

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM291172 (2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfuran-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM172369 (US9090596, 50) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The experiments were carried out on the FLIPR TETRA.RTM. platform from Molecular Devices. After the basal level had been read, the compounds were add... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM291181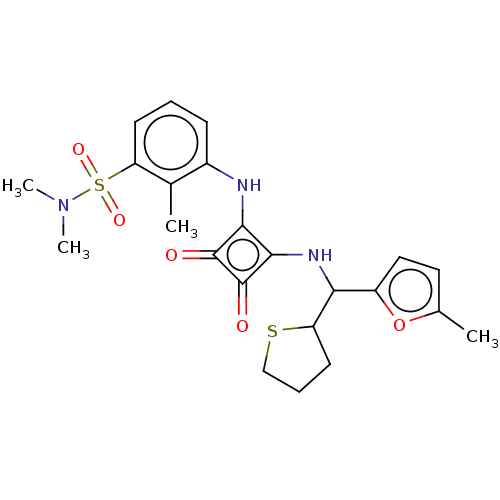 (6-chloro-2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM172372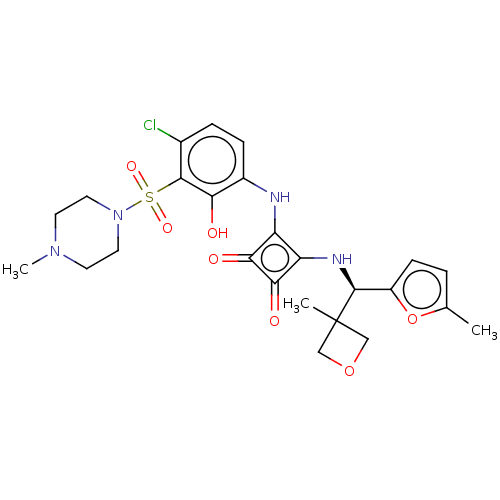 (US9090596, 53) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The experiments were carried out on the FLIPR TETRA.RTM. platform from Molecular Devices. After the basal level had been read, the compounds were add... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM291172 (2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfuran-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM236791 (US9388149, 22 | US9580412, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM291172 (2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfuran-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453825 (CHEMBL4215407 | US10556883, Compound 19a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in NHEK assessed as reduction in LPS/TPA-stimulated TNFalpha production preincubated for 1 hr followed by LPS/TPA stimulation for ... | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM172342 (US9090596, 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The experiments were carried out on the FLIPR TETRA.RTM. platform from Molecular Devices. After the basal level had been read, the compounds were add... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453834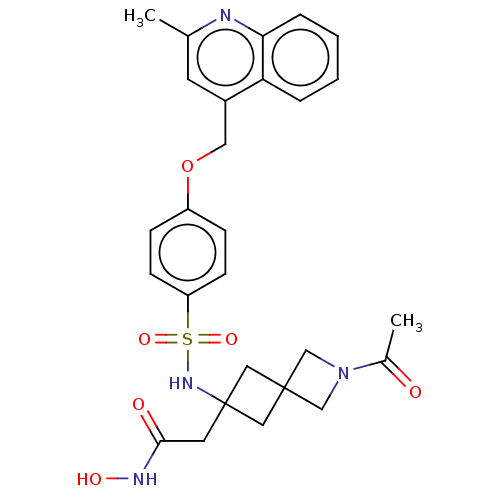 (CHEMBL4208289) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453825 (CHEMBL4215407 | US10556883, Compound 19a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of recombinant human TACE catalytic domain using Mca-Pro-Leu-Ala-Gln-Ala-Val-Dpa-Arg-Ser-Ser-Ser-Arg-NH2 as substrate after 2 hrs by fluor... | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453839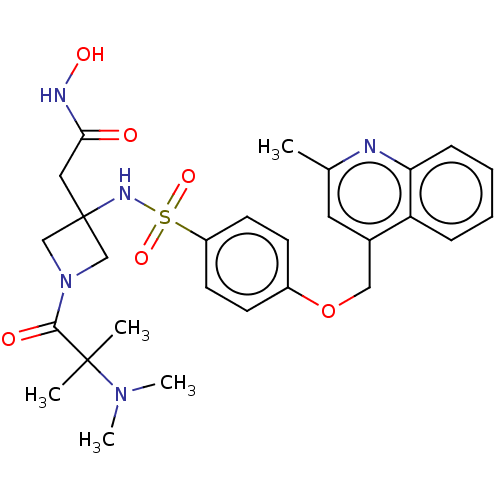 (CHEMBL4209541 | US10556883, Compound 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453838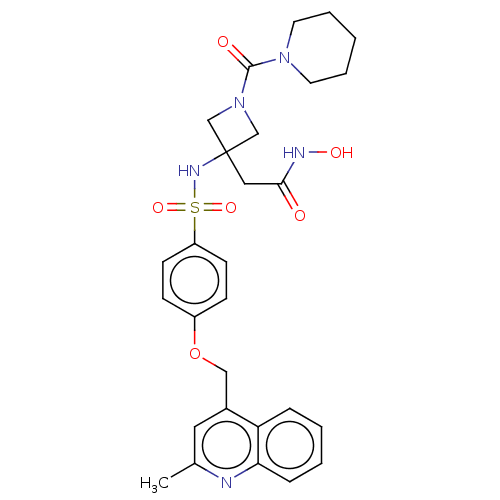 (CHEMBL4206825) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM172328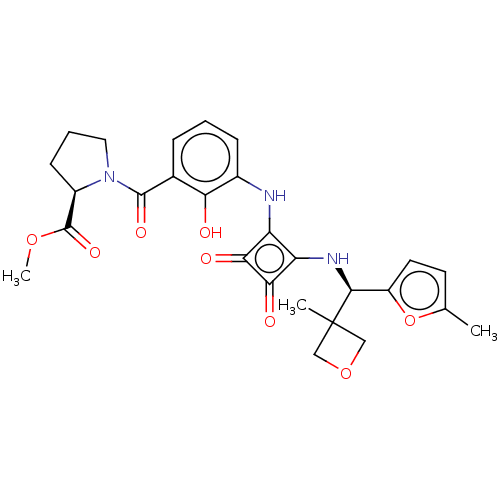 (US9090596, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The experiments were carried out on the FLIPR TETRA.RTM. platform from Molecular Devices. After the basal level had been read, the compounds were add... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM236795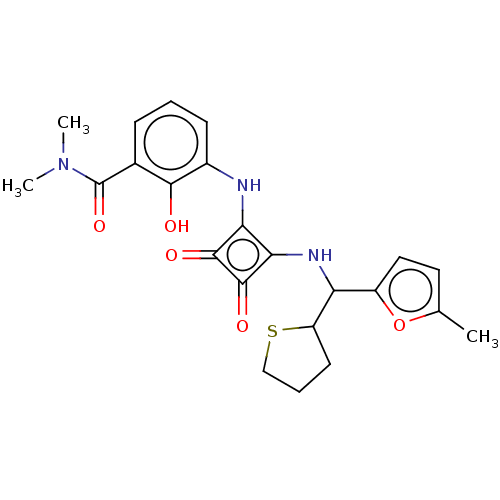 (US9388149, 2 (Diastereoisomer 1) | US9388149, 2 (D...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453847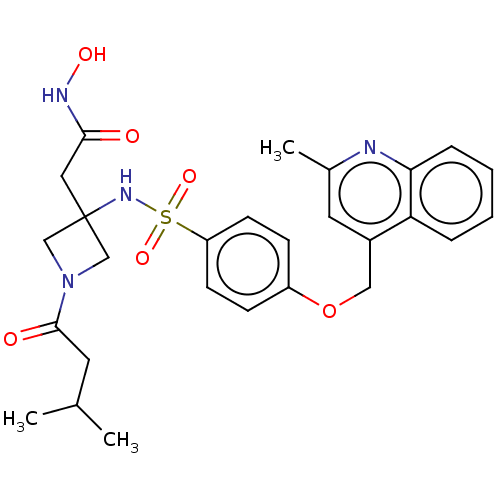 (CHEMBL4216577) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50453823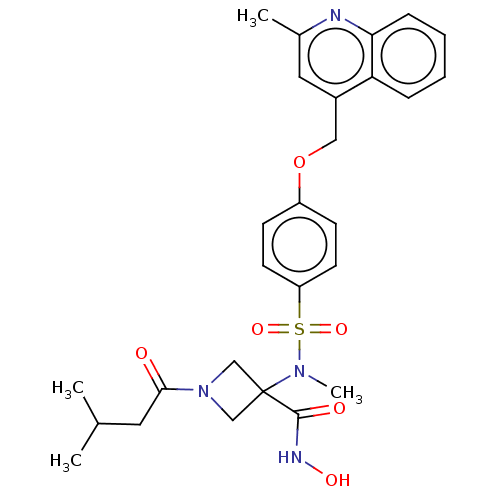 (CHEMBL4208519 | US10556883, Compound 19d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of MMP12 (unknown origin) | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453837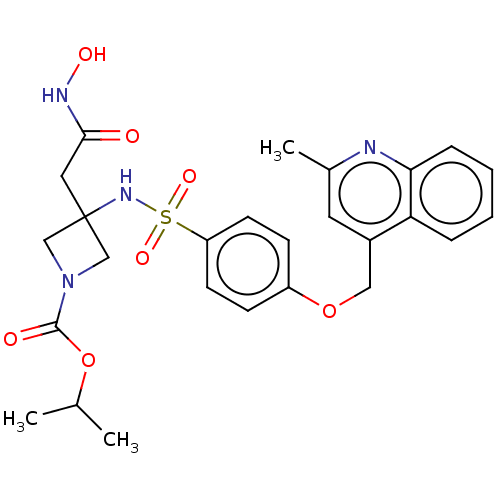 (CHEMBL4211704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453823 (CHEMBL4208519 | US10556883, Compound 19d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of recombinant human TACE catalytic domain using Mca-Pro-Leu-Ala-Gln-Ala-Val-Dpa-Arg-Ser-Ser-Ser-Arg-NH2 as substrate after 2 hrs by fluor... | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM236789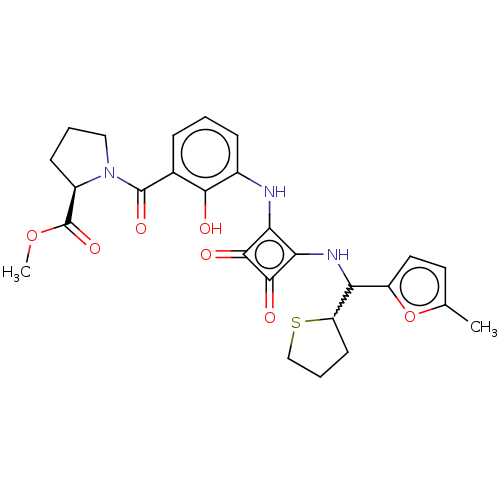 (US9388149, 20 | US9580412, Example 20) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453835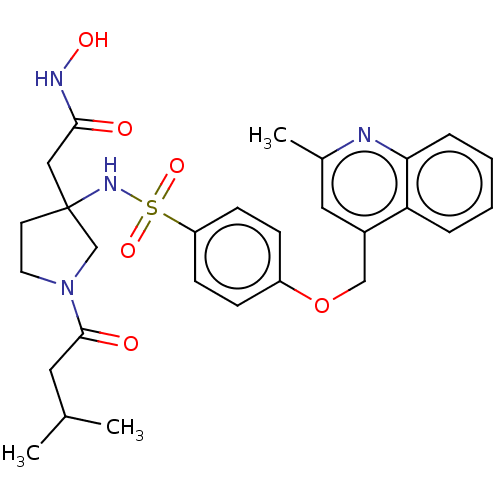 (CHEMBL4203428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM236795 (US9388149, 2 (Diastereoisomer 1) | US9388149, 2 (D...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453835 (CHEMBL4203428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453826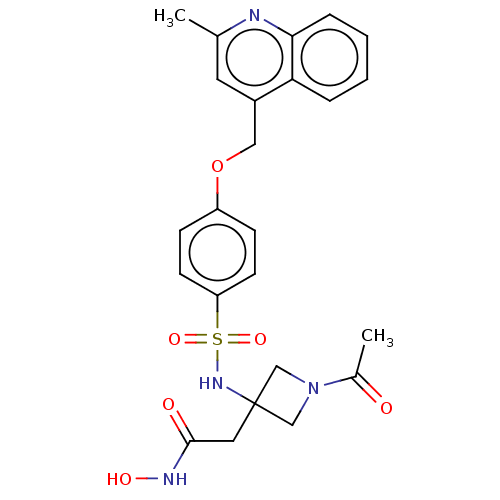 (CHEMBL4217309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM236788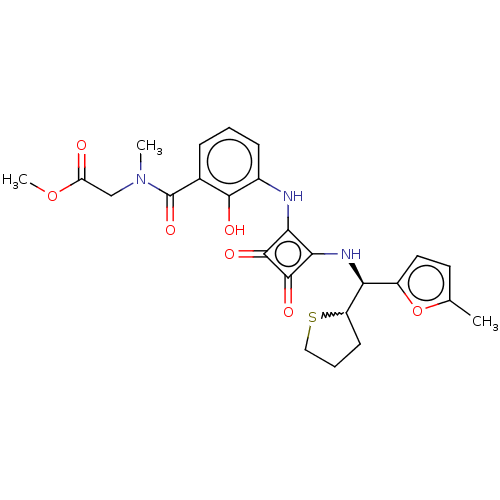 (US9388149, 19 | US9580412, Example 19) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM236789 (US9388149, 20 | US9580412, Example 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453825 (CHEMBL4215407 | US10556883, Compound 19a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM236791 (US9388149, 22 | US9580412, Example 22) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM291177 (US9580412, Example 6 | US9580412, Example 7 | meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453856 (CHEMBL4212130) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453851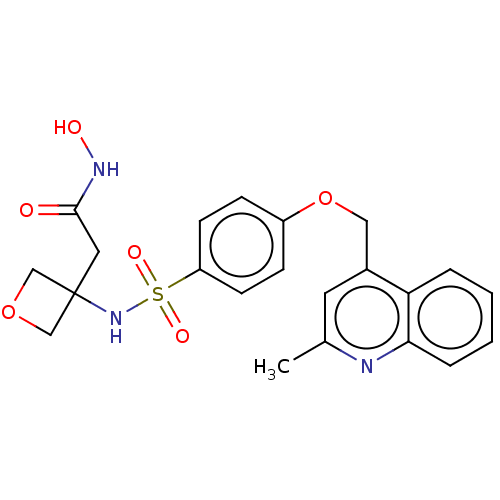 (CHEMBL4207783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM236788 (US9388149, 19 | US9580412, Example 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453836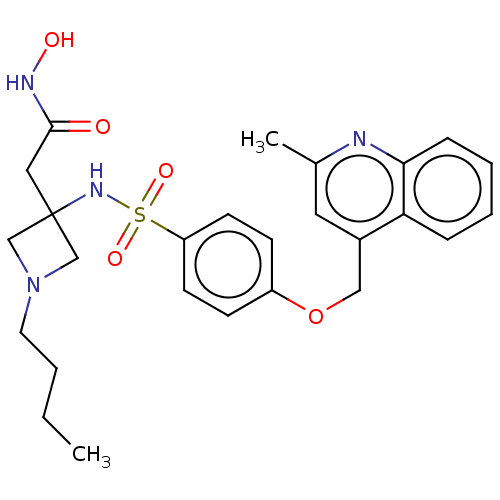 (CHEMBL4217104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453833 (CHEMBL4212683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50453825 (CHEMBL4215407 | US10556883, Compound 19a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of MMP12 (unknown origin) | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236795 (US9388149, 2 (Diastereoisomer 1) | US9388149, 2 (D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236787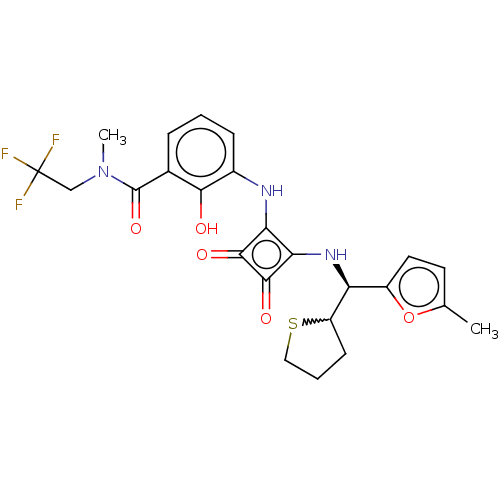 (US9388149, 18 | US9580412, Example 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236795 (US9388149, 2 (Diastereoisomer 1) | US9388149, 2 (D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236787 (US9388149, 18 | US9580412, Example 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453843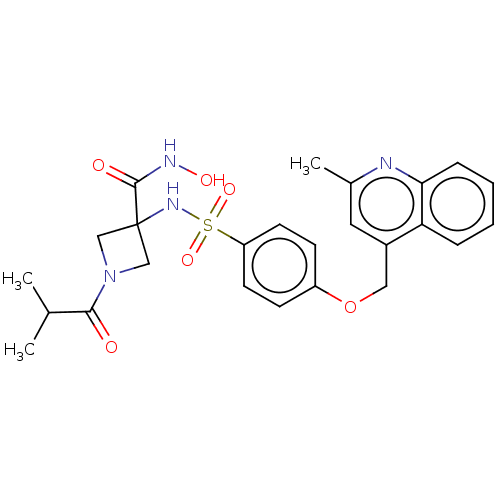 (CHEMBL4210459 | US10556883, Compound 7b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in human PBMC assessed as reduction in LPS-induced TNFalpha production incubated overnight by HTRF assay | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM291181 (6-chloro-2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236813 (US9388149, 10 (Diastereoisomer 1) | US9388149, 10 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236791 (US9388149, 22 | US9580412, Example 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236791 (US9388149, 22 | US9580412, Example 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50461084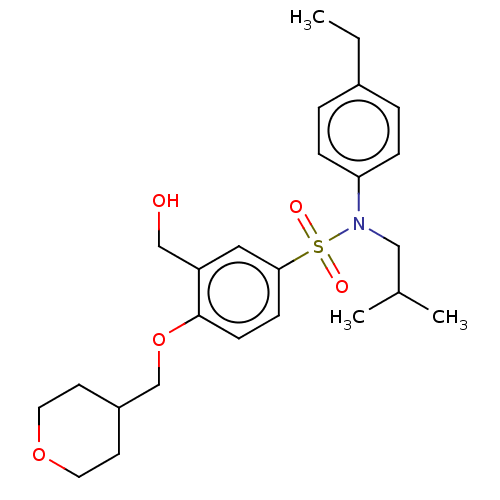 (CHEMBL4225088) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health Curated by ChEMBL | Assay Description Inverse agonist activity at RORgamma (unknown origin) expressed in human HeLa-derived HG5LN cells transfected with the GAL4 DNA-binding domain fused ... | Bioorg Med Chem Lett 28: 1269-1273 (2018) Article DOI: 10.1016/j.bmcl.2018.03.041 BindingDB Entry DOI: 10.7270/Q2WD437Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50461063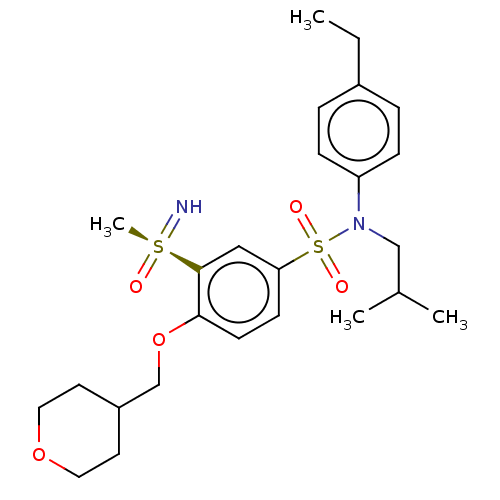 (CHEMBL4226803 | US10457637, Compound 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health Curated by ChEMBL | Assay Description Inverse agonist activity at RORgamma in human CD4+ T cells assessed as inhibition of antiCD-28 antibody stimulated IL-17A production after 4 days by ... | Bioorg Med Chem Lett 28: 1269-1273 (2018) Article DOI: 10.1016/j.bmcl.2018.03.041 BindingDB Entry DOI: 10.7270/Q2WD437Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50453823 (CHEMBL4208519 | US10556883, Compound 19d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of TACE in NHEK assessed as reduction in LPS/TPA-stimulated TNFalpha production preincubated for 1 hr followed by LPS/TPA stimulation for ... | Bioorg Med Chem 26: 945-956 (2018) Article DOI: 10.1016/j.bmc.2017.07.054 BindingDB Entry DOI: 10.7270/Q2RX9FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM419139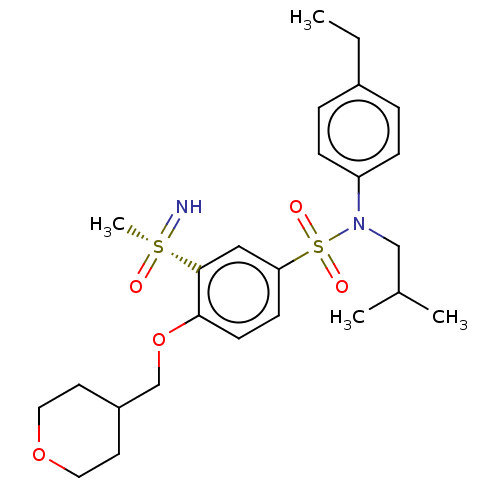 (BDBM50461078 | N-(4-ethylphenyl)-N- isobutyl-3- me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestle Skin Health Curated by ChEMBL | Assay Description Inverse agonist activity at RORgamma in human CD4+ T cells assessed as inhibition of antiCD-28 antibody stimulated IL-17A production after 4 days by ... | Bioorg Med Chem Lett 28: 1269-1273 (2018) Article DOI: 10.1016/j.bmcl.2018.03.041 BindingDB Entry DOI: 10.7270/Q2WD437Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236788 (US9388149, 19 | US9580412, Example 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236788 (US9388149, 19 | US9580412, Example 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 362 total ) | Next | Last >> |