

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472916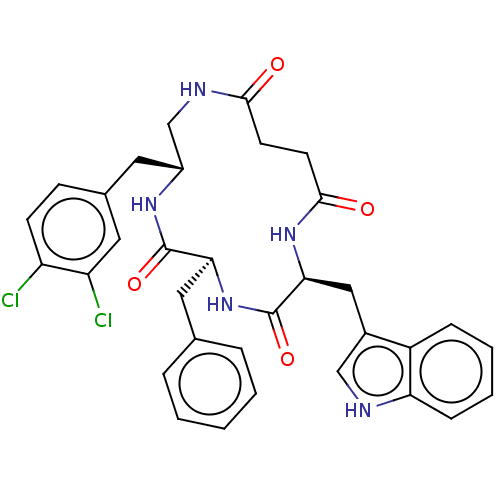 (CHEMBL223221 | MEN-11690) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472916 (CHEMBL223221 | MEN-11690) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473829 (CHEMBL389459 | MEN-11995) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473828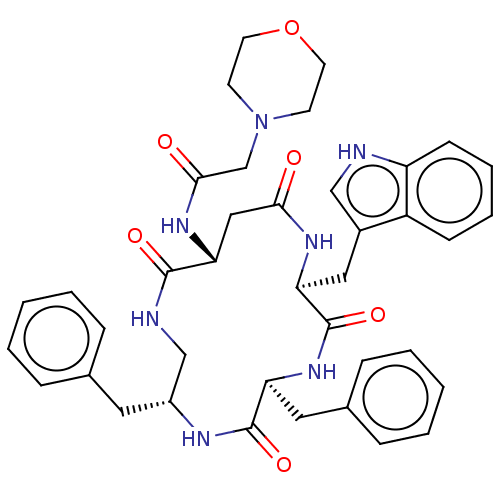 (CHEMBL116812 | MEN-11990) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473824 (CHEMBL406024 | MEN-11959) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50366377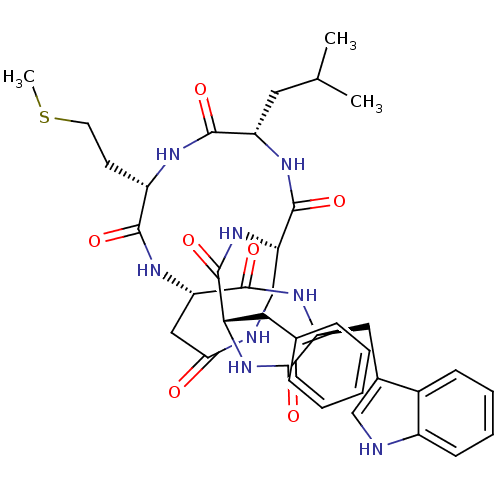 (CHEMBL334721 | MEN-10627) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473818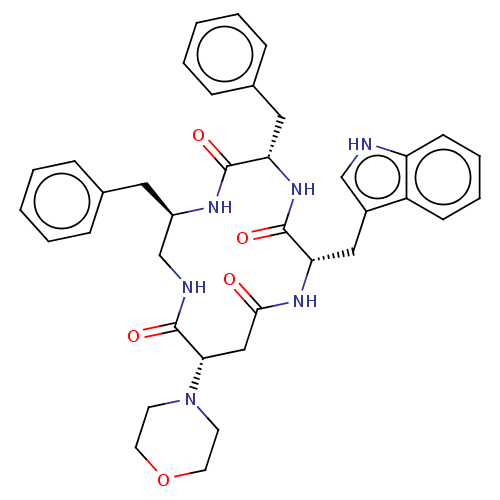 (CHEMBL406287 | MEN-11842) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473833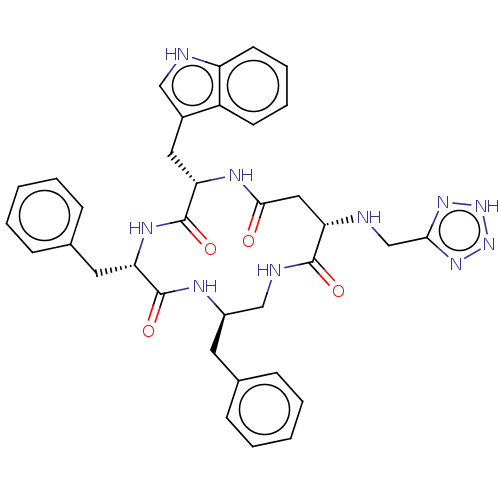 (CHEMBL420859 | MEN-11830) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472917 (CHEMBL323884 | MEN-11749) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472917 (CHEMBL323884 | MEN-11749) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404020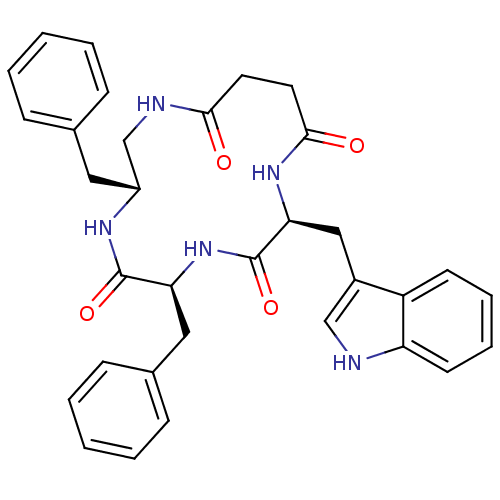 (CHEMBL435324 | MEN-11558) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404020 (CHEMBL435324 | MEN-11558) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472911 (CHEMBL407782 | MEN-11420) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473823 (CHEMBL420308 | MEN-11691) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473822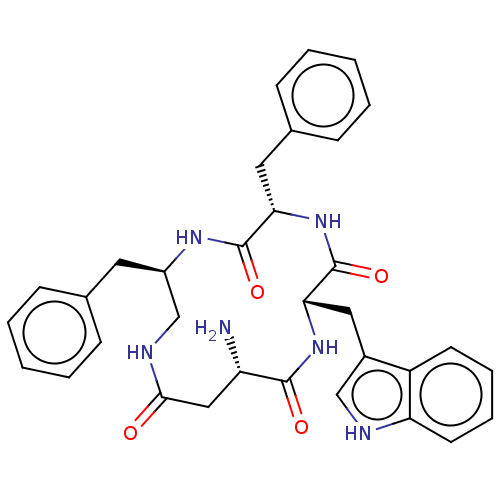 (CHEMBL438559 | MEN-11799) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473836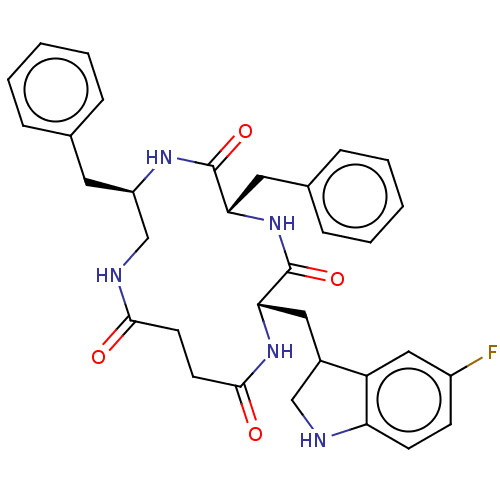 (CHEMBL324313 | MEN-11701) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473830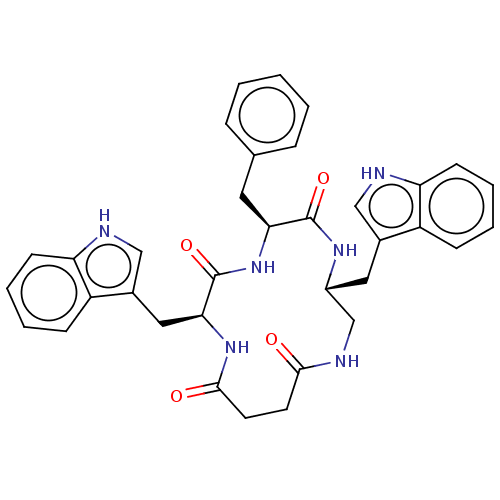 (CHEMBL323677 | MEN-11703) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473839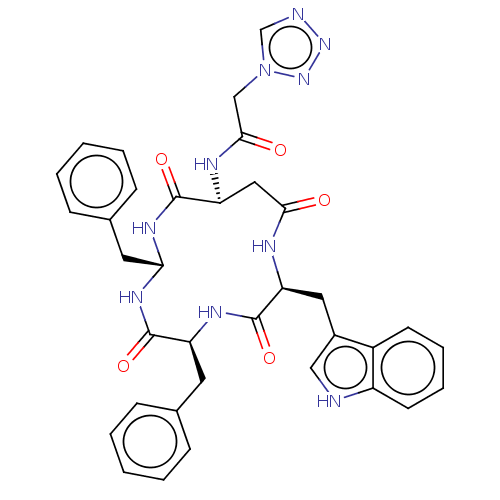 (CHEMBL116871 | MEN-11987) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473821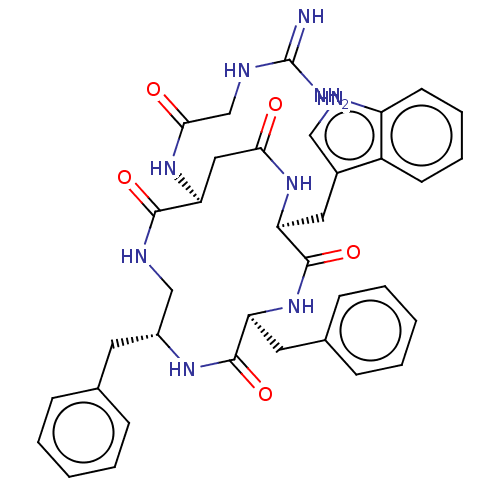 (CHEMBL114068 | MEN-11986) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473825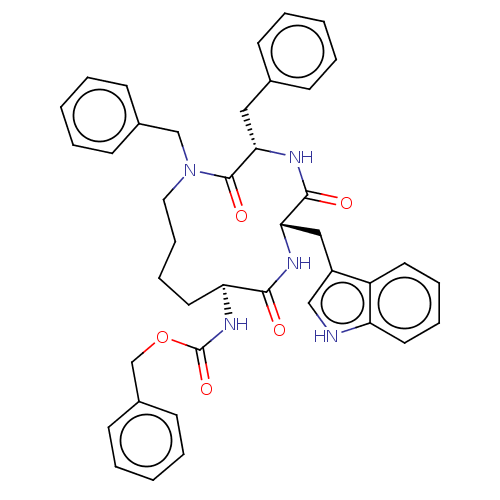 (CHEMBL113765 | MEN-11983) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473831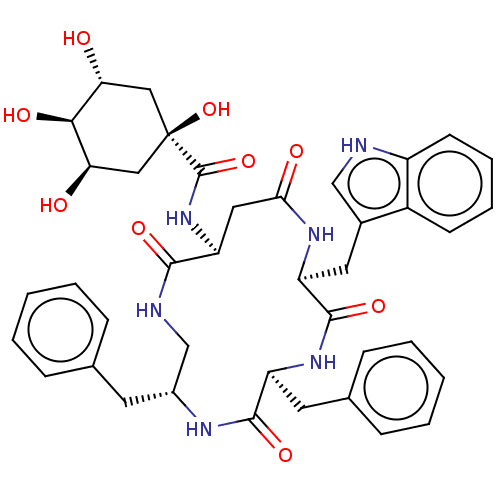 (CHEMBL432986 | MEN-11757) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472912 (CHEMBL116564 | MEN-11712) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472912 (CHEMBL116564 | MEN-11712) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473837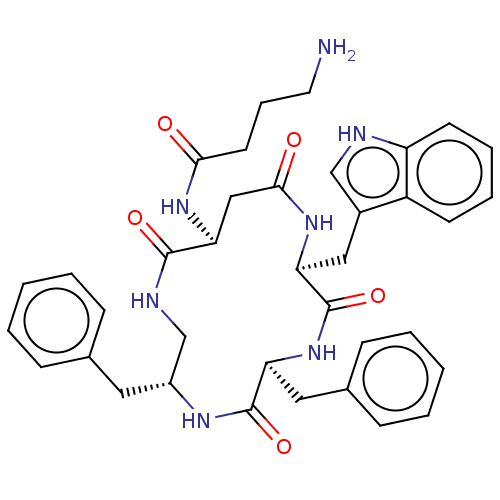 (CHEMBL324532 | MEN-11877) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472908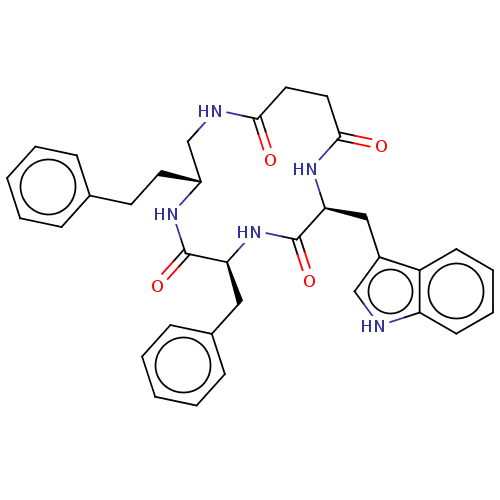 (CHEMBL336053) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472910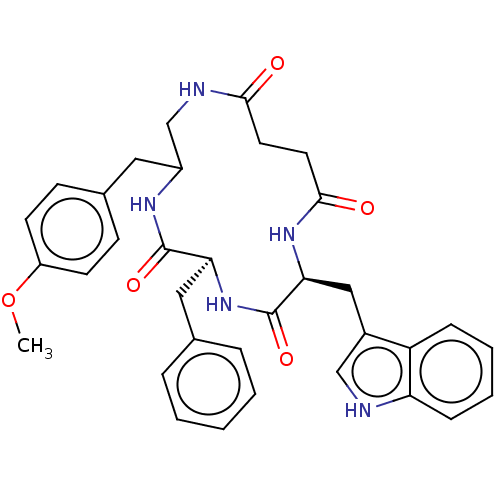 (CHEMBL263194) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473838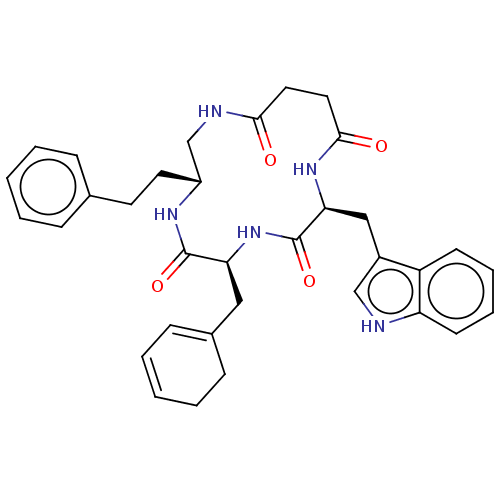 (CHEMBL116629 | MEN-11708) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473827 (CHEMBL323836 | MEN-11668) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473832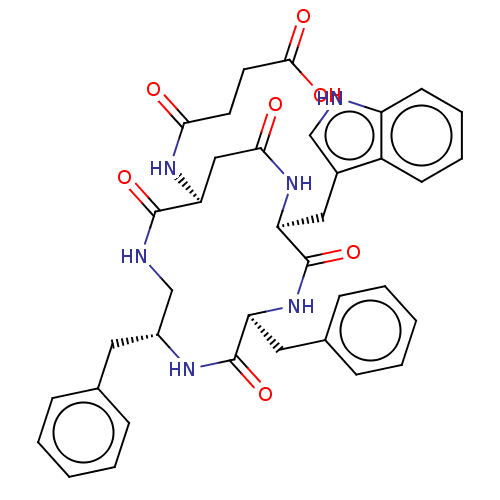 (CHEMBL116969 | MEN-11755) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472913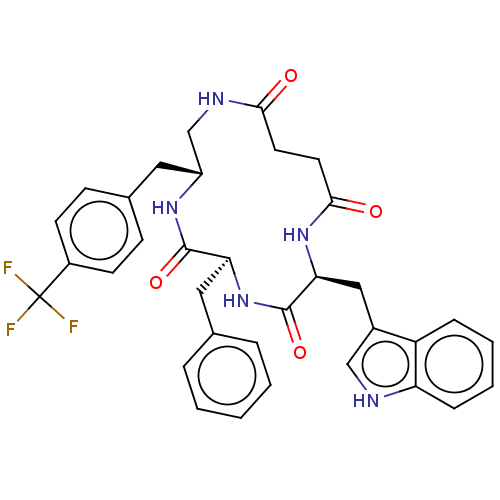 (CHEMBL2112675) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473834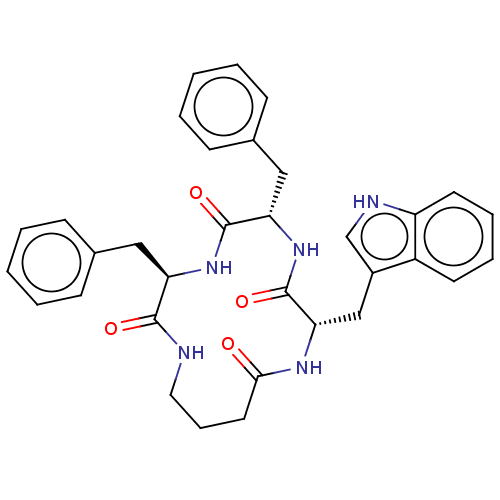 (CHEMBL324106 | MEN-11733) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473826 (CHEMBL116855 | MEN-11715) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472914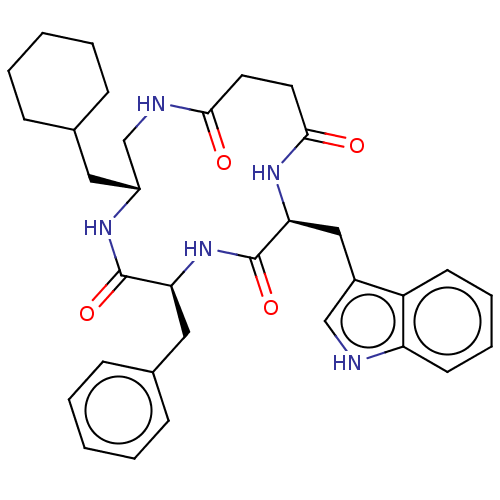 (CHEMBL114442 | MEN-11667) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472914 (CHEMBL114442 | MEN-11667) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473820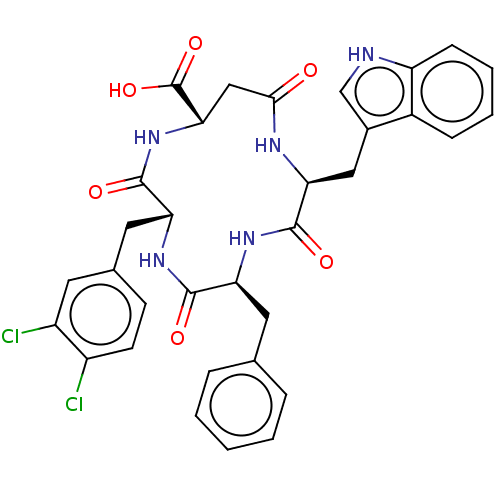 (CHEMBL117039 | MEN-11716) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473819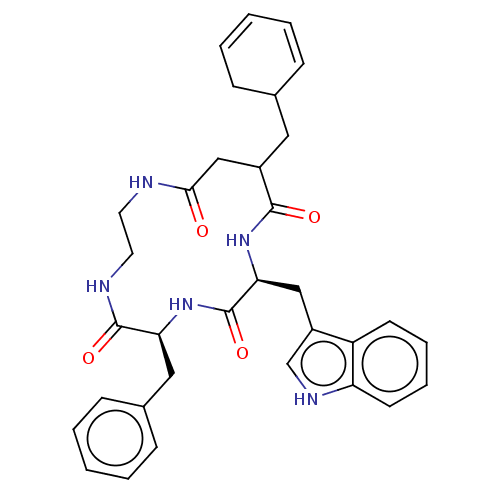 (CHEMBL326790 | MEN-11532) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472919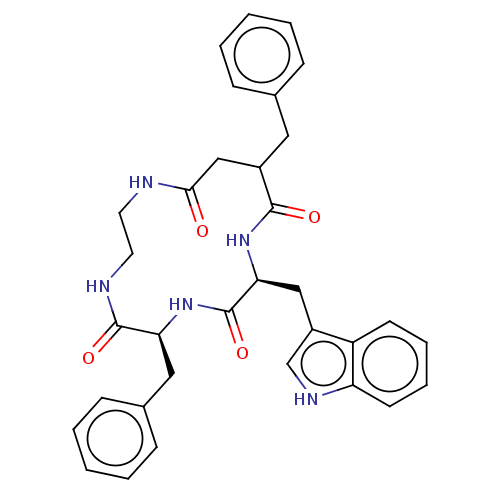 (CHEMBL337628) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472915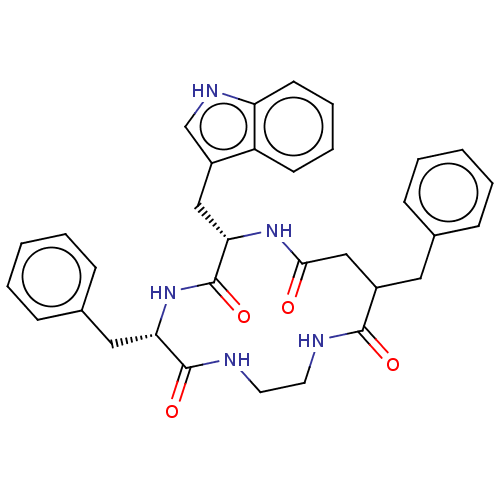 (CHEMBL114036 | MEN-11540) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472915 (CHEMBL114036 | MEN-11540) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472918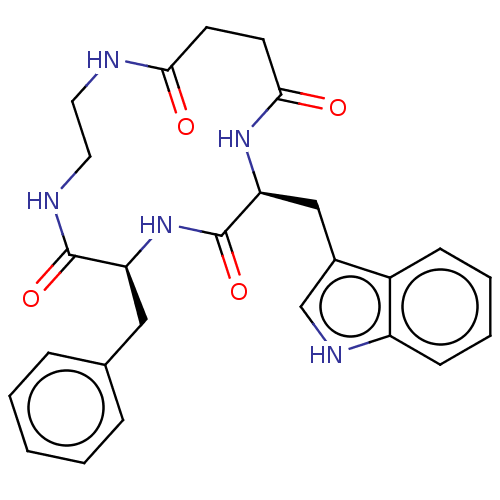 (CHEMBL324804 | MEN-11970) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472907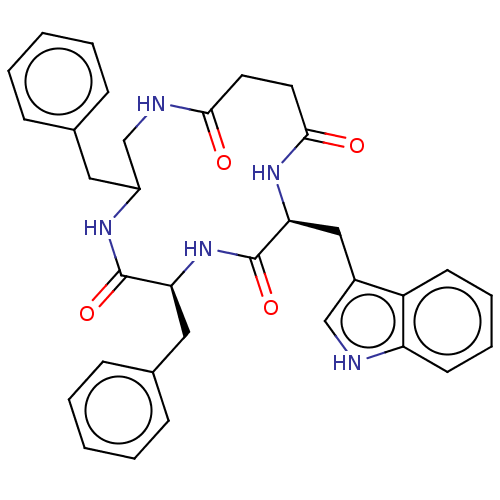 (CHEMBL131024) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472918 (CHEMBL324804 | MEN-11970) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472909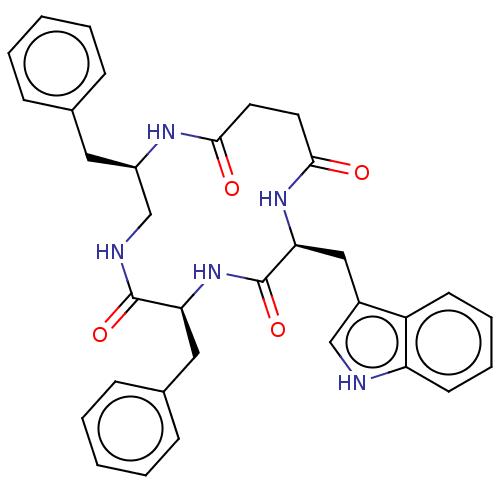 (CHEMBL2112669) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472906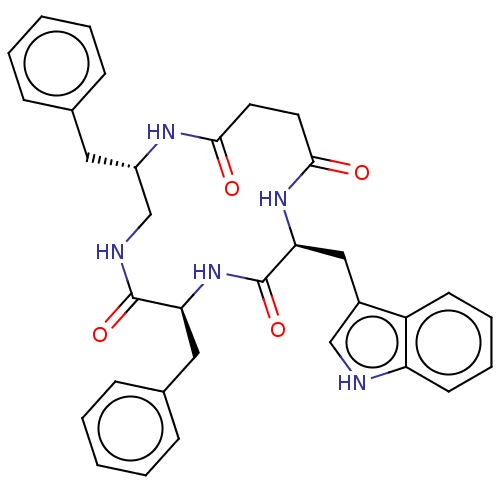 (CHEMBL2111858) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Binding affinity towards Tachykinin receptor 2 | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50473835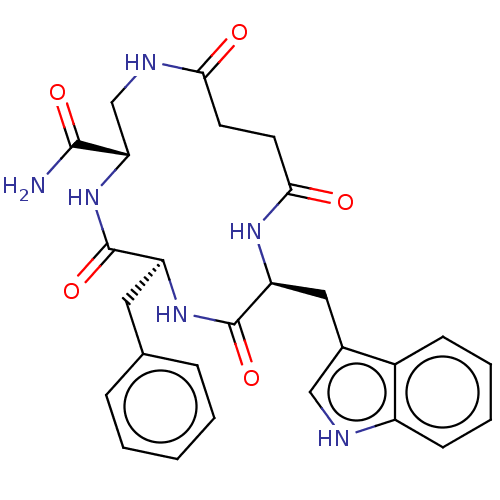 (CHEMBL420685 | MEN-11523) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472911 (CHEMBL407782 | MEN-11420) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Compound was evaluated for Kd value against [3H]-SR- 48968 on the Tyr206Phe mutated human NK-2 receptor | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472911 (CHEMBL407782 | MEN-11420) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Compound was evaluated for Kd value against [125I]NKA on the Tyr266Phe mutated human NK-2 receptor | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50404020 (CHEMBL435324 | MEN-11558) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Compound was evaluated for Kd value against [125I]NKA on the Tyr266Phe mutated human NK-2 receptor | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472911 (CHEMBL407782 | MEN-11420) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Compound was evaluated for Kd value against [3H]-SR- 48968 on the Phe270Ala mutated human NK-2 receptor | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472911 (CHEMBL407782 | MEN-11420) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Compound was evaluated for Kd value against [3H]-SR- 48968 on the Tyr266Phe mutated human NK-2 receptor | J Med Chem 43: 4041-4 (2000) Article DOI: 10.1021/jm0010217 BindingDB Entry DOI: 10.7270/Q2Z322CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |