 Found 49 hits with Last Name = 'sanguinetti' and Initial = 'm'
Found 49 hits with Last Name = 'sanguinetti' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50006878

((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(C)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-12-14-18(15-13-16)25-24(30)27-22-23(29)28(2)20-11-7-6-10-19(20)21(26-22)17-8-4-3-5-9-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552035
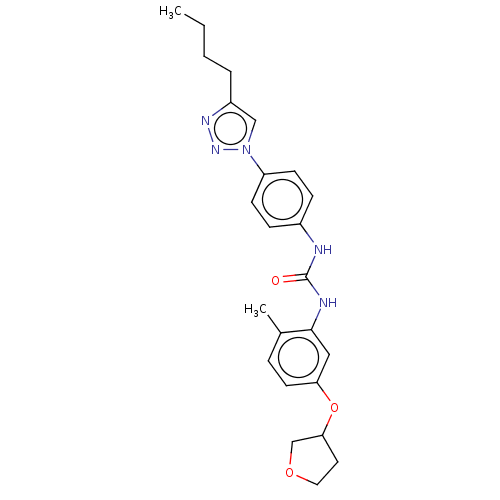
(CHEMBL4792698)Show SMILES CCCCc1cn(nn1)-c1ccc(NC(=O)Nc2cc(OC3CCOC3)ccc2C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061207
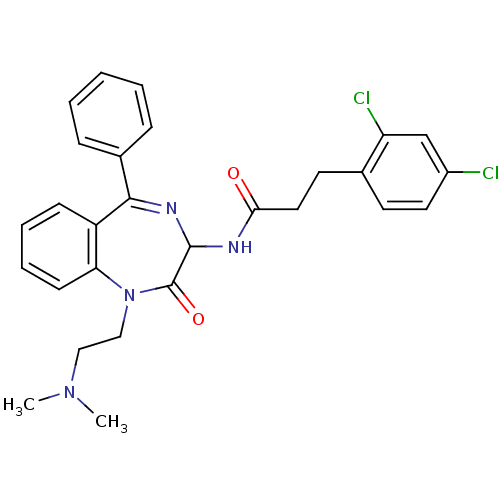
(3-(2,4-Dichloro-phenyl)-N-[1-(2-dimethylamino-ethy...)Show SMILES CN(C)CCN1c2ccccc2C(=NC(NC(=O)CCc2ccc(Cl)cc2Cl)C1=O)c1ccccc1 |c:13| Show InChI InChI=1S/C28H28Cl2N4O2/c1-33(2)16-17-34-24-11-7-6-10-22(24)26(20-8-4-3-5-9-20)32-27(28(34)36)31-25(35)15-13-19-12-14-21(29)18-23(19)30/h3-12,14,18,27H,13,15-17H2,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552040
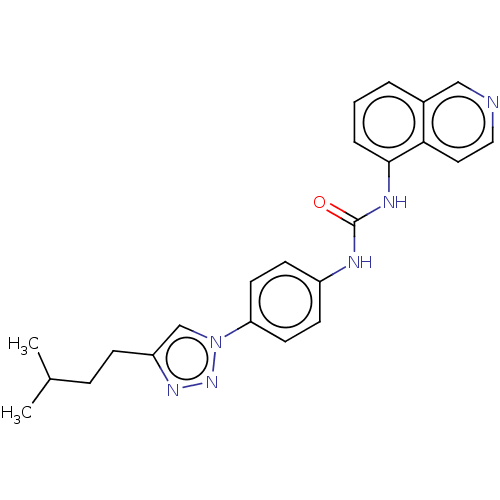
(CHEMBL4764675)Show SMILES CC(C)CCc1cn(nn1)-c1ccc(NC(=O)Nc2cccc3cnccc23)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552034
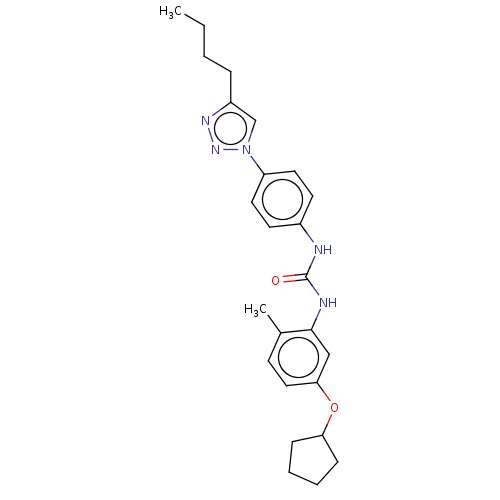
(CHEMBL4743218)Show SMILES CCCCc1cn(nn1)-c1ccc(NC(=O)Nc2cc(OC3CCCC3)ccc2C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552041
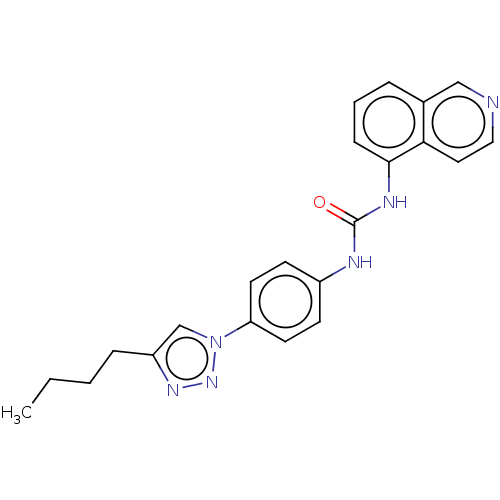
(CHEMBL4744776)Show SMILES CCCCc1cn(nn1)-c1ccc(NC(=O)Nc2cccc3cnccc23)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061220
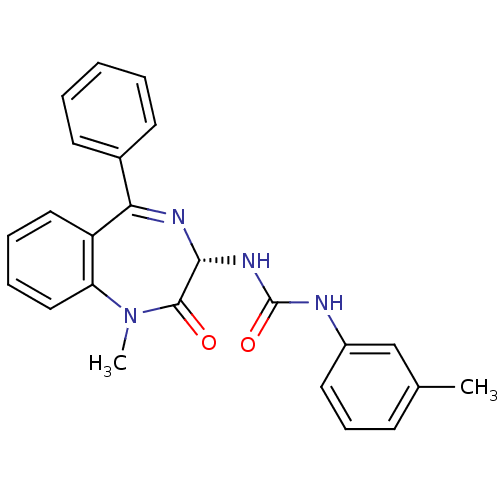
(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50537058

(CHEMBL4531719)Show InChI InChI=1S/C20H23N5O/c1-3-4-8-17-14-25(24-23-17)18-12-10-16(11-13-18)21-20(26)22-19-9-6-5-7-15(19)2/h5-7,9-14H,3-4,8H2,1-2H3,(H2,21,22,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552022
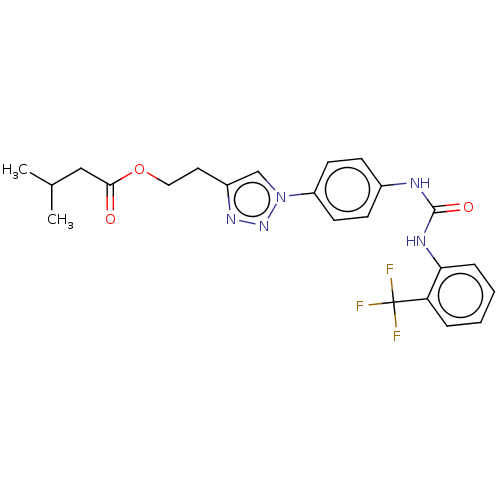
(CHEMBL4785115)Show SMILES CC(C)CC(=O)OCCc1cn(nn1)-c1ccc(NC(=O)Nc2ccccc2C(F)(F)F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552031
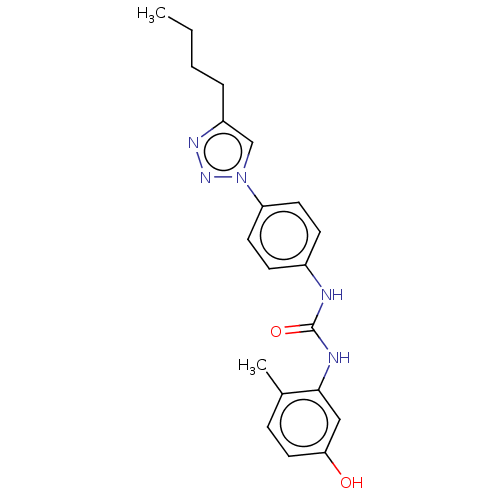
(CHEMBL4741968)Show SMILES CCCCc1cn(nn1)-c1ccc(NC(=O)Nc2cc(O)ccc2C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552032
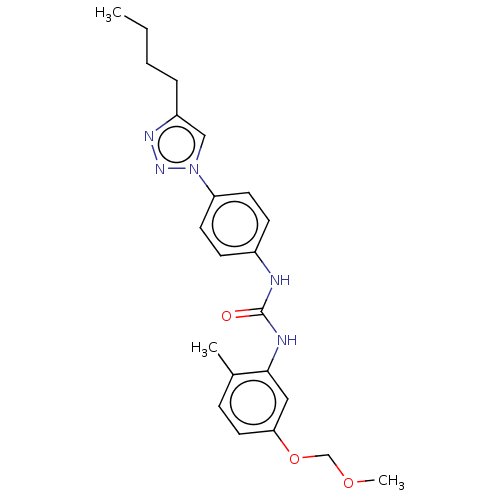
(CHEMBL4742331)Show SMILES CCCCc1cn(nn1)-c1ccc(NC(=O)Nc2cc(OCOC)ccc2C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552024
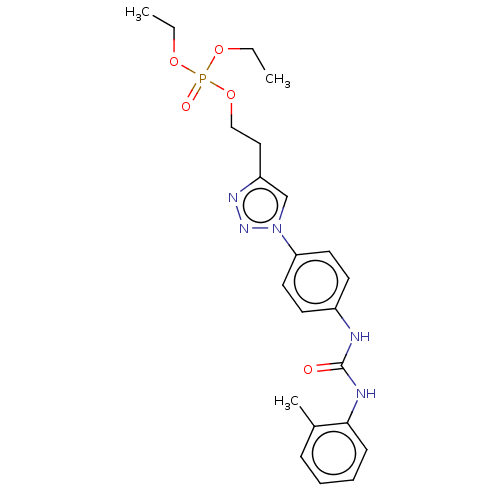
(CHEMBL4741684)Show SMILES CCOP(=O)(OCC)OCCc1cn(nn1)-c1ccc(NC(=O)Nc2ccccc2C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552033
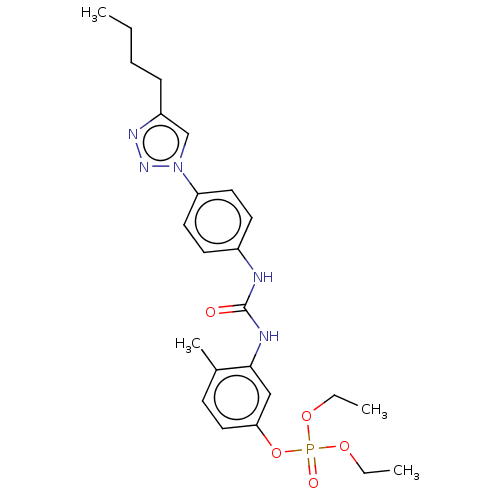
(CHEMBL4787707)Show SMILES CCCCc1cn(nn1)-c1ccc(NC(=O)Nc2cc(OP(=O)(OCC)OCC)ccc2C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061224
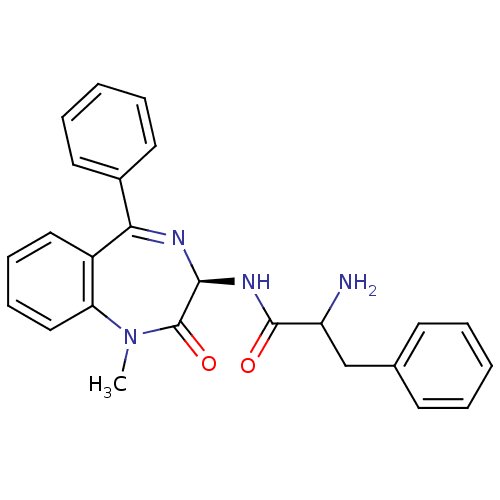
(2-Amino-N-((R)-1-methyl-2-oxo-5-phenyl-2,3-dihydro...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)C(N)Cc2ccccc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C25H24N4O2/c1-29-21-15-9-8-14-19(21)22(18-12-6-3-7-13-18)27-23(25(29)31)28-24(30)20(26)16-17-10-4-2-5-11-17/h2-15,20,23H,16,26H2,1H3,(H,28,30)/t20?,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061227
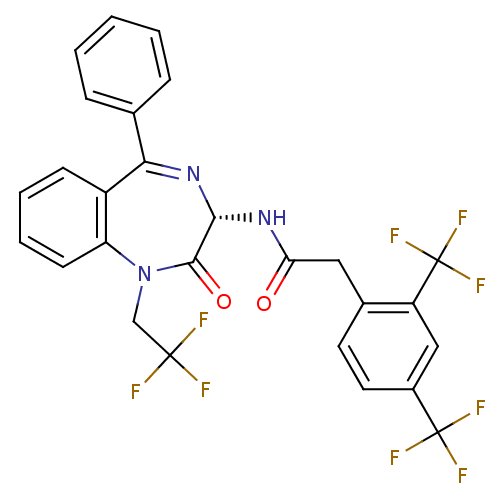
(2-(2,4-Bis-trifluoromethyl-phenyl)-N-[(S)-2-oxo-5-...)Show SMILES FC(F)(F)CN1c2ccccc2C(=N[C@H](NC(=O)Cc2ccc(cc2C(F)(F)F)C(F)(F)F)C1=O)c1ccccc1 |c:13| Show InChI InChI=1S/C27H18F9N3O2/c28-25(29,30)14-39-20-9-5-4-8-18(20)22(15-6-2-1-3-7-15)38-23(24(39)41)37-21(40)12-16-10-11-17(26(31,32)33)13-19(16)27(34,35)36/h1-11,13,23H,12,14H2,(H,37,40)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061222

(3-Cyclohexyl-N-((R)-1-methyl-2-oxo-5-phenyl-2,3-di...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)CCC2CCCCC2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C25H29N3O2/c1-28-21-15-9-8-14-20(21)23(19-12-6-3-7-13-19)27-24(25(28)30)26-22(29)17-16-18-10-4-2-5-11-18/h3,6-9,12-15,18,24H,2,4-5,10-11,16-17H2,1H3,(H,26,29)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061216
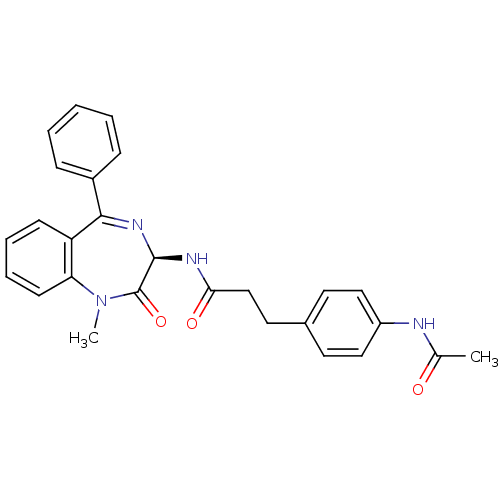
(3-(4-Acetylamino-phenyl)-N-((R)-1-methyl-2-oxo-5-p...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)CCc2ccc(NC(C)=O)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C27H26N4O3/c1-18(32)28-21-15-12-19(13-16-21)14-17-24(33)29-26-27(34)31(2)23-11-7-6-10-22(23)25(30-26)20-8-4-3-5-9-20/h3-13,15-16,26H,14,17H2,1-2H3,(H,28,32)(H,29,33)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061209
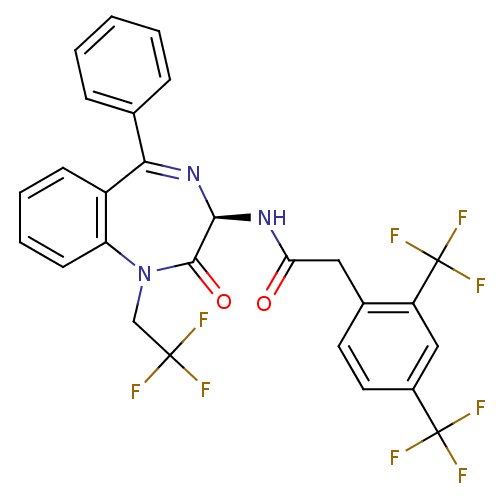
(2-(2,4-Bis-trifluoromethyl-phenyl)-N-[(R)-2-oxo-5-...)Show SMILES FC(F)(F)CN1c2ccccc2C(=N[C@@H](NC(=O)Cc2ccc(cc2C(F)(F)F)C(F)(F)F)C1=O)c1ccccc1 |c:13| Show InChI InChI=1S/C27H18F9N3O2/c28-25(29,30)14-39-20-9-5-4-8-18(20)22(15-6-2-1-3-7-15)38-23(24(39)41)37-21(40)12-16-10-11-17(26(31,32)33)13-19(16)27(34,35)36/h1-11,13,23H,12,14H2,(H,37,40)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061225
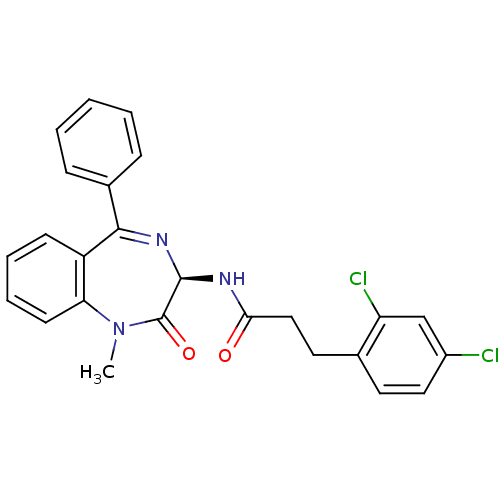
(3-(2,4-Dichloro-phenyl)-N-((R)-1-methyl-2-oxo-5-ph...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)CCc2ccc(Cl)cc2Cl)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C25H21Cl2N3O2/c1-30-21-10-6-5-9-19(21)23(17-7-3-2-4-8-17)29-24(25(30)32)28-22(31)14-12-16-11-13-18(26)15-20(16)27/h2-11,13,15,24H,12,14H2,1H3,(H,28,31)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061222

(3-Cyclohexyl-N-((R)-1-methyl-2-oxo-5-phenyl-2,3-di...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)CCC2CCCCC2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C25H29N3O2/c1-28-21-15-9-8-14-20(21)23(19-12-6-3-7-13-19)27-24(25(28)30)26-22(29)17-16-18-10-4-2-5-11-18/h3,6-9,12-15,18,24H,2,4-5,10-11,16-17H2,1H3,(H,26,29)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061208
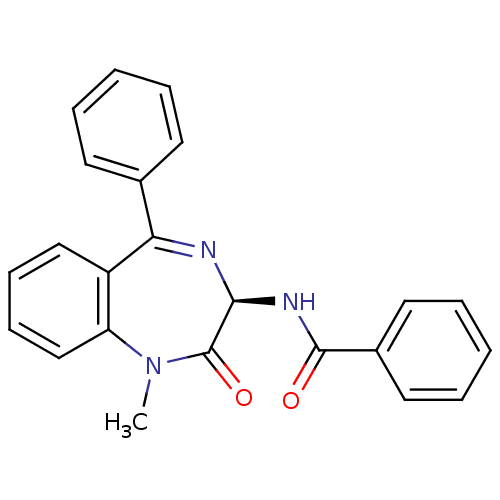
(CHEMBL128031 | N-((R)-1-Methyl-2-oxo-5-phenyl-2,3-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)c2ccccc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C23H19N3O2/c1-26-19-15-9-8-14-18(19)20(16-10-4-2-5-11-16)24-21(23(26)28)25-22(27)17-12-6-3-7-13-17/h2-15,21H,1H3,(H,25,27)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061218
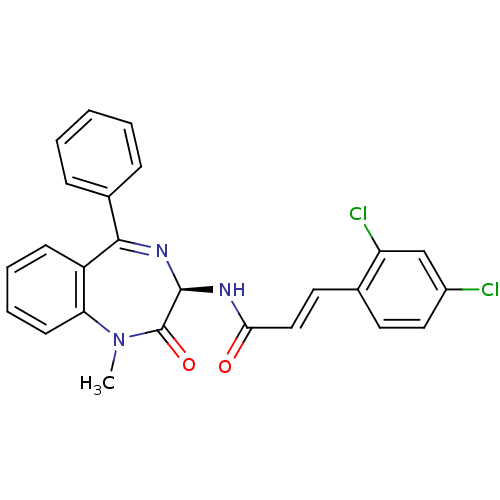
((E)-3-(2,4-Dichloro-phenyl)-N-((R)-1-methyl-2-oxo-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)\C=C\c2ccc(Cl)cc2Cl)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C25H19Cl2N3O2/c1-30-21-10-6-5-9-19(21)23(17-7-3-2-4-8-17)29-24(25(30)32)28-22(31)14-12-16-11-13-18(26)15-20(16)27/h2-15,24H,1H3,(H,28,31)/b14-12+/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061217
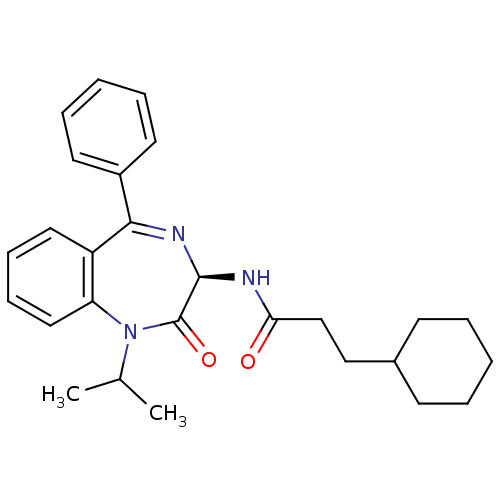
(3-Cyclohexyl-N-((R)-1-isopropyl-2-oxo-5-phenyl-2,3...)Show SMILES CC(C)N1c2ccccc2C(=N[C@@H](NC(=O)CCC2CCCCC2)C1=O)c1ccccc1 |c:11| Show InChI InChI=1S/C27H33N3O2/c1-19(2)30-23-16-10-9-15-22(23)25(21-13-7-4-8-14-21)29-26(27(30)32)28-24(31)18-17-20-11-5-3-6-12-20/h4,7-10,13-16,19-20,26H,3,5-6,11-12,17-18H2,1-2H3,(H,28,31)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061212

((R)-N-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)CCc2ccccc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C25H23N3O2/c1-28-21-15-9-8-14-20(21)23(19-12-6-3-7-13-19)27-24(25(28)30)26-22(29)17-16-18-10-4-2-5-11-18/h2-15,24H,16-17H2,1H3,(H,26,29)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061214
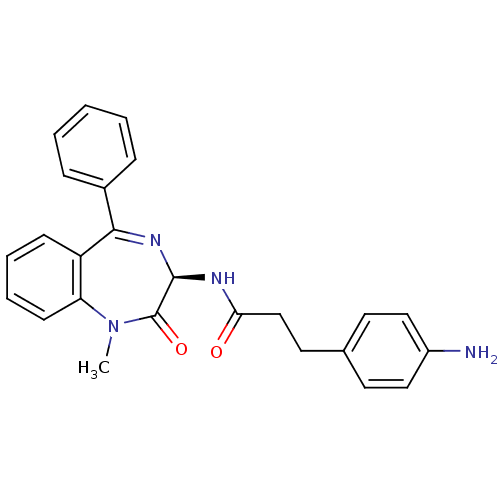
(3-(4-Amino-phenyl)-N-((R)-1-methyl-2-oxo-5-phenyl-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)CCc2ccc(N)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C25H24N4O2/c1-29-21-10-6-5-9-20(21)23(18-7-3-2-4-8-18)28-24(25(29)31)27-22(30)16-13-17-11-14-19(26)15-12-17/h2-12,14-15,24H,13,16,26H2,1H3,(H,27,30)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
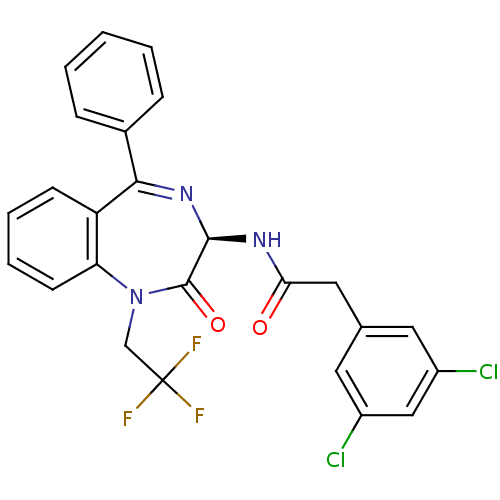
(Homo sapiens (Human)) | BDBM50061207

(3-(2,4-Dichloro-phenyl)-N-[1-(2-dimethylamino-ethy...)Show SMILES CN(C)CCN1c2ccccc2C(=NC(NC(=O)CCc2ccc(Cl)cc2Cl)C1=O)c1ccccc1 |c:13| Show InChI InChI=1S/C28H28Cl2N4O2/c1-33(2)16-17-34-24-11-7-6-10-22(24)26(20-8-4-3-5-9-20)32-27(28(34)36)31-25(35)15-13-19-12-14-21(29)18-23(19)30/h3-12,14,18,27H,13,15-17H2,1-2H3,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061206
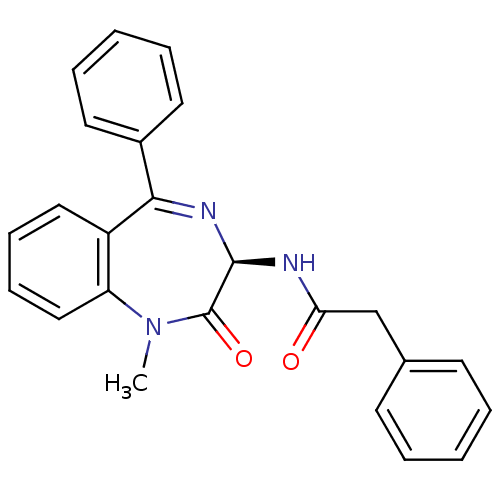
(CHEMBL124306 | N-((R)-1-Methyl-2-oxo-5-phenyl-2,3-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Cc2ccccc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H21N3O2/c1-27-20-15-9-8-14-19(20)22(18-12-6-3-7-13-18)26-23(24(27)29)25-21(28)16-17-10-4-2-5-11-17/h2-15,23H,16H2,1H3,(H,25,28)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061205
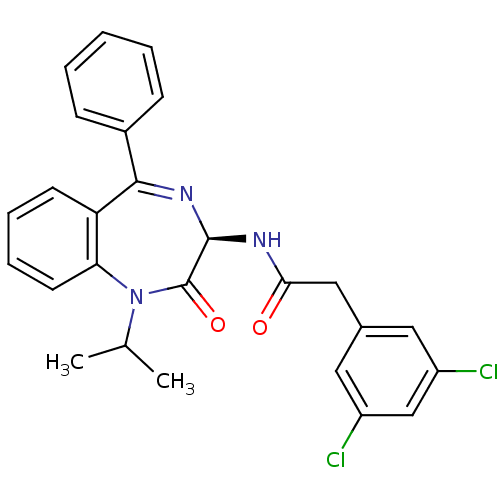
(2-(3,5-Dichloro-phenyl)-N-((R)-1-isopropyl-2-oxo-5...)Show SMILES CC(C)N1c2ccccc2C(=N[C@@H](NC(=O)Cc2cc(Cl)cc(Cl)c2)C1=O)c1ccccc1 |c:11| Show InChI InChI=1S/C26H23Cl2N3O2/c1-16(2)31-22-11-7-6-10-21(22)24(18-8-4-3-5-9-18)30-25(26(31)33)29-23(32)14-17-12-19(27)15-20(28)13-17/h3-13,15-16,25H,14H2,1-2H3,(H,29,32)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552037
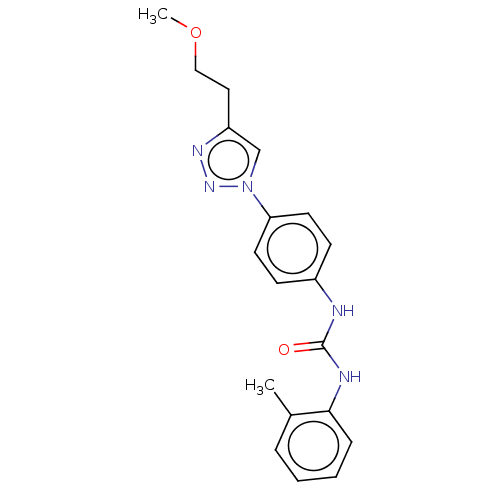
(CHEMBL4784714) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552023
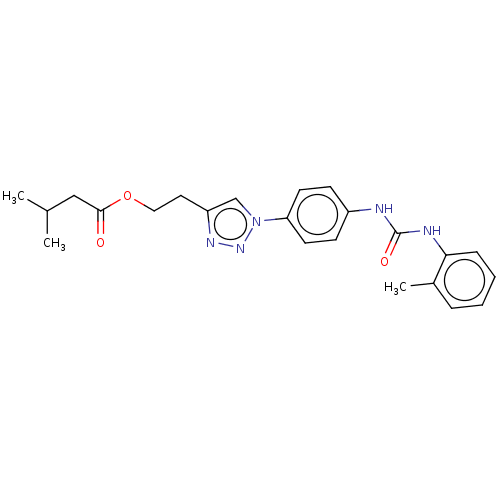
(CHEMBL4799050)Show SMILES CC(C)CC(=O)OCCc1cn(nn1)-c1ccc(NC(=O)Nc2ccccc2C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061218

((E)-3-(2,4-Dichloro-phenyl)-N-((R)-1-methyl-2-oxo-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)\C=C\c2ccc(Cl)cc2Cl)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C25H19Cl2N3O2/c1-30-21-10-6-5-9-19(21)23(17-7-3-2-4-8-17)29-24(25(30)32)28-22(31)14-12-16-11-13-18(26)15-20(16)27/h2-15,24H,1H3,(H,28,31)/b14-12+/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552038
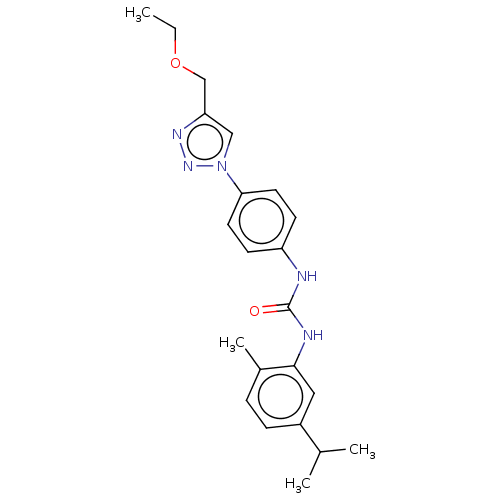
(CHEMBL4790390)Show SMILES CCOCc1cn(nn1)-c1ccc(NC(=O)Nc2cc(ccc2C)C(C)C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552039
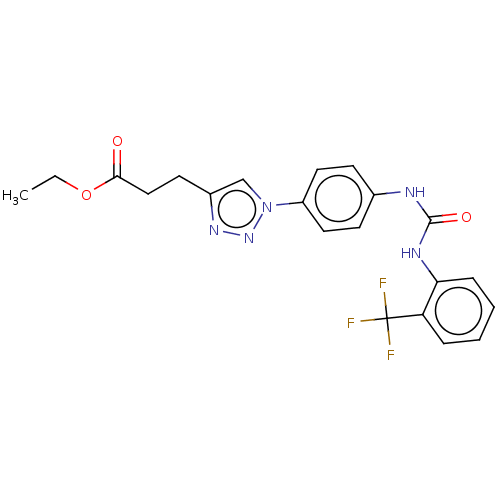
(CHEMBL4795681)Show SMILES CCOC(=O)CCc1cn(nn1)-c1ccc(NC(=O)Nc2ccccc2C(F)(F)F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061213
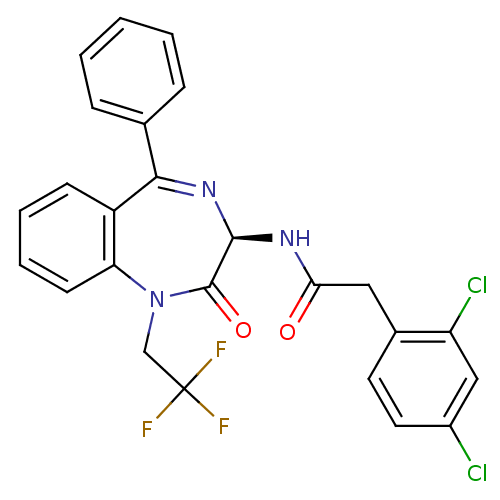
(2-(2,4-Dichloro-phenyl)-N-[(R)-2-oxo-5-phenyl-1-(2...)Show SMILES FC(F)(F)CN1c2ccccc2C(=N[C@@H](NC(=O)Cc2ccc(Cl)cc2Cl)C1=O)c1ccccc1 |c:13| Show InChI InChI=1S/C25H18Cl2F3N3O2/c26-17-11-10-16(19(27)13-17)12-21(34)31-23-24(35)33(14-25(28,29)30)20-9-5-4-8-18(20)22(32-23)15-6-2-1-3-7-15/h1-11,13,23H,12,14H2,(H,31,34)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552036
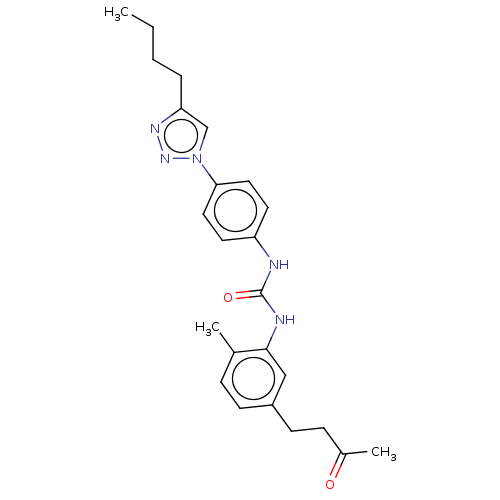
(CHEMBL4761720)Show SMILES CCCCc1cn(nn1)-c1ccc(NC(=O)Nc2cc(CCC(C)=O)ccc2C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552027
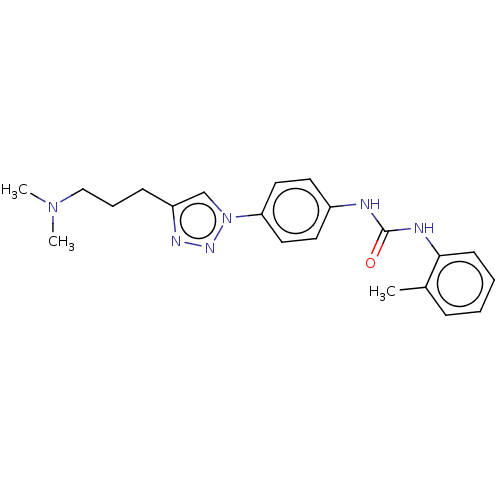
(CHEMBL4742051)Show SMILES CN(C)CCCc1cn(nn1)-c1ccc(NC(=O)Nc2ccccc2C)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061221
(2-(3,5-Dichloro-phenyl)-N-[(R)-2-oxo-5-phenyl-1-(2...)Show SMILES FC(F)(F)CN1c2ccccc2C(=N[C@@H](NC(=O)Cc2cc(Cl)cc(Cl)c2)C1=O)c1ccccc1 |c:13| Show InChI InChI=1S/C25H18Cl2F3N3O2/c26-17-10-15(11-18(27)13-17)12-21(34)31-23-24(35)33(14-25(28,29)30)20-9-5-4-8-19(20)22(32-23)16-6-2-1-3-7-16/h1-11,13,23H,12,14H2,(H,31,34)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50006878

((R)-1-(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2ccc(C)cc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-12-14-18(15-13-16)25-24(30)27-22-23(29)28(2)20-11-7-6-10-19(20)21(26-22)17-8-4-3-5-9-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552021
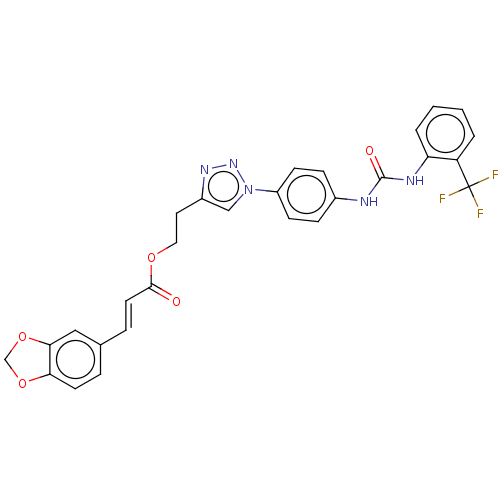
(CHEMBL4764209)Show SMILES FC(F)(F)c1ccccc1NC(=O)Nc1ccc(cc1)-n1cc(CCOC(=O)\C=C\c2ccc3OCOc3c2)nn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061209

(2-(2,4-Bis-trifluoromethyl-phenyl)-N-[(R)-2-oxo-5-...)Show SMILES FC(F)(F)CN1c2ccccc2C(=N[C@@H](NC(=O)Cc2ccc(cc2C(F)(F)F)C(F)(F)F)C1=O)c1ccccc1 |c:13| Show InChI InChI=1S/C27H18F9N3O2/c28-25(29,30)14-39-20-9-5-4-8-18(20)22(15-6-2-1-3-7-15)38-23(24(39)41)37-21(40)12-16-10-11-17(26(31,32)33)13-19(16)27(34,35)36/h1-11,13,23H,12,14H2,(H,37,40)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061224

(2-Amino-N-((R)-1-methyl-2-oxo-5-phenyl-2,3-dihydro...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)C(N)Cc2ccccc2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C25H24N4O2/c1-29-21-15-9-8-14-19(21)22(18-12-6-3-7-13-18)27-23(25(29)31)28-24(30)20(26)16-17-10-4-2-5-11-17/h2-15,20,23H,16,26H2,1H3,(H,28,30)/t20?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50061220

(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV. |
J Med Chem 40: 3865-8 (1998)
Article DOI: 10.1021/jm970517u
BindingDB Entry DOI: 10.7270/Q2Q81C6H |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552025
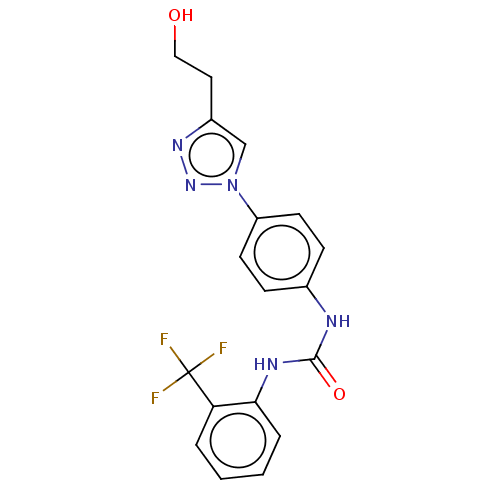
(CHEMBL4786545)Show SMILES OCCc1cn(nn1)-c1ccc(NC(=O)Nc2ccccc2C(F)(F)F)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552026
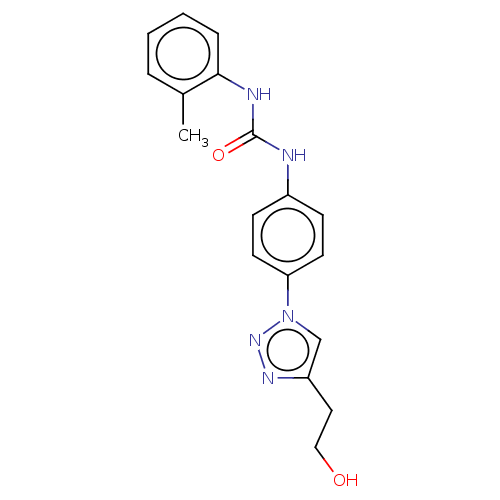
(CHEMBL4783844) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552028
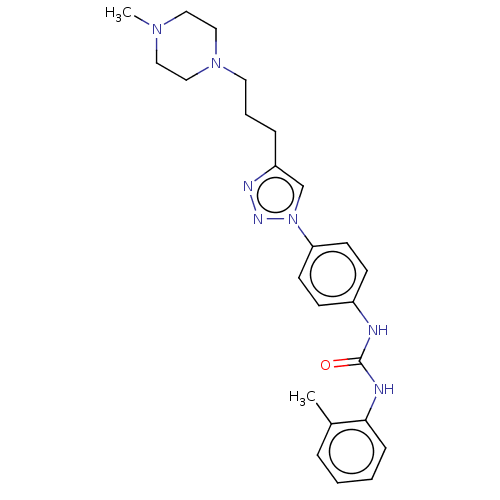
(CHEMBL4764635)Show SMILES CN1CCN(CCCc2cn(nn2)-c2ccc(NC(=O)Nc3ccccc3C)cc2)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552029
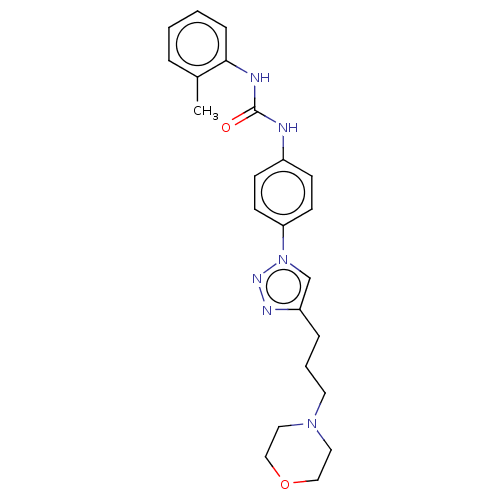
(CHEMBL4758562)Show SMILES Cc1ccccc1NC(=O)Nc1ccc(cc1)-n1cc(CCCN2CCOCC2)nn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
ATP-dependent RNA helicase DDX3X
(Homo sapiens (Human)) | BDBM50552030

(CHEMBL4743223)Show SMILES Cc1ccccc1NC(=O)Nc1ccc(cc1)-n1cc(CCN2CCOCC2)nn1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DDX3X using dsRNA as substrate measured every 30 sec for 40 mins by FRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112319
BindingDB Entry DOI: 10.7270/Q23J3HMG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50376592

(CHEMBL406931)Show SMILES COc1ccc2nccc(C(=O)CC[C@@H]3CCN(CC#Cc4cc(F)cc(F)c4F)C[C@@H]3C(O)=O)c2c1 Show InChI InChI=1S/C28H25F3N2O4/c1-37-20-5-6-25-22(15-20)21(8-10-32-25)26(34)7-4-17-9-12-33(16-23(17)28(35)36)11-2-3-18-13-19(29)14-24(30)27(18)31/h5-6,8,10,13-15,17,23H,4,7,9,11-12,16H2,1H3,(H,35,36)/t17-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Activation of human ERG1 isoform 1 expressed in Xenopus laevis oocytes assessed as increase in peak tail current magnitude by two electrode voltage c... |
Proc Natl Acad Sci USA 104: 13827-32 (2007)
Article DOI: 10.1073/pnas.0703934104
BindingDB Entry DOI: 10.7270/Q2GT5P29 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50376592

(CHEMBL406931)Show SMILES COc1ccc2nccc(C(=O)CC[C@@H]3CCN(CC#Cc4cc(F)cc(F)c4F)C[C@@H]3C(O)=O)c2c1 Show InChI InChI=1S/C28H25F3N2O4/c1-37-20-5-6-25-22(15-20)21(8-10-32-25)26(34)7-4-17-9-12-33(16-23(17)28(35)36)11-2-3-18-13-19(29)14-24(30)27(18)31/h5-6,8,10,13-15,17,23H,4,7,9,11-12,16H2,1H3,(H,35,36)/t17-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Activation of human ERG1 isoform 1 expressed in Xenopus laevis oocytes assessed as shifting of voltage dependence of inactivation towards positive po... |
Proc Natl Acad Sci USA 104: 13827-32 (2007)
Article DOI: 10.1073/pnas.0703934104
BindingDB Entry DOI: 10.7270/Q2GT5P29 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data