 Found 166 hits with Last Name = 'sanyal' and Initial = 's'
Found 166 hits with Last Name = 'sanyal' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50405531
(CHEMBL5270713)Show InChI InChI=1S/C20H33NO3/c1-16(2)21-13-19(22)15-24-20-9-7-17(8-10-20)11-12-23-14-18-5-3-4-6-18/h7-10,16,18-19,21-22H,3-6,11-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human trypsin and the control activity being 7.3 umol/min/mg |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552941

(CHEMBL4764710)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CCC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552934

(CHEMBL4744727)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552931

(CHEMBL4755227)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552948

(CHEMBL4779920)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccncn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552950

(CHEMBL4783395)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552940

(CHEMBL4749009)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552930
(CHEMBL4744926)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCOc2c1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552937

(CHEMBL4788473)Show SMILES CCOC(=O)N1CCCc2nc(ccc12)[C@@H](C)NC(=O)c1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552949

(CHEMBL4779248)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(C)n1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552927

(CHEMBL4799004)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCCc2c1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552929
(CHEMBL4780616)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCC(F)(F)c2c1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552932

(CHEMBL4777831)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1cc2CCCN(C(=O)c3cccc(Cl)c3)c2cn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552926
(CHEMBL4791214)Show SMILES Clc1ccc(cc1)C(=O)NCc1ccc2N(CCCc2c1)C(=O)c1cccc(Cl)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538500
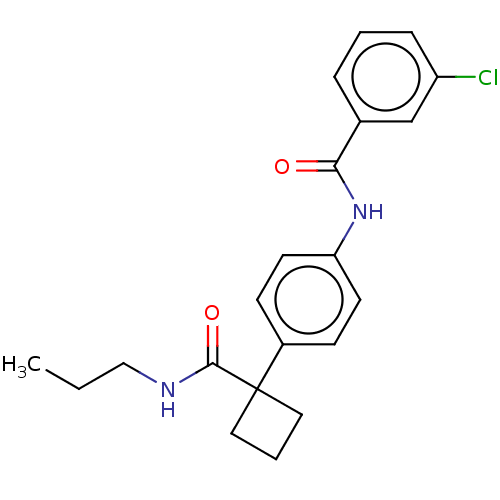
(CHEMBL4636709)Show SMILES CCCNC(=O)C1(CCC1)c1ccc(NC(=O)c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C21H23ClN2O2/c1-2-13-23-20(26)21(11-4-12-21)16-7-9-18(10-8-16)24-19(25)15-5-3-6-17(22)14-15/h3,5-10,14H,2,4,11-13H2,1H3,(H,23,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552939
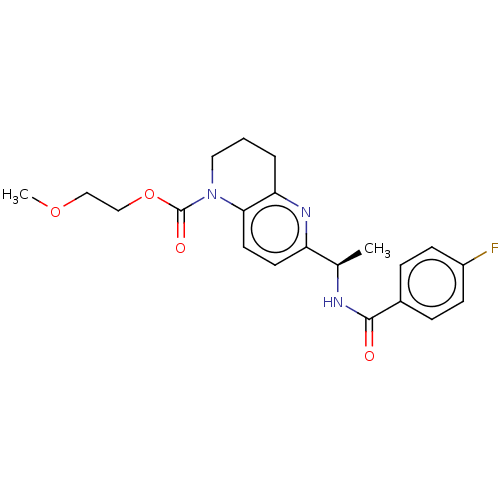
(CHEMBL4789491)Show SMILES COCCOC(=O)N1CCCc2nc(ccc12)[C@@H](C)NC(=O)c1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552951
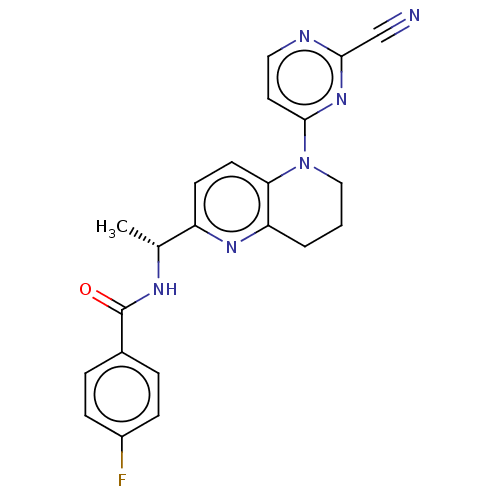
(CHEMBL4764265)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(n1)C#N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552925
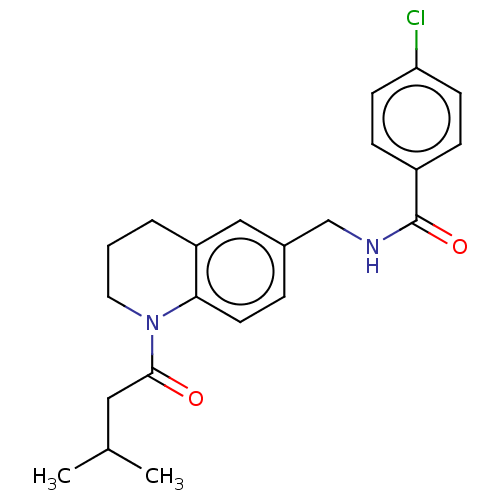
(CHEMBL4748177)Show SMILES CC(C)CC(=O)N1CCCc2cc(CNC(=O)c3ccc(Cl)cc3)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552942
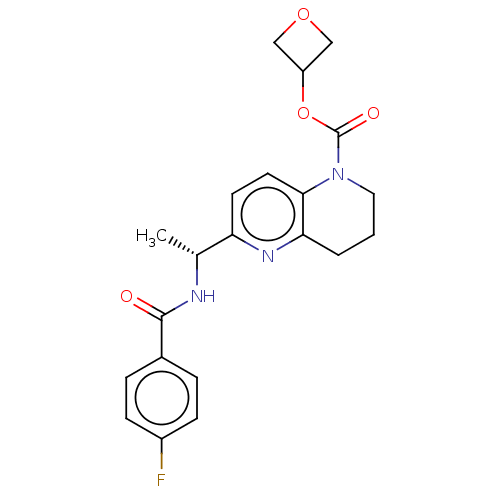
(CHEMBL4757898)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1COC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552935
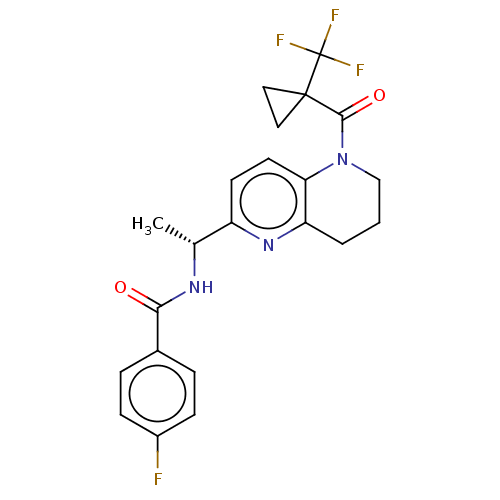
(CHEMBL4762148)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)C1(CC1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552936
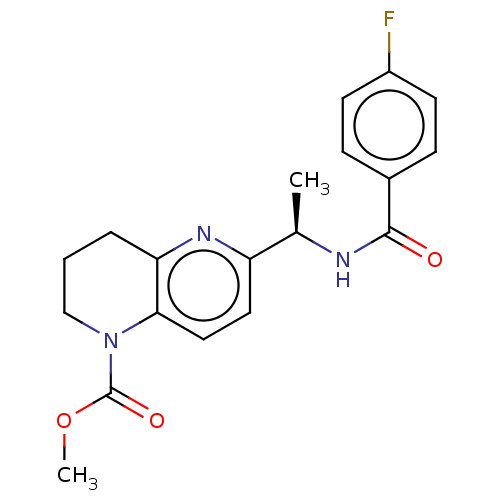
(CHEMBL4747995)Show SMILES COC(=O)N1CCCc2nc(ccc12)[C@@H](C)NC(=O)c1ccc(F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552947

(CHEMBL4750629)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ncccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552928
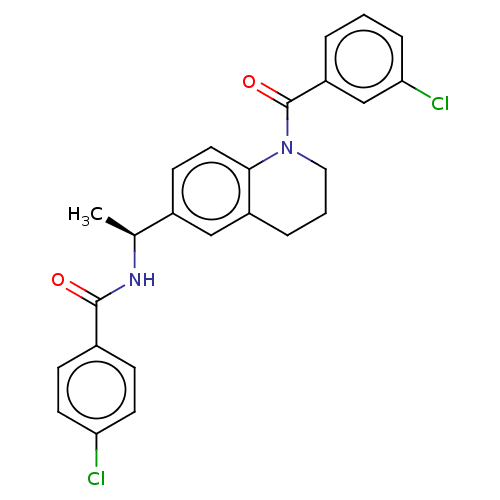
(CHEMBL4788204)Show SMILES C[C@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCCc2c1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552934

(CHEMBL4744727)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in LPS and IFN-gamma-stimulated human Whole blood assessed as reduction in kynurenine production using isotope-labeled tryptophan ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50405536
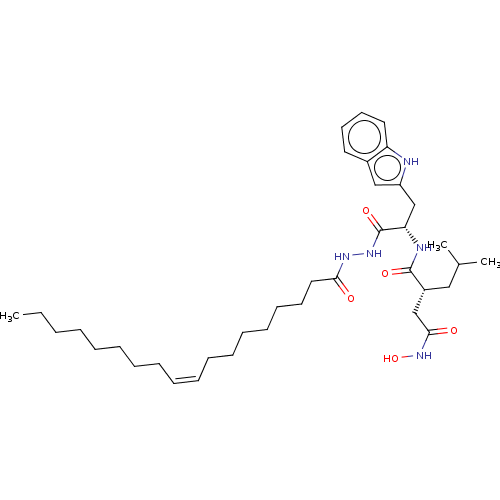
(CHEMBL5283314)Show InChI InChI=1S/C17H27NO3/c1-13(2)18-9-16(19)12-21-17-7-5-15(6-8-17)11-20-10-14-3-4-14/h5-8,13-14,16,18-19H,3-4,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acrosin and control activity being 11.3 umol/min/mg |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552946
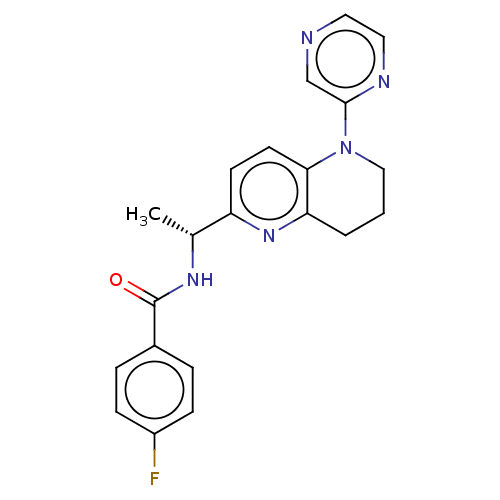
(CHEMBL4799199)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1cnccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50405532
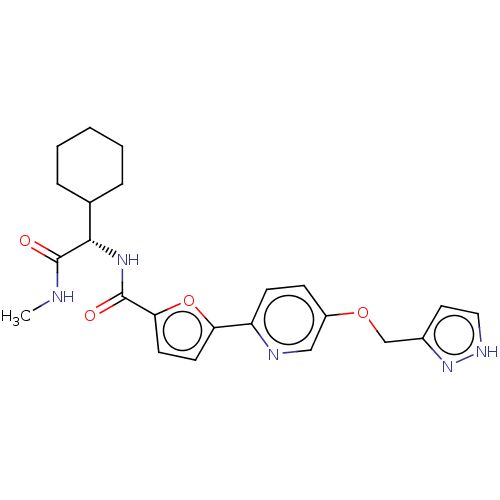
(CHEMBL584683)Show InChI InChI=1S/C19H29NO3/c21-18(12-20-17-6-7-17)14-23-19-8-4-15(5-9-19)10-11-22-13-16-2-1-3-16/h4-5,8-9,16-18,20-21H,1-3,6-7,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human trypsin and the control activity being 7.3 umol/min/mg |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552950

(CHEMBL4783395)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(n1)C(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in LPS and IFN-gamma-stimulated human Whole blood assessed as reduction in kynurenine production using isotope-labeled tryptophan ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552945
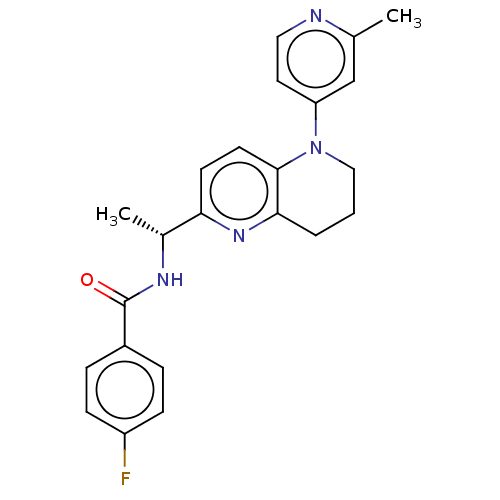
(CHEMBL4747474)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(C)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552924
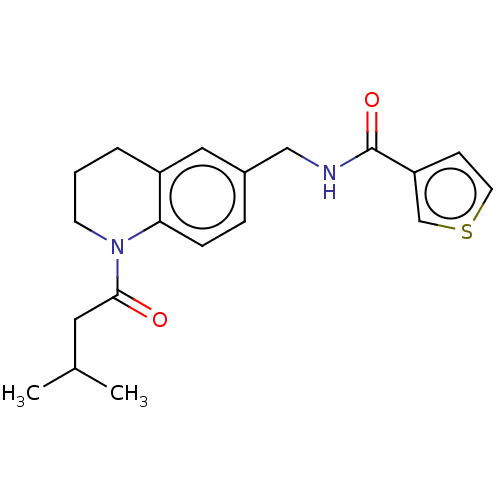
(CHEMBL4788767) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552940

(CHEMBL4749009)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in LPS and IFN-gamma-stimulated human Whole blood assessed as reduction in kynurenine production using isotope-labeled tryptophan ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50405534
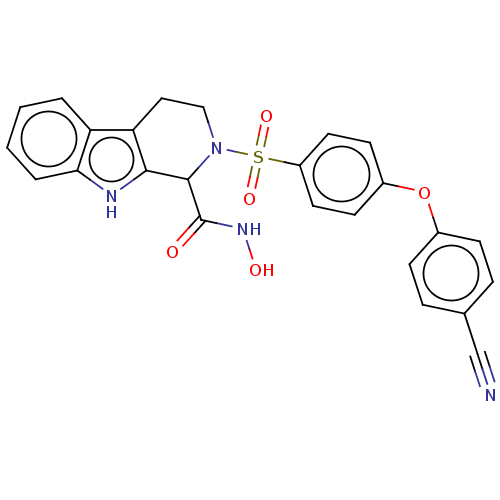
(CHEMBL5271490)Show SMILES COc1ccc(CCNCC(O)COc2ccc(CCOCC3CCC3)cc2)cc1OC Show InChI InChI=1S/C26H37NO5/c1-29-25-11-8-21(16-26(25)30-2)12-14-27-17-23(28)19-32-24-9-6-20(7-10-24)13-15-31-18-22-4-3-5-22/h6-11,16,22-23,27-28H,3-5,12-15,17-19H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human trypsin and the control activity being 7.3 umol/min/mg |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50552948

(CHEMBL4779920)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccncn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552938
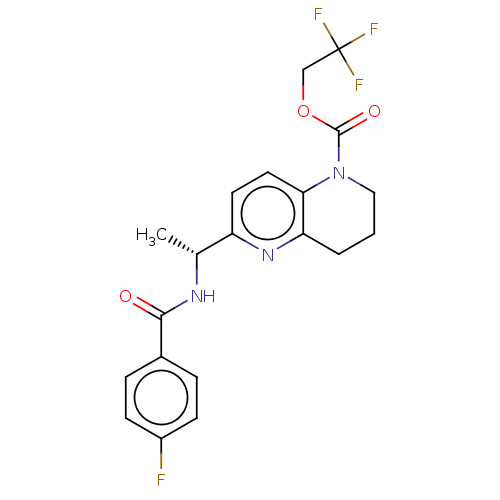
(CHEMBL4761407)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OCC(F)(F)F |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 281 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552944
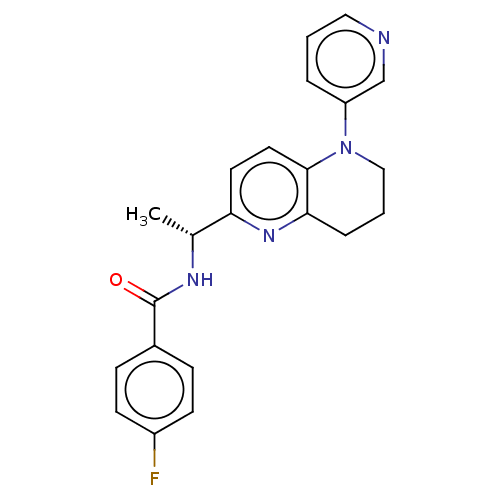
(CHEMBL4757764)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1cccnc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50405537
(CHEMBL5269640)Show SMILES CC(CCc1ccc(F)cc1)NCC(O)COc1ccc(CCOCC2CCC2)cc1 Show InChI InChI=1S/C26H36FNO3/c1-20(5-6-21-7-11-24(27)12-8-21)28-17-25(29)19-31-26-13-9-22(10-14-26)15-16-30-18-23-3-2-4-23/h7-14,20,23,25,28-29H,2-6,15-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 376 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human trypsin and the control activity being 7.3 umol/min/mg |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50405547
(CHEMBL5291044)Show InChI InChI=1S/C20H20NO/c1-16-15-21(13-14-22)12-11-17(16)9-10-19-7-4-6-18-5-2-3-8-20(18)19/h2-12,15,22H,13-14H2,1H3/q+1/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human trypsin and the control activity being 7.3 umol/min/mg |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50241504
(3-hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxylic aci...)Show SMILES Cc1cc(=O)c(O)c(o1)C(=O)NCc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C20H17NO4/c1-13-11-17(22)18(23)19(25-13)20(24)21-12-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-11,23H,12H2,1H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acrosin and control activity being 11.3 umol/min/mg |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50552945

(CHEMBL4747474)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccnc(C)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [35S]-MK499 binding to human ERG expressed in HEK293 cells incubated for 2 hrs by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50552927

(CHEMBL4799004)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCCc2c1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [35S]-MK499 binding to human ERG expressed in HEK293 cells incubated for 2 hrs by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50552928

(CHEMBL4788204)Show SMILES C[C@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCCc2c1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [35S]-MK499 binding to human ERG expressed in HEK293 cells incubated for 2 hrs by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552933

(CHEMBL4777112)Show SMILES CC(C)CC(=O)N1CCCc2cc(CNC(=O)c3ccc(Cl)cc3)cnc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552943
(CHEMBL4756334)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)c1ccccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50250904
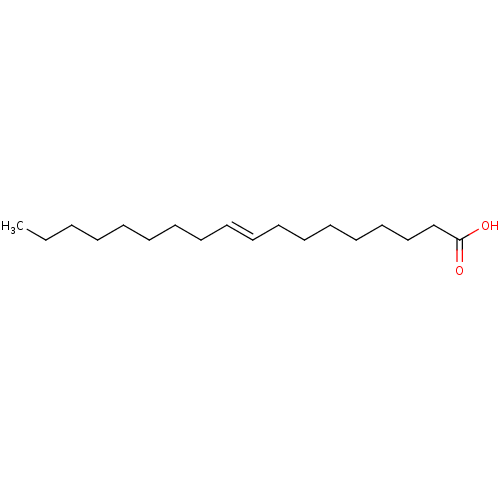
(CHEMBL460657 | Elaidinsaeure | elaidic acid | tran...)Show InChI InChI=1S/C18H34O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h9-10H,2-8,11-17H2,1H3,(H,19,20)/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human trypsin and the control activity being 7.3 umol/min/mg |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50552924

(CHEMBL4788767) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in LPS and IFN-gamma-stimulated human Whole blood assessed as reduction in kynurenine production using isotope-labeled tryptophan ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50405533
(CHEMBL5282693)Show InChI InChI=1S/C19H31NO5S/c1-15(2)20-12-17(21)13-25-18-7-5-16(6-8-18)9-11-26(22,23)14-19-4-3-10-24-19/h5-8,15,17,19-21H,3-4,9-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acrosin and control activity being 11.3 umol/min/mg |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50552940

(CHEMBL4749009)Show SMILES C[C@@H](NC(=O)c1ccc(F)cc1)c1ccc2N(CCCc2n1)C(=O)OC1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50552930

(CHEMBL4744926)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCOc2c1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [35S]-MK499 binding to human ERG expressed in HEK293 cells incubated for 2 hrs by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50552931

(CHEMBL4755227)Show SMILES C[C@@H](NC(=O)c1ccc(Cl)cc1)c1ccc2N(CCCc2n1)C(=O)c1cccc(Cl)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [35S]-MK499 binding to human ERG expressed in HEK293 cells incubated for 2 hrs by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00525
BindingDB Entry DOI: 10.7270/Q20G3PS5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82186
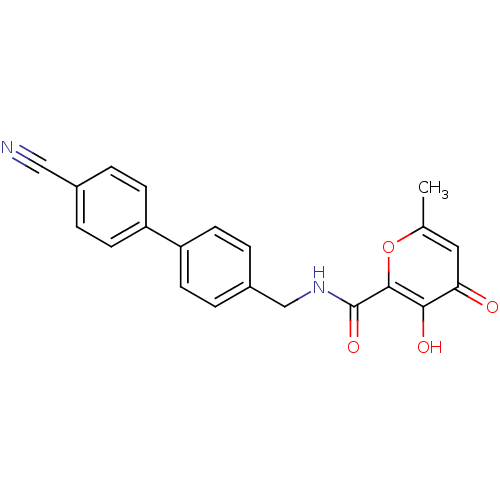
(AM-5)Show SMILES Cc1cc(=O)c(O)c(o1)C(=O)NCc1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C21H16N2O4/c1-13-10-18(24)19(25)20(27-13)21(26)23-12-15-4-8-17(9-5-15)16-6-2-14(11-22)3-7-16/h2-10,25H,12H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human acrosin and control activity being 11.3 umol/min/mg |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data