13-5-7)11(10)4-9/h1-2,4,7-8,13H,3,5-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401002
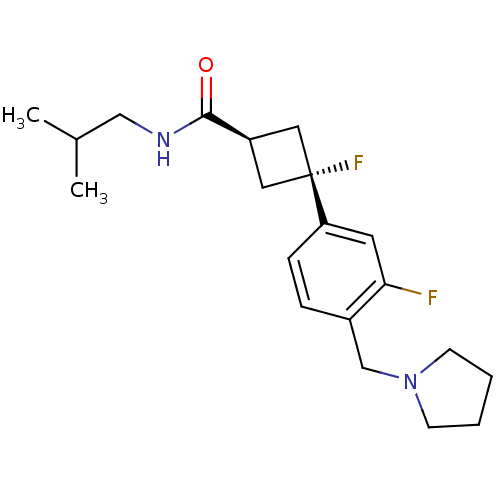
(CHEMBL2206292)Show SMILES CC(C)CNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:7.6,wD:9.9,(6.68,-19.62,;6.28,-18.13,;4.79,-17.73,;7.37,-17.04,;8.85,-17.44,;9.94,-16.35,;9.54,-14.87,;11.43,-16.75,;12.76,-15.98,;13.53,-17.32,;14.3,-18.65,;12.2,-18.09,;14.87,-16.55,;14.87,-15.01,;16.2,-14.24,;17.54,-15.01,;18.87,-14.24,;20.2,-15.01,;20.36,-16.54,;21.87,-16.86,;22.64,-15.52,;21.61,-14.38,;17.54,-16.55,;18.87,-17.32,;16.2,-17.32,)| Show InChI InChI=1S/C20H28F2N2O/c1-14(2)12-23-19(25)16-10-20(22,11-16)17-6-5-15(18(21)9-17)13-24-7-3-4-8-24/h5-6,9,14,16H,3-4,7-8,10-13H2,1-2H3,(H,23,25)/t16-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401002

(CHEMBL2206292)Show SMILES CC(C)CNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:7.6,wD:9.9,(6.68,-19.62,;6.28,-18.13,;4.79,-17.73,;7.37,-17.04,;8.85,-17.44,;9.94,-16.35,;9.54,-14.87,;11.43,-16.75,;12.76,-15.98,;13.53,-17.32,;14.3,-18.65,;12.2,-18.09,;14.87,-16.55,;14.87,-15.01,;16.2,-14.24,;17.54,-15.01,;18.87,-14.24,;20.2,-15.01,;20.36,-16.54,;21.87,-16.86,;22.64,-15.52,;21.61,-14.38,;17.54,-16.55,;18.87,-17.32,;16.2,-17.32,)| Show InChI InChI=1S/C20H28F2N2O/c1-14(2)12-23-19(25)16-10-20(22,11-16)17-6-5-15(18(21)9-17)13-24-7-3-4-8-24/h5-6,9,14,16H,3-4,7-8,10-13H2,1-2H3,(H,23,25)/t16-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401007
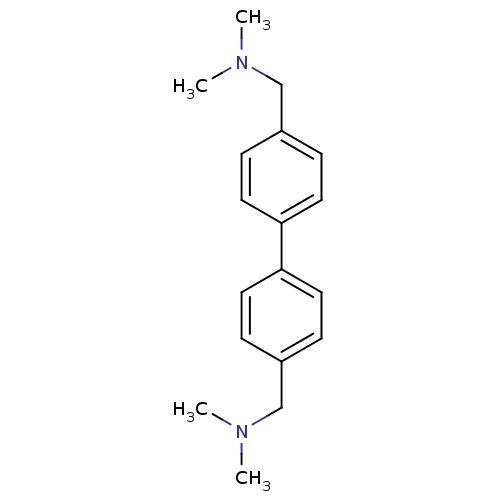
(CHEMBL209478)Show InChI InChI=1S/C18H24N2/c1-19(2)13-15-5-9-17(10-6-15)18-11-7-16(8-12-18)14-20(3)4/h5-12H,13-14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401007

(CHEMBL209478)Show InChI InChI=1S/C18H24N2/c1-19(2)13-15-5-9-17(10-6-15)18-11-7-16(8-12-18)14-20(3)4/h5-12H,13-14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50167469
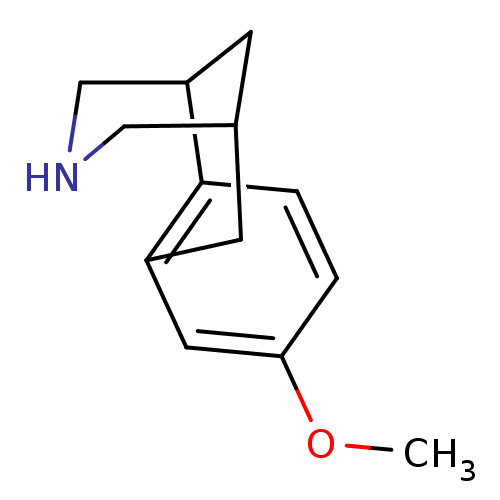
(5-Methoxy-11-aza-tricyclo[7.3.1.0*2,7*]trideca-2,4...)Show InChI InChI=1S/C13H17NO/c1-15-12-2-3-13-10(6-12)4-9-5-11(13)8-14-7-9/h2-3,6,9,11,14H,4-5,7-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 in human HEK293 cells using [3H]- nicotine as radioligand |
Bioorg Med Chem Lett 15: 2974-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.036
BindingDB Entry DOI: 10.7270/Q25Q4WWB |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401002

(CHEMBL2206292)Show SMILES CC(C)CNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:7.6,wD:9.9,(6.68,-19.62,;6.28,-18.13,;4.79,-17.73,;7.37,-17.04,;8.85,-17.44,;9.94,-16.35,;9.54,-14.87,;11.43,-16.75,;12.76,-15.98,;13.53,-17.32,;14.3,-18.65,;12.2,-18.09,;14.87,-16.55,;14.87,-15.01,;16.2,-14.24,;17.54,-15.01,;18.87,-14.24,;20.2,-15.01,;20.36,-16.54,;21.87,-16.86,;22.64,-15.52,;21.61,-14.38,;17.54,-16.55,;18.87,-17.32,;16.2,-17.32,)| Show InChI InChI=1S/C20H28F2N2O/c1-14(2)12-23-19(25)16-10-20(22,11-16)17-6-5-15(18(21)9-17)13-24-7-3-4-8-24/h5-6,9,14,16H,3-4,7-8,10-13H2,1-2H3,(H,23,25)/t16-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401002

(CHEMBL2206292)Show SMILES CC(C)CNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:7.6,wD:9.9,(6.68,-19.62,;6.28,-18.13,;4.79,-17.73,;7.37,-17.04,;8.85,-17.44,;9.94,-16.35,;9.54,-14.87,;11.43,-16.75,;12.76,-15.98,;13.53,-17.32,;14.3,-18.65,;12.2,-18.09,;14.87,-16.55,;14.87,-15.01,;16.2,-14.24,;17.54,-15.01,;18.87,-14.24,;20.2,-15.01,;20.36,-16.54,;21.87,-16.86,;22.64,-15.52,;21.61,-14.38,;17.54,-16.55,;18.87,-17.32,;16.2,-17.32,)| Show InChI InChI=1S/C20H28F2N2O/c1-14(2)12-23-19(25)16-10-20(22,11-16)17-6-5-15(18(21)9-17)13-24-7-3-4-8-24/h5-6,9,14,16H,3-4,7-8,10-13H2,1-2H3,(H,23,25)/t16-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401004

(CHEMBL2206291)Show SMILES CCN(C)C(=O)[C@H]1C[C@H](C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:6.5,8.10,(5.81,-12.97,;6.58,-14.3,;8.12,-14.3,;8.89,-12.97,;8.89,-15.64,;8.12,-16.97,;10.43,-15.64,;11.51,-14.55,;12.6,-15.64,;11.51,-16.72,;14.14,-15.64,;14.91,-14.3,;16.45,-14.3,;17.22,-15.64,;18.76,-15.64,;19.53,-14.3,;21.06,-14.14,;21.39,-12.63,;20.05,-11.86,;18.91,-12.89,;16.45,-16.97,;17.22,-18.3,;14.91,-16.97,)| Show InChI InChI=1S/C19H27FN2O/c1-3-21(2)19(23)17-10-16(11-17)14-6-7-15(18(20)12-14)13-22-8-4-5-9-22/h6-7,12,16-17H,3-5,8-11,13H2,1-2H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401004

(CHEMBL2206291)Show SMILES CCN(C)C(=O)[C@H]1C[C@H](C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:6.5,8.10,(5.81,-12.97,;6.58,-14.3,;8.12,-14.3,;8.89,-12.97,;8.89,-15.64,;8.12,-16.97,;10.43,-15.64,;11.51,-14.55,;12.6,-15.64,;11.51,-16.72,;14.14,-15.64,;14.91,-14.3,;16.45,-14.3,;17.22,-15.64,;18.76,-15.64,;19.53,-14.3,;21.06,-14.14,;21.39,-12.63,;20.05,-11.86,;18.91,-12.89,;16.45,-16.97,;17.22,-18.3,;14.91,-16.97,)| Show InChI InChI=1S/C19H27FN2O/c1-3-21(2)19(23)17-10-16(11-17)14-6-7-15(18(20)12-14)13-22-8-4-5-9-22/h6-7,12,16-17H,3-5,8-11,13H2,1-2H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 in human HEK293 cells using [3H]- nicotine as radioligand |
Bioorg Med Chem Lett 15: 2974-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.036
BindingDB Entry DOI: 10.7270/Q25Q4WWB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174193
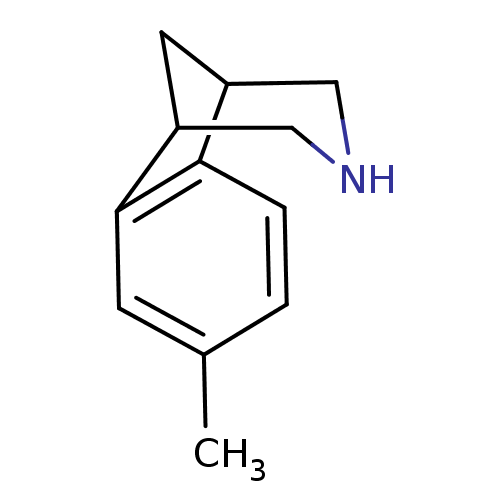
(4-Methyl-10-aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),...)Show InChI InChI=1S/C12H15N/c1-8-2-3-11-9-5-10(7-13-6-9)12(11)4-8/h2-4,9-10,13H,5-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401003
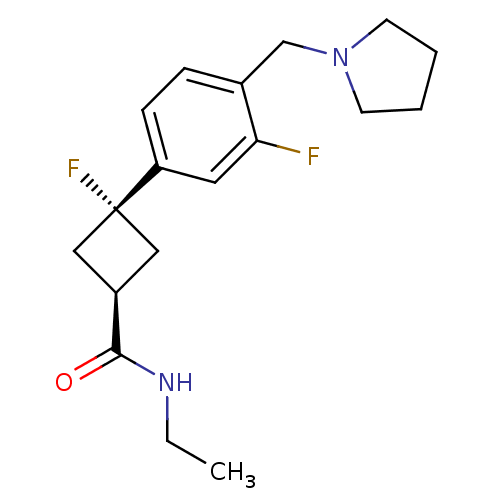
(CHEMBL2151197)Show SMILES CCNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:5.4,wD:7.7,(15.4,-34.66,;16.73,-35.43,;18.07,-34.66,;19.4,-35.43,;19.4,-36.97,;20.73,-34.66,;21.13,-33.17,;22.62,-33.57,;23.84,-34.51,;22.22,-35.06,;23.95,-32.81,;25.29,-33.58,;26.62,-32.81,;26.62,-31.26,;27.95,-30.48,;29.28,-31.25,;29.46,-32.78,;30.97,-33.09,;31.73,-31.76,;30.7,-30.62,;25.28,-30.5,;25.28,-28.96,;23.95,-31.27,)| Show InChI InChI=1S/C18H24F2N2O/c1-2-21-17(23)14-10-18(20,11-14)15-6-5-13(16(19)9-15)12-22-7-3-4-8-22/h5-6,9,14H,2-4,7-8,10-12H2,1H3,(H,21,23)/t14-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401003

(CHEMBL2151197)Show SMILES CCNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:5.4,wD:7.7,(15.4,-34.66,;16.73,-35.43,;18.07,-34.66,;19.4,-35.43,;19.4,-36.97,;20.73,-34.66,;21.13,-33.17,;22.62,-33.57,;23.84,-34.51,;22.22,-35.06,;23.95,-32.81,;25.29,-33.58,;26.62,-32.81,;26.62,-31.26,;27.95,-30.48,;29.28,-31.25,;29.46,-32.78,;30.97,-33.09,;31.73,-31.76,;30.7,-30.62,;25.28,-30.5,;25.28,-28.96,;23.95,-31.27,)| Show InChI InChI=1S/C18H24F2N2O/c1-2-21-17(23)14-10-18(20,11-14)15-6-5-13(16(19)9-15)12-22-7-3-4-8-22/h5-6,9,14H,2-4,7-8,10-12H2,1H3,(H,21,23)/t14-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histone H3 receptor expressed in human HEK293 cells assessed as inhibition of forskolin-induced cAMP release pretreated ... |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174178
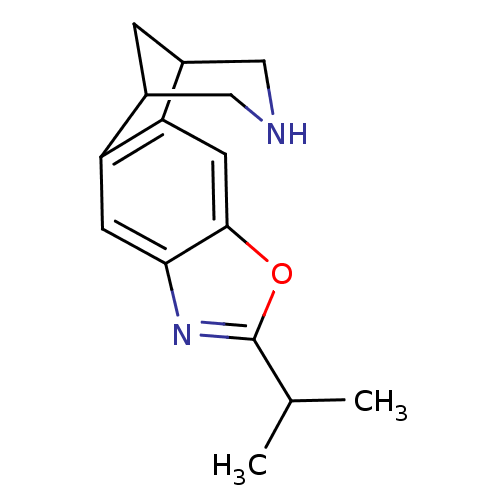
(6-isopropyl-7-oxa-5,13-diazatetracyclo[9.3.1.02,10...)Show InChI InChI=1S/C15H18N2O/c1-8(2)15-17-13-4-11-9-3-10(7-16-6-9)12(11)5-14(13)18-15/h4-5,8-10,16H,3,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50167462
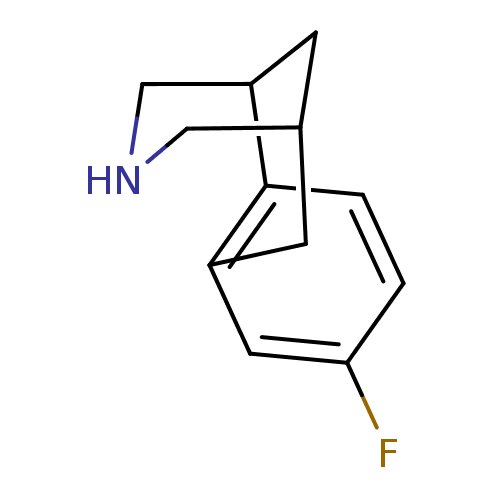
(5-Fluoro-11-aza-tricyclo[7.3.1.0*2,7*]trideca-2,4,...)Show InChI InChI=1S/C12H14FN/c13-11-1-2-12-9(5-11)3-8-4-10(12)7-14-6-8/h1-2,5,8,10,14H,3-4,6-7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 in human HEK293 cells using [3H]- nicotine as radioligand |
Bioorg Med Chem Lett 15: 2974-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.036
BindingDB Entry DOI: 10.7270/Q25Q4WWB |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50174188
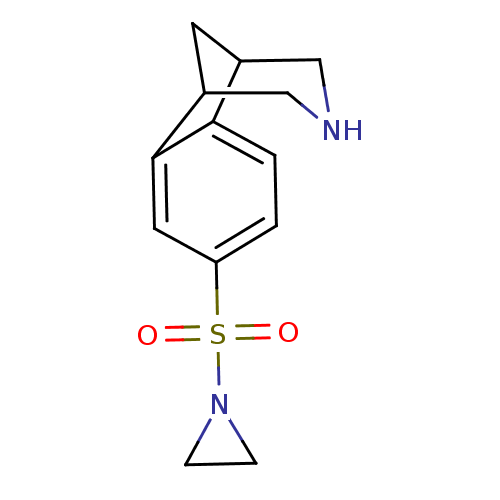
(4-(Aziridine-1-sulfonyl)-10-aza-tricyclo[6.3.1.0*2...)Show InChI InChI=1S/C13H16N2O2S/c16-18(17,15-3-4-15)11-1-2-12-9-5-10(8-14-7-9)13(12)6-11/h1-2,6,9-10,14H,3-5,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for human Nicotinic acetylcholine receptor alpha4-beta2 expressed in HEK 293 cells using [3H]nicotine |
Bioorg Med Chem Lett 15: 4889-97 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.035
BindingDB Entry DOI: 10.7270/Q2MS3TJC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401001

(CHEMBL2206288)Show SMILES C([C@H]1C[C@H](C1)c1ccc(CN2CCCC2)cc1)N1CCOCC1 |r,wU:3.5,1.0,(12.44,-17.63,;13.93,-17.23,;14.7,-15.89,;16.03,-16.66,;15.26,-18,;17.52,-16.26,;18.61,-17.35,;20.1,-16.95,;20.5,-15.47,;21.98,-15.07,;23.07,-16.16,;22.83,-17.68,;24.2,-18.38,;25.29,-17.29,;24.59,-15.92,;19.41,-14.38,;17.92,-14.78,;11.35,-16.54,;11.75,-15.05,;10.66,-13.96,;9.18,-14.36,;8.78,-15.85,;9.87,-16.93,)| Show InChI InChI=1S/C20H30N2O/c1-2-8-21(7-1)15-17-3-5-19(6-4-17)20-13-18(14-20)16-22-9-11-23-12-10-22/h3-6,18,20H,1-2,7-16H2/t18-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401001

(CHEMBL2206288)Show SMILES C([C@H]1C[C@H](C1)c1ccc(CN2CCCC2)cc1)N1CCOCC1 |r,wU:3.5,1.0,(12.44,-17.63,;13.93,-17.23,;14.7,-15.89,;16.03,-16.66,;15.26,-18,;17.52,-16.26,;18.61,-17.35,;20.1,-16.95,;20.5,-15.47,;21.98,-15.07,;23.07,-16.16,;22.83,-17.68,;24.2,-18.38,;25.29,-17.29,;24.59,-15.92,;19.41,-14.38,;17.92,-14.78,;11.35,-16.54,;11.75,-15.05,;10.66,-13.96,;9.18,-14.36,;8.78,-15.85,;9.87,-16.93,)| Show InChI InChI=1S/C20H30N2O/c1-2-8-21(7-1)15-17-3-5-19(6-4-17)20-13-18(14-20)16-22-9-11-23-12-10-22/h3-6,18,20H,1-2,7-16H2/t18-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50401003

(CHEMBL2151197)Show SMILES CCNC(=O)[C@H]1C[C@](F)(C1)c1ccc(CN2CCCC2)c(F)c1 |r,wU:5.4,wD:7.7,(15.4,-34.66,;16.73,-35.43,;18.07,-34.66,;19.4,-35.43,;19.4,-36.97,;20.73,-34.66,;21.13,-33.17,;22.62,-33.57,;23.84,-34.51,;22.22,-35.06,;23.95,-32.81,;25.29,-33.58,;26.62,-32.81,;26.62,-31.26,;27.95,-30.48,;29.28,-31.25,;29.46,-32.78,;30.97,-33.09,;31.73,-31.76,;30.7,-30.62,;25.28,-30.5,;25.28,-28.96,;23.95,-31.27,)| Show InChI InChI=1S/C18H24F2N2O/c1-2-21-17(23)14-10-18(20,11-14)15-6-5-13(16(19)9-15)12-22-7-3-4-8-22/h5-6,9,14H,2-4,7-8,10-12H2,1H3,(H,21,23)/t14-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-n-alpha-methylhistamine from human histone H3 receptor homogenate after 60 mins by scintillation counting |
J Med Chem 54: 7602-20 (2011)
Article DOI: 10.1021/jm200939b
BindingDB Entry DOI: 10.7270/Q27D2W9G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data