 Found 30 hits with Last Name = 'rose' and Initial = 'ja'
Found 30 hits with Last Name = 'rose' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA gyrase subunit B
(Staphylococcus aureus) | BDBM50388101
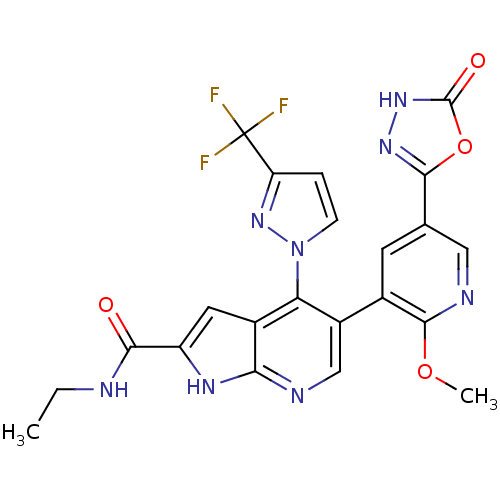
(CHEMBL2059380)Show SMILES CCNC(=O)c1cc2c(c(cnc2[nH]1)-c1cc(cnc1OC)-c1n[nH]c(=O)o1)-n1ccc(n1)C(F)(F)F Show InChI InChI=1S/C22H17F3N8O4/c1-3-26-18(34)14-7-12-16(33-5-4-15(32-33)22(23,24)25)13(9-27-17(12)29-14)11-6-10(8-28-20(11)36-2)19-30-31-21(35)37-19/h4-9H,3H2,1-2H3,(H,26,34)(H,27,29)(H,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase B expressed in Escherichia coli BL21(DE3) cells assessed ATP hydrolysis activity after 18 to 24 hrs by... |
Bioorg Med Chem Lett 22: 5150-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.128
BindingDB Entry DOI: 10.7270/Q2D79CG0 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Staphylococcus aureus) | BDBM50388100
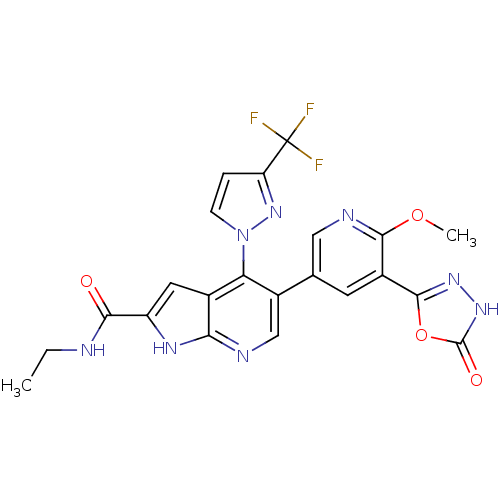
(CHEMBL2059379)Show SMILES CCNC(=O)c1cc2c(c(cnc2[nH]1)-c1cnc(OC)c(c1)-c1n[nH]c(=O)o1)-n1ccc(n1)C(F)(F)F Show InChI InChI=1S/C22H17F3N8O4/c1-3-26-18(34)14-7-11-16(33-5-4-15(32-33)22(23,24)25)13(9-27-17(11)29-14)10-6-12(19(36-2)28-8-10)20-30-31-21(35)37-20/h4-9H,3H2,1-2H3,(H,26,34)(H,27,29)(H,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase B expressed in Escherichia coli BL21(DE3) cells assessed ATP hydrolysis activity after 18 to 24 hrs by... |
Bioorg Med Chem Lett 22: 5150-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.128
BindingDB Entry DOI: 10.7270/Q2D79CG0 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Staphylococcus aureus) | BDBM50388092

(CHEMBL2059196)Show SMILES CCNC(=O)c1cc2c(c(cnc2[nH]1)-c1cncc(c1)C(O)=O)-n1ccc(n1)C(F)(F)F Show InChI InChI=1S/C20H15F3N6O3/c1-2-25-18(30)14-6-12-16(29-4-3-15(28-29)20(21,22)23)13(9-26-17(12)27-14)10-5-11(19(31)32)8-24-7-10/h3-9H,2H2,1H3,(H,25,30)(H,26,27)(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase B expressed in Escherichia coli BL21(DE3) cells assessed ATP hydrolysis activity after 18 to 24 hrs by... |
Bioorg Med Chem Lett 22: 5150-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.128
BindingDB Entry DOI: 10.7270/Q2D79CG0 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Staphylococcus aureus) | BDBM50388097

(CHEMBL2059375)Show SMILES CCNC(=O)c1cc2c(c(cnc2[nH]1)-c1cncc(c1)C(N)=O)-n1ccc(n1)C(F)(F)F Show InChI InChI=1S/C20H16F3N7O2/c1-2-26-19(32)14-6-12-16(30-4-3-15(29-30)20(21,22)23)13(9-27-18(12)28-14)10-5-11(17(24)31)8-25-7-10/h3-9H,2H2,1H3,(H2,24,31)(H,26,32)(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase B expressed in Escherichia coli BL21(DE3) cells assessed ATP hydrolysis activity after 18 to 24 hrs by... |
Bioorg Med Chem Lett 22: 5150-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.128
BindingDB Entry DOI: 10.7270/Q2D79CG0 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Staphylococcus aureus) | BDBM50388098
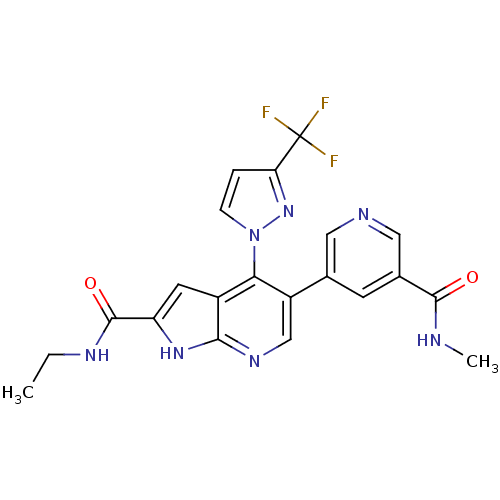
(CHEMBL2059376)Show SMILES CCNC(=O)c1cc2c(c(cnc2[nH]1)-c1cncc(c1)C(=O)NC)-n1ccc(n1)C(F)(F)F Show InChI InChI=1S/C21H18F3N7O2/c1-3-27-20(33)15-7-13-17(31-5-4-16(30-31)21(22,23)24)14(10-28-18(13)29-15)11-6-12(9-26-8-11)19(32)25-2/h4-10H,3H2,1-2H3,(H,25,32)(H,27,33)(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase B expressed in Escherichia coli BL21(DE3) cells assessed ATP hydrolysis activity after 18 to 24 hrs by... |
Bioorg Med Chem Lett 22: 5150-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.128
BindingDB Entry DOI: 10.7270/Q2D79CG0 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Staphylococcus aureus) | BDBM50388099

(CHEMBL2059377)Show SMILES CCNC(=O)c1cc2c(c(cnc2[nH]1)-c1cncc(c1)C#N)-n1ccc(n1)C(F)(F)F Show InChI InChI=1S/C20H14F3N7O/c1-2-26-19(31)15-6-13-17(30-4-3-16(29-30)20(21,22)23)14(10-27-18(13)28-15)12-5-11(7-24)8-25-9-12/h3-6,8-10H,2H2,1H3,(H,26,31)(H,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase B expressed in Escherichia coli BL21(DE3) cells assessed ATP hydrolysis activity after 18 to 24 hrs by... |
Bioorg Med Chem Lett 22: 5150-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.128
BindingDB Entry DOI: 10.7270/Q2D79CG0 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Staphylococcus aureus) | BDBM50388095

(CHEMBL2059373)Show SMILES OC(=O)c1cncc(c1)-c1cnc2[nH]c(cc2c1-n1ccc(n1)C(F)(F)F)C(=O)NC1CC1 Show InChI InChI=1S/C21H15F3N6O3/c22-21(23,24)16-3-4-30(29-16)17-13-6-15(19(31)27-12-1-2-12)28-18(13)26-9-14(17)10-5-11(20(32)33)8-25-7-10/h3-9,12H,1-2H2,(H,26,28)(H,27,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase B expressed in Escherichia coli BL21(DE3) cells assessed ATP hydrolysis activity after 18 to 24 hrs by... |
Bioorg Med Chem Lett 22: 5150-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.128
BindingDB Entry DOI: 10.7270/Q2D79CG0 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Staphylococcus aureus) | BDBM50388093

(CHEMBL2059197)Show SMILES CCCNC(=O)c1cc2c(c(cnc2[nH]1)-c1cncc(c1)C(O)=O)-n1ccc(n1)C(F)(F)F Show InChI InChI=1S/C21H17F3N6O3/c1-2-4-26-19(31)15-7-13-17(30-5-3-16(29-30)21(22,23)24)14(10-27-18(13)28-15)11-6-12(20(32)33)9-25-8-11/h3,5-10H,2,4H2,1H3,(H,26,31)(H,27,28)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase B expressed in Escherichia coli BL21(DE3) cells assessed ATP hydrolysis activity after 18 to 24 hrs by... |
Bioorg Med Chem Lett 22: 5150-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.128
BindingDB Entry DOI: 10.7270/Q2D79CG0 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Staphylococcus aureus) | BDBM50388096

(CHEMBL2059374)Show SMILES CCNC(=O)c1cc2c(c(cnc2[nH]1)-c1cncc(c1)C(O)=O)-n1nc(cc1C)C(F)(F)F |(24.55,-4.68,;25.31,-6.02,;26.85,-6.02,;27.62,-7.35,;26.85,-8.69,;29.16,-7.35,;30.07,-8.61,;31.54,-8.13,;32.88,-8.9,;34.21,-8.13,;34.21,-6.58,;32.87,-5.82,;31.54,-6.58,;30.07,-6.1,;35.54,-8.9,;35.54,-10.44,;36.88,-11.21,;38.21,-10.44,;38.2,-8.89,;36.87,-8.13,;39.54,-8.11,;40.87,-8.87,;39.53,-6.57,;32.88,-10.44,;34.13,-11.34,;33.65,-12.81,;32.11,-12.81,;31.63,-11.34,;30.17,-10.86,;34.55,-14.05,;33.93,-15.46,;36.09,-13.89,;35.31,-15.38,)| Show InChI InChI=1S/C21H17F3N6O3/c1-3-26-19(31)15-6-13-17(30-10(2)4-16(29-30)21(22,23)24)14(9-27-18(13)28-15)11-5-12(20(32)33)8-25-7-11/h4-9H,3H2,1-2H3,(H,26,31)(H,27,28)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase B expressed in Escherichia coli BL21(DE3) cells assessed ATP hydrolysis activity after 18 to 24 hrs by... |
Bioorg Med Chem Lett 22: 5150-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.128
BindingDB Entry DOI: 10.7270/Q2D79CG0 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Staphylococcus aureus) | BDBM50388094
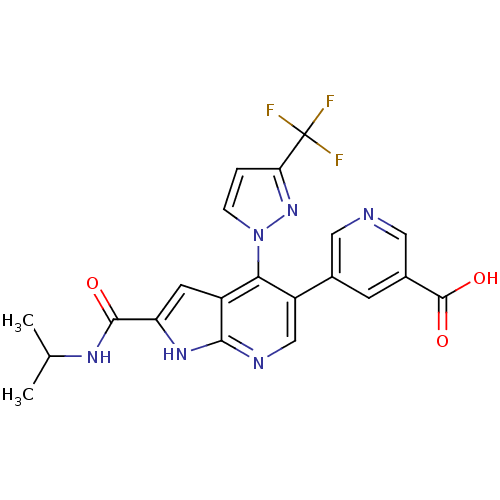
(CHEMBL2059198)Show SMILES CC(C)NC(=O)c1cc2c(c(cnc2[nH]1)-c1cncc(c1)C(O)=O)-n1ccc(n1)C(F)(F)F Show InChI InChI=1S/C21H17F3N6O3/c1-10(2)27-19(31)15-6-13-17(30-4-3-16(29-30)21(22,23)24)14(9-26-18(13)28-15)11-5-12(20(32)33)8-25-7-11/h3-10H,1-2H3,(H,26,28)(H,27,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus DNA gyrase B expressed in Escherichia coli BL21(DE3) cells assessed ATP hydrolysis activity after 18 to 24 hrs by... |
Bioorg Med Chem Lett 22: 5150-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.128
BindingDB Entry DOI: 10.7270/Q2D79CG0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404642
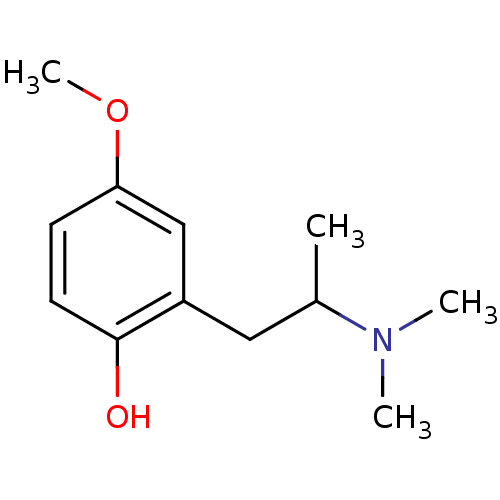
(CHEMBL175328)Show InChI InChI=1S/C12H19NO2/c1-9(13(2)3)7-10-8-11(15-4)5-6-12(10)14/h5-6,8-9,14H,7H2,1-4H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic against 5-hydroxytryptamine 2B receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM29135

(CHEMBL11608 | cid_5610 | p-Tyramine | tyramine)Show InChI InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity of corresponding methoxy compound against serotonin 5-HT receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404640
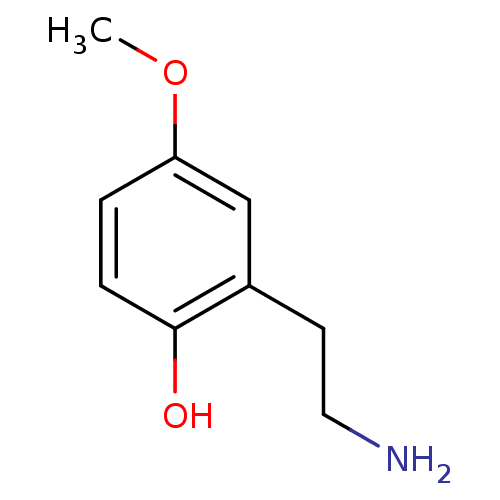
(CHEMBL175204)Show InChI InChI=1S/C9H13NO2/c1-12-8-2-3-9(11)7(6-8)4-5-10/h2-3,6,11H,4-5,10H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against 5-hydroxytryptamine 2B receptor obtained from rat stomach fundus preparation |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404641

(CHEMBL174563)Show InChI InChI=1S/C11H17NO2/c1-12(2)7-6-9-8-10(14-3)4-5-11(9)13/h4-5,8,13H,6-7H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 302 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic against 5-hydroxytryptamine 2B receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404645

(CHEMBL175205)Show InChI InChI=1S/C10H15NO2/c1-7(11)5-8-6-9(13-2)3-4-10(8)12/h3-4,6-7,12H,5,11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against 5-hydroxytryptamine 2B receptor obtained from rat stomach fundus preparation |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404648

(3-O-Methyl-Alpha-Methyldopamine | CHEMBL1347)Show InChI InChI=1S/C10H15NO2/c1-7(11)5-8-3-4-9(12)10(6-8)13-2/h3-4,6-7,12H,5,11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic against 5-hydroxytryptamine 2B receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404646
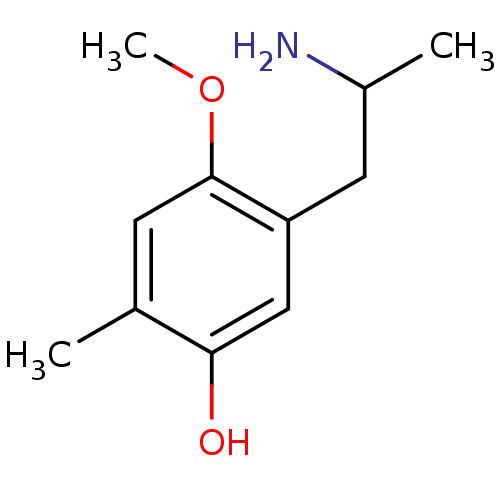
(CHEMBL177800)Show InChI InChI=1S/C11H17NO2/c1-7-4-11(14-3)9(5-8(2)12)6-10(7)13/h4,6,8,13H,5,12H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against 5-hydroxytryptamine 2B receptor obtained from rat stomach fundus preparation |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404647
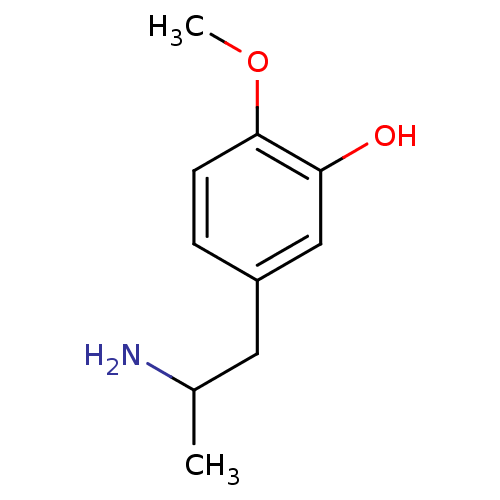
(CHEMBL174723)Show InChI InChI=1S/C10H15NO2/c1-7(11)5-8-3-4-10(13-2)9(12)6-8/h3-4,6-7,12H,5,11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic against 5-hydroxytryptamine 2B receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404643

(CHEMBL175272)Show InChI InChI=1S/C10H15NO2/c1-7(11)5-8-3-4-9(13-2)6-10(8)12/h3-4,6-7,12H,5,11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic against 5-hydroxytryptamine 2B receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404639

(CHEMBL174449)Show InChI InChI=1S/C18H23NO2/c1-19(2)12-11-16-13-17(20-3)9-10-18(16)21-14-15-7-5-4-6-8-15/h4-10,13H,11-12,14H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 302 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic against 5-hydroxytryptamine 2B receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404641

(CHEMBL174563)Show InChI InChI=1S/C11H17NO2/c1-12(2)7-6-9-8-10(14-3)4-5-11(9)13/h4-5,8,13H,6-7H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against 5-hydroxytryptamine 2B receptor obtained from rat stomach fundus preparation |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404644

(CHEMBL174380)Show InChI InChI=1S/C11H17NO2/c1-7-4-10(13)9(5-8(2)12)6-11(7)14-3/h4,6,8,13H,5,12H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic against 5-hydroxytryptamine 2B receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404647

(CHEMBL174723)Show InChI InChI=1S/C10H15NO2/c1-7(11)5-8-3-4-10(13-2)9(12)6-8/h3-4,6-7,12H,5,11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against 5-hydroxytryptamine 2B receptor obtained from rat stomach fundus preparation |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404639

(CHEMBL174449)Show InChI InChI=1S/C18H23NO2/c1-19(2)12-11-16-13-17(20-3)9-10-18(16)21-14-15-7-5-4-6-8-15/h4-10,13H,11-12,14H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 3.63E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against 5-hydroxytryptamine 2B receptor obtained from rat stomach fundus preparation |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404640

(CHEMBL175204)Show InChI InChI=1S/C9H13NO2/c1-12-8-2-3-9(11)7(6-8)4-5-10/h2-3,6,11H,4-5,10H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic against 5-hydroxytryptamine 2B receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404642

(CHEMBL175328)Show InChI InChI=1S/C12H19NO2/c1-9(13(2)3)7-10-8-11(15-4)5-6-12(10)14/h5-6,8-9,14H,7H2,1-4H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against 5-hydroxytryptamine 2B receptor obtained from rat stomach fundus preparation |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404645

(CHEMBL175205)Show InChI InChI=1S/C10H15NO2/c1-7(11)5-8-6-9(13-2)3-4-10(8)12/h3-4,6-7,12H,5,11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic against 5-hydroxytryptamine 2B receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404644

(CHEMBL174380)Show InChI InChI=1S/C11H17NO2/c1-7-4-10(13)9(5-8(2)12)6-11(7)14-3/h4,6,8,13H,5,12H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 36.3 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against 5-hydroxytryptamine 2B receptor obtained from rat stomach fundus preparation |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404648

(3-O-Methyl-Alpha-Methyldopamine | CHEMBL1347)Show InChI InChI=1S/C10H15NO2/c1-7(11)5-8-3-4-9(12)10(6-8)13-2/h3-4,6-7,12H,5,11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic activity against 5-hydroxytryptamine 2B receptor obtained from rat stomach fundus preparation |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(RAT) | BDBM50404646

(CHEMBL177800)Show InChI InChI=1S/C11H17NO2/c1-7-4-11(14-3)9(5-8(2)12)6-10(7)13/h4,6,8,13H,5,12H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonistic against 5-hydroxytryptamine 2B receptor |
J Med Chem 23: 990-4 (1980)
BindingDB Entry DOI: 10.7270/Q2668FCW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data