

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190623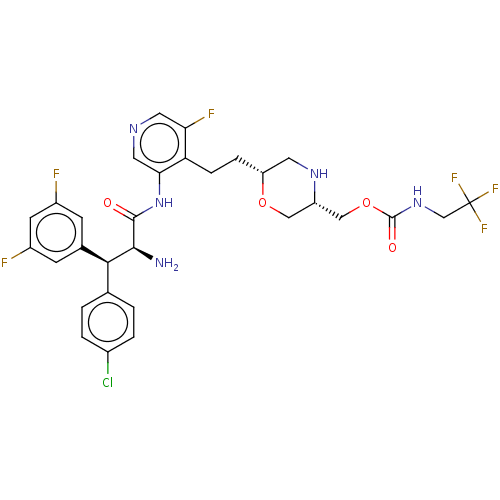 (CHEMBL3828743) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190624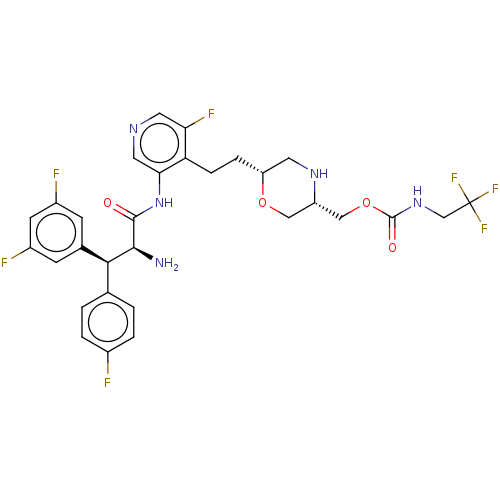 (CHEMBL3828417) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190625 (CHEMBL3828552) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190638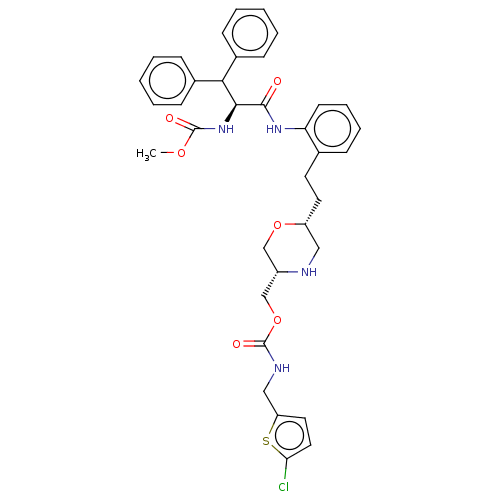 (CHEMBL3828119) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190631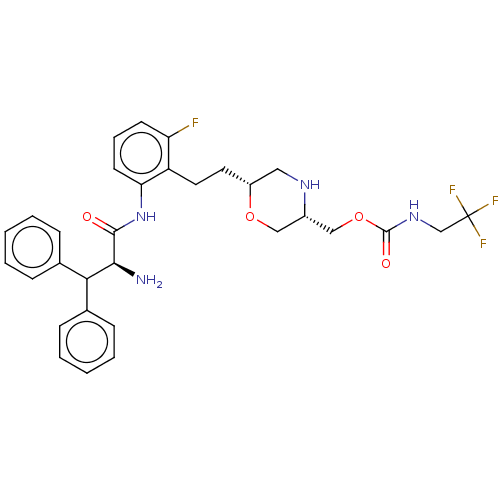 (CHEMBL3828166) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190639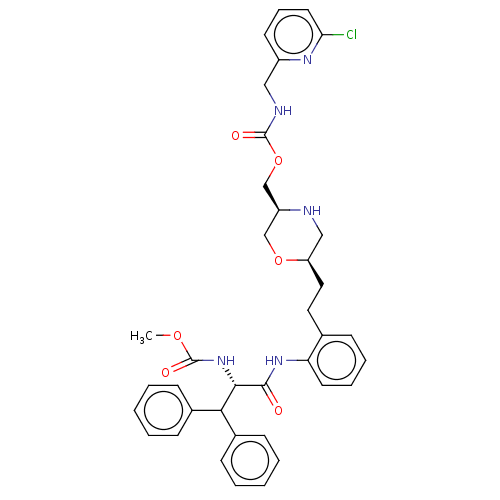 (CHEMBL3827524) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190629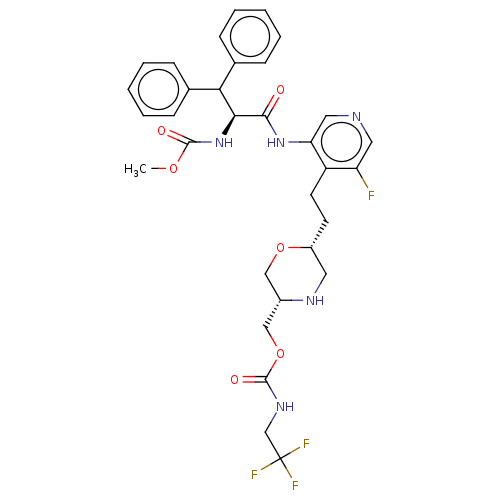 (CHEMBL3828678) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190636 (CHEMBL3828275) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190641 (CHEMBL3827205) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190640 (CHEMBL3828355) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190642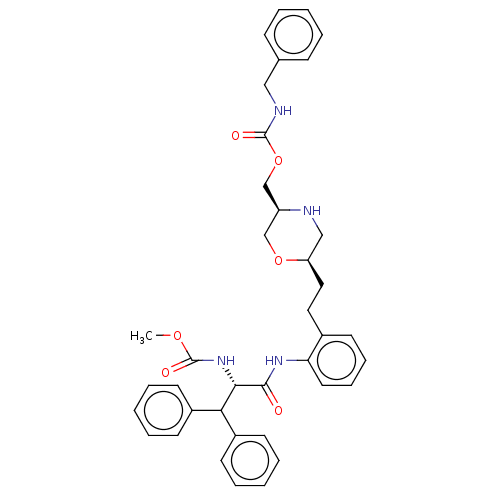 (CHEMBL3827353) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190627 (CHEMBL3827958) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190626 (CHEMBL3827319) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190637 (CHEMBL3827348) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190628 (CHEMBL3827975) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190632 (CHEMBL3827450) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190630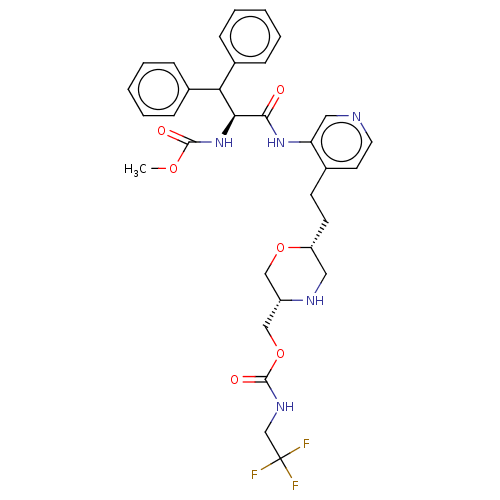 (CHEMBL3827960) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190633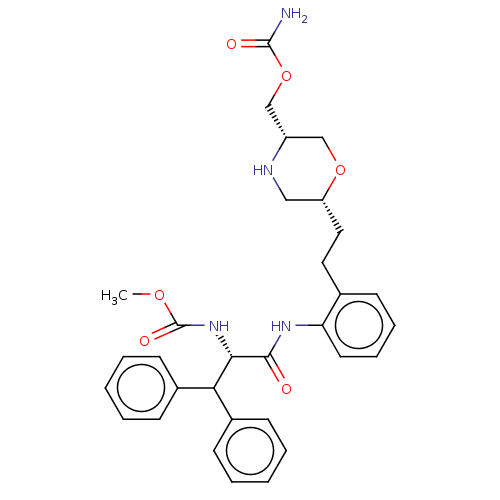 (CHEMBL3827407) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190634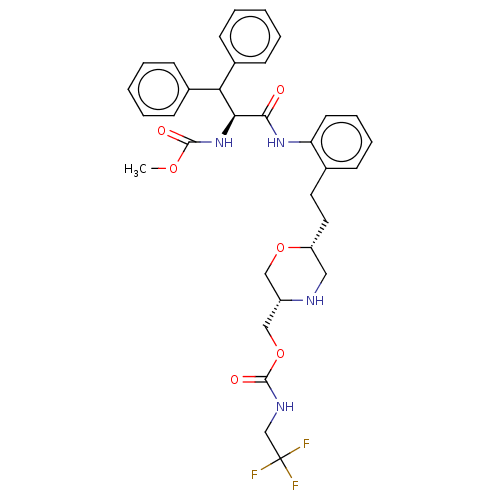 (CHEMBL3828494) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50190635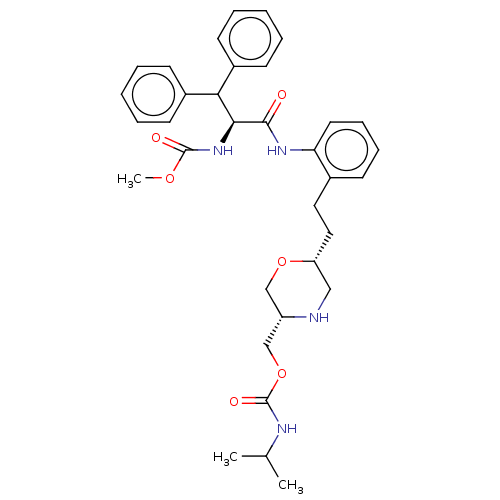 (CHEMBL3827668) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 protease expressed in Escherichia coli assessed as reduction in product formation preincubated for 30 mins followed by a... | ACS Med Chem Lett 7: 702-7 (2016) Article DOI: 10.1021/acsmedchemlett.6b00135 BindingDB Entry DOI: 10.7270/Q28W3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||