

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (RAT) | BDBM50070888 (CHEMBL298725 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010423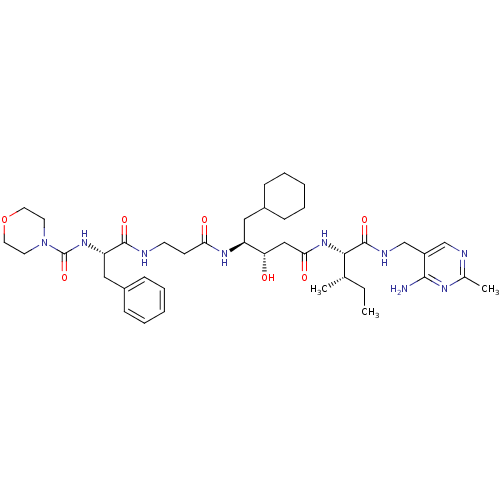 ((Morpholinocarbonyl)-Phe-beta-Ala-ACHPA-Ile N-[(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50069575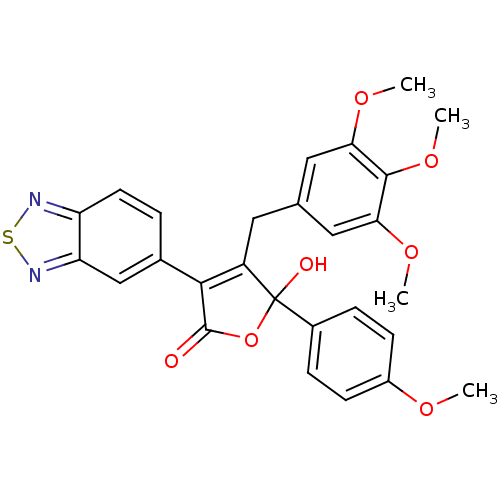 (3-Benzo[1,2,5]thiadiazol-5-yl-5-hydroxy-5-(4-metho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to rat aorta membrane Endothelin A receptor | Bioorg Med Chem Lett 8: 17-22 (1999) BindingDB Entry DOI: 10.7270/Q23B5Z9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070884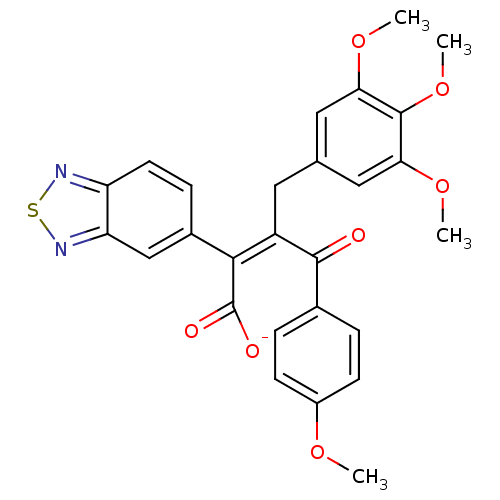 (CHEMBL48907 | Sodium; (Z)-2-benzo[1,2,5]thiadiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010416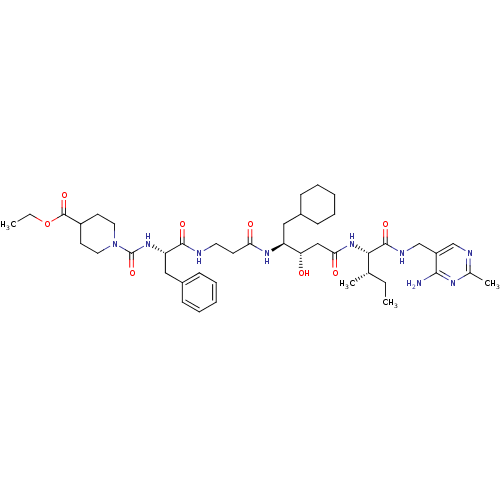 (CHEMBL324692 | [[4-(Ethoxycarbonyl)piperdino]carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50034267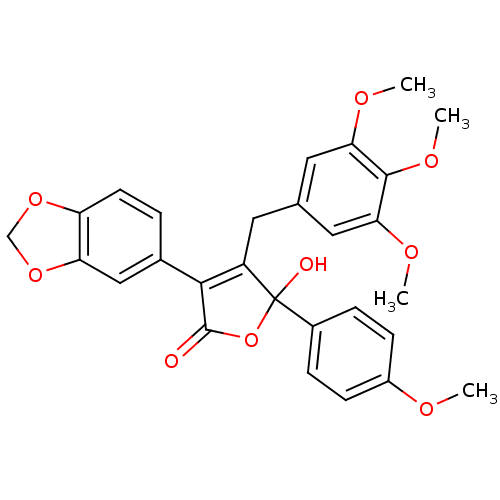 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to rat aorta membrane Endothelin A receptor | Bioorg Med Chem Lett 8: 17-22 (1999) BindingDB Entry DOI: 10.7270/Q23B5Z9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010424 (BOC-Phe-Ala-ACHPA-Ile N-[(4-amino-2-methyl-5-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010419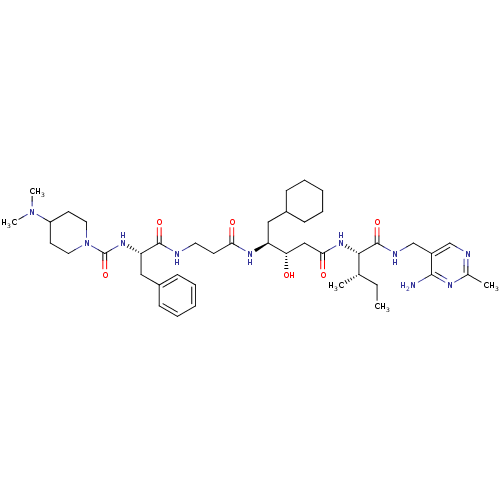 (4-Dimethylamino-piperidine-1-carboxylic acid {1-[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010415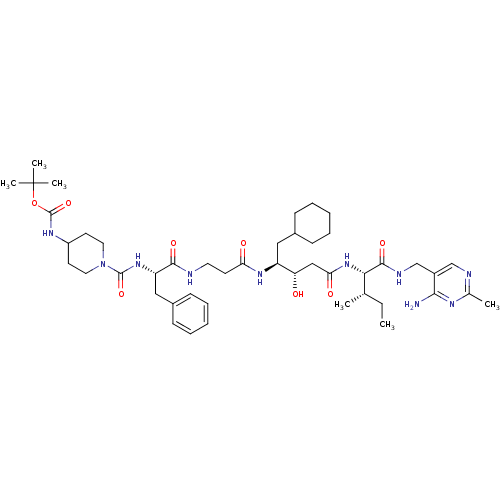 ((1-{1-[2-(3-{1-[(4-Amino-2-methyl-pyrimidin-5-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010408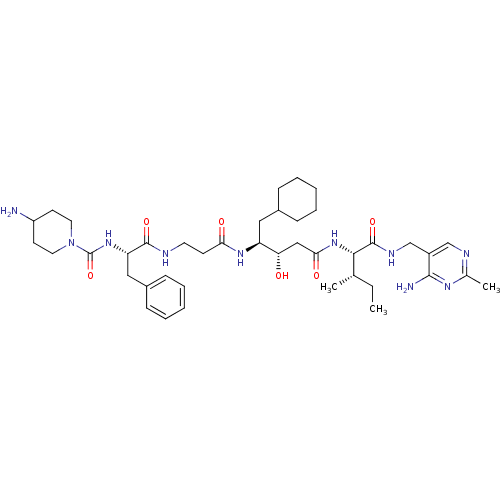 (4-Amino-piperidine-1-carboxylic acid {1-[2-(3-{1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070886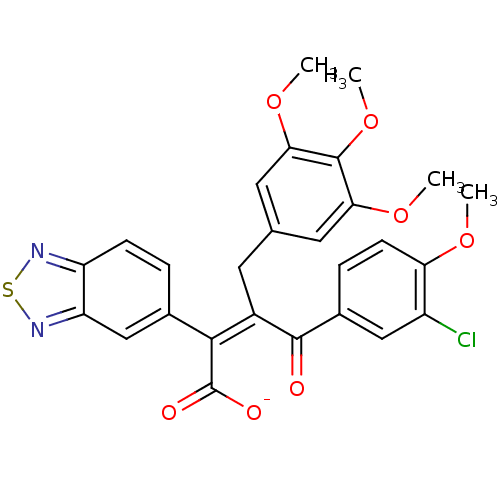 (CHEMBL51234 | Sodium; (Z)-2-benzo[1,2,5]thiadiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010431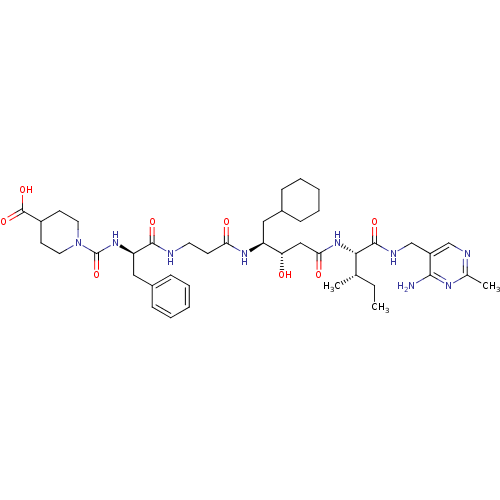 (CHEMBL320726 | [4-(Carboxypiperidino)carbonyl]-Phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010410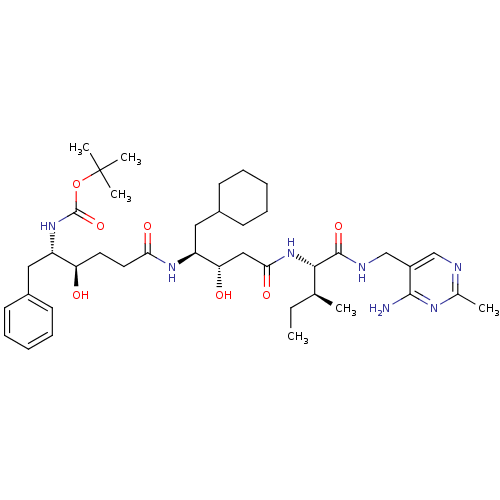 (CHEMBL323006 | [4-(3-{1-[(4-Amino-2-methyl-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50034263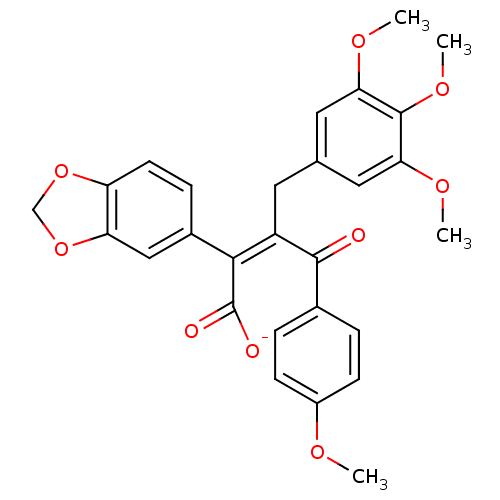 (CHEMBL25438 | PD-156707 | Sodium; (Z)-2-benzo[1,3]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070881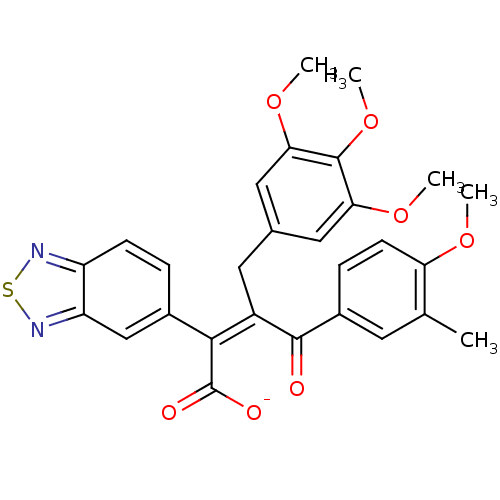 (CHEMBL295483 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50051007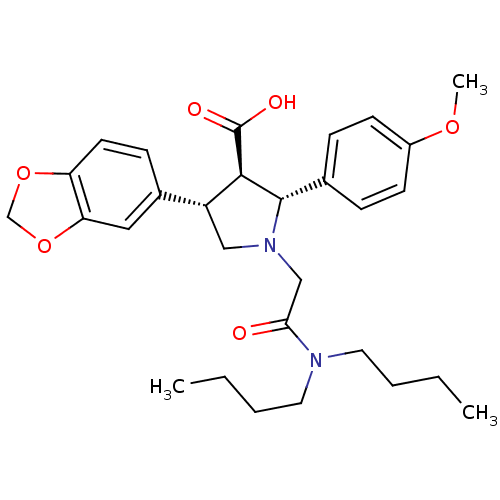 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010421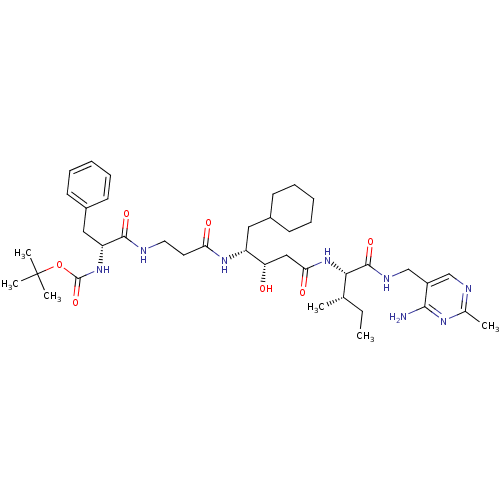 (BOC-Phe-beta-Ala-ACHPA-Ile N-[(4-amino-2-methyl-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17941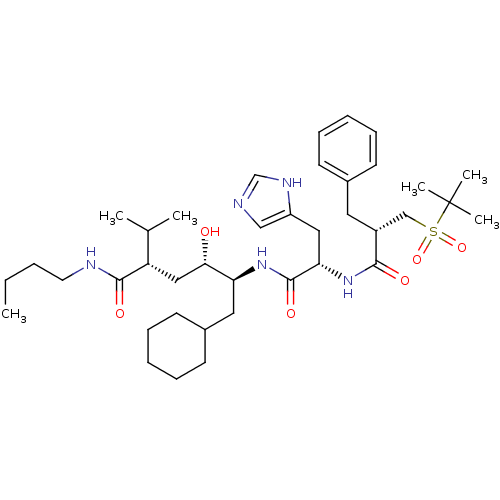 ((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibition of human renin. | J Med Chem 37: 486-97 (1994) BindingDB Entry DOI: 10.7270/Q2MW2HTW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50368156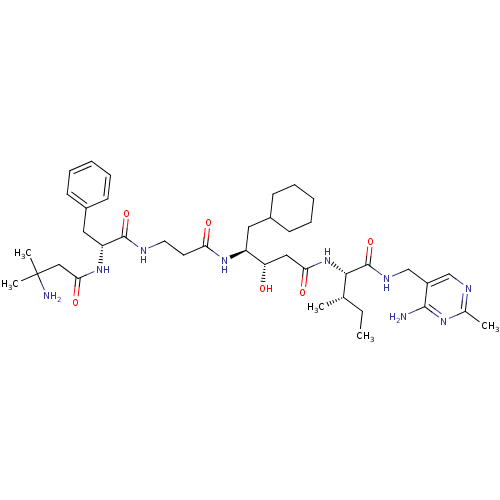 (CHEMBL1203096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010426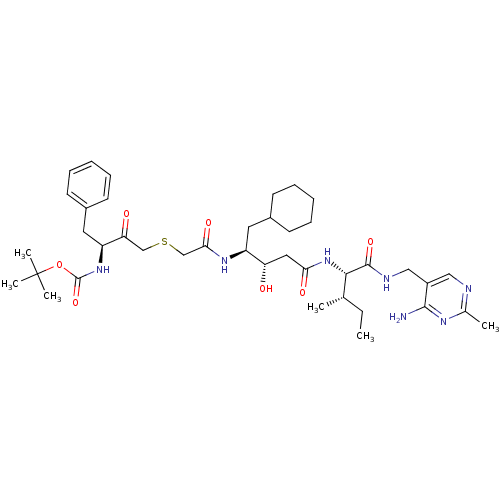 (CHEMBL324588 | {3-[(3-{1-[(4-Amino-2-methyl-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010433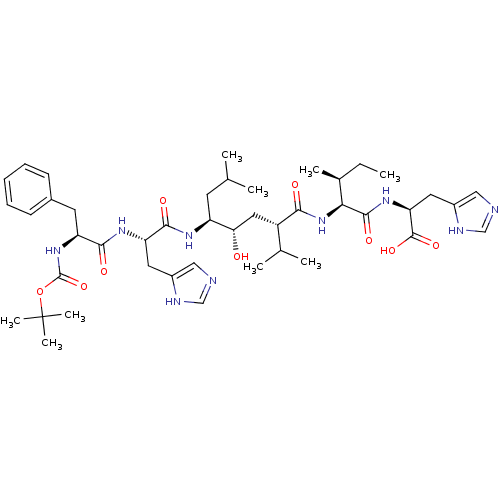 (BOC-Phe-His-Leu-psi-[CHOHCH2]Val-Ile-His-OH | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50040384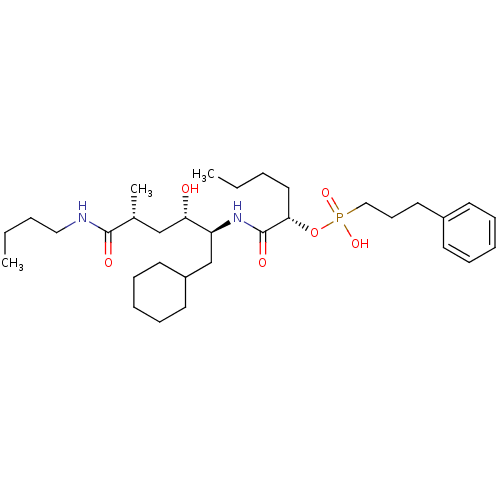 ((3-Phenyl-propyl)-phosphonic acid mono-[(S)-1-((1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibition of human renin. | J Med Chem 37: 486-97 (1994) BindingDB Entry DOI: 10.7270/Q2MW2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010427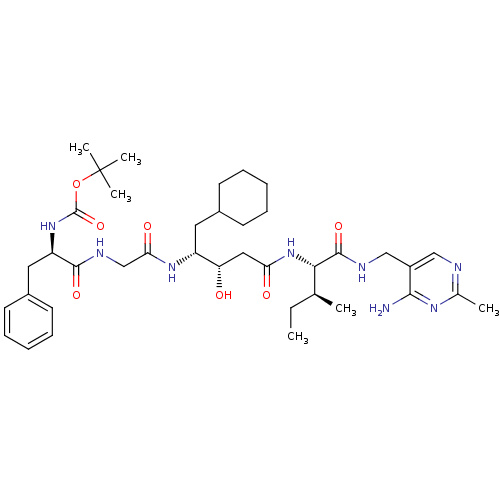 (BOC-Phe-Gly-ACHPA-Ile N-[(4-amino-2-methyl-5-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002969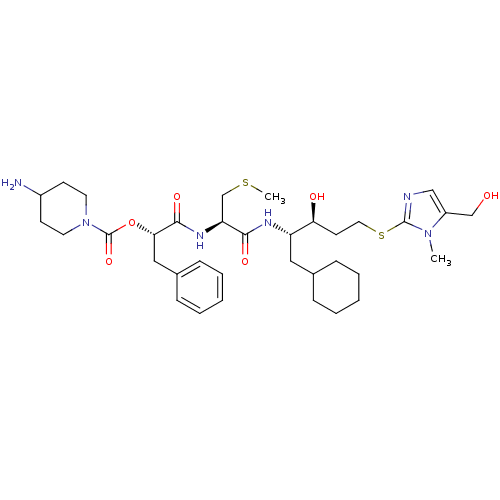 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50040390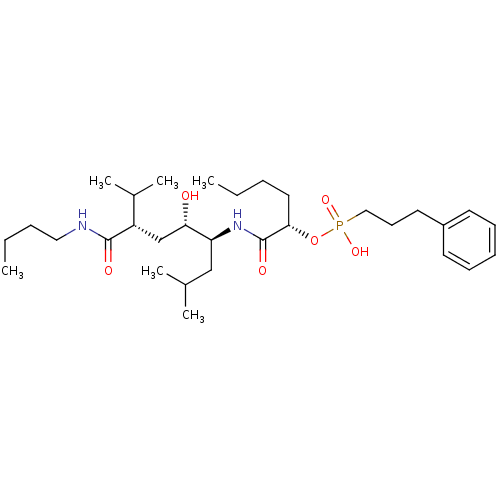 ((3-Phenyl-propyl)-phosphonic acid mono-[(S)-1-((1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibition of human renin. | J Med Chem 37: 486-97 (1994) BindingDB Entry DOI: 10.7270/Q2MW2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010432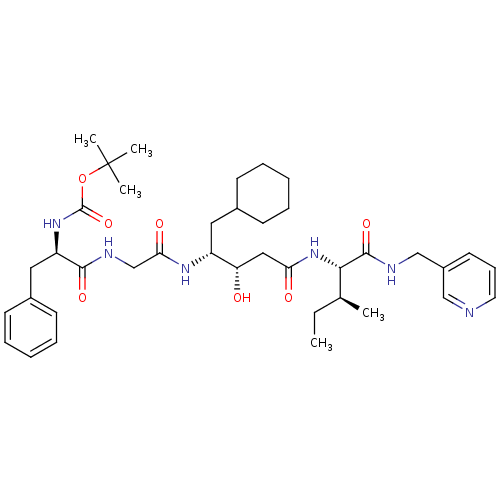 (BOC-Phe-Gly-ACHPA-Ile N-(3-Pyridylmethyl)amide | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010429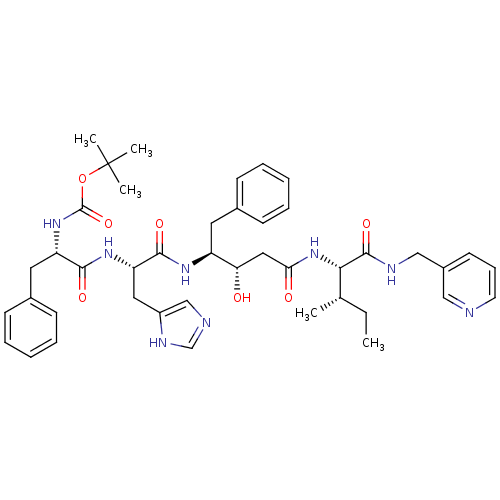 (CHEMBL323967 | {1-[1-(1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070882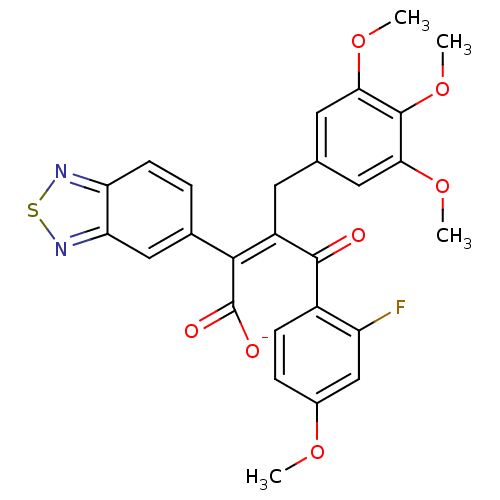 ((Z)-2-Benzo[1,2,5]thiadiazol-5-yl-4-(2-fluoro-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070877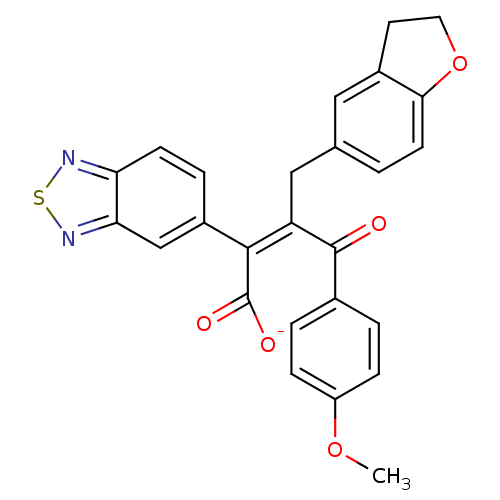 (CHEMBL262000 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070890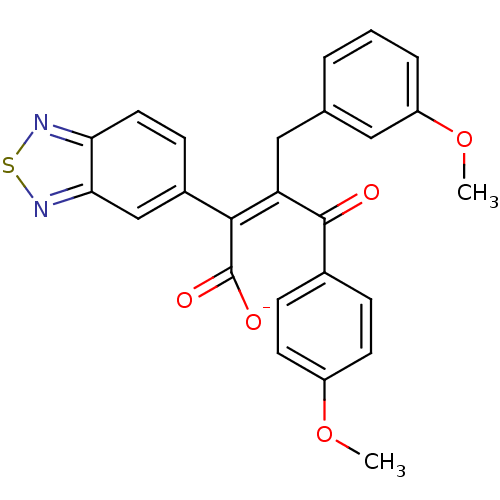 (CHEMBL51890 | Sodium; (Z)-2-benzo[1,2,5]thiadiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070871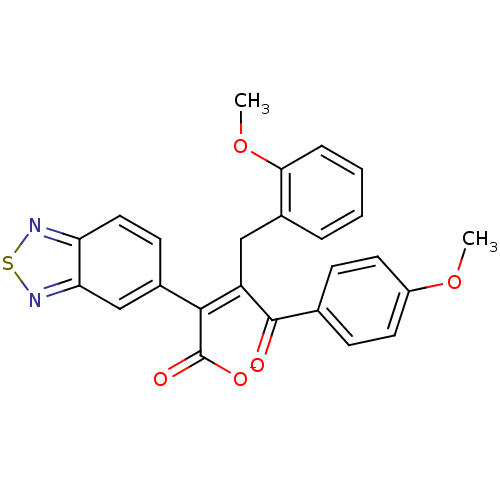 (CHEMBL431160 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50075421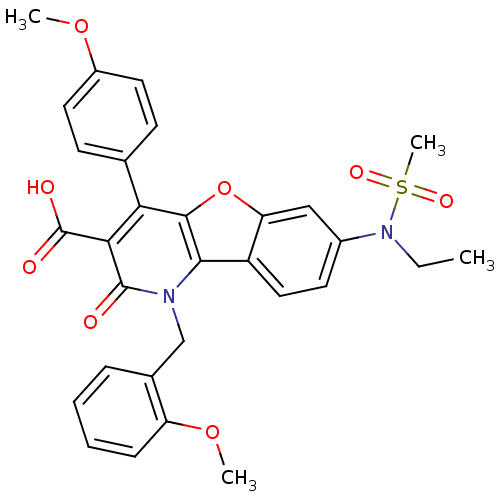 (7-(Ethyl-methanesulfonyl-amino)-1-(2-methoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Ability to displace [125I]-Endothelin-1 from endothelin B receptor in porcine kidney membranes | Bioorg Med Chem Lett 9: 619-22 (1999) BindingDB Entry DOI: 10.7270/Q2DB811P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070873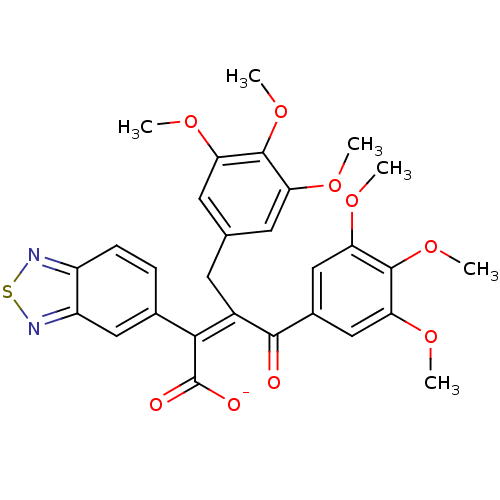 (CHEMBL301577 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50040388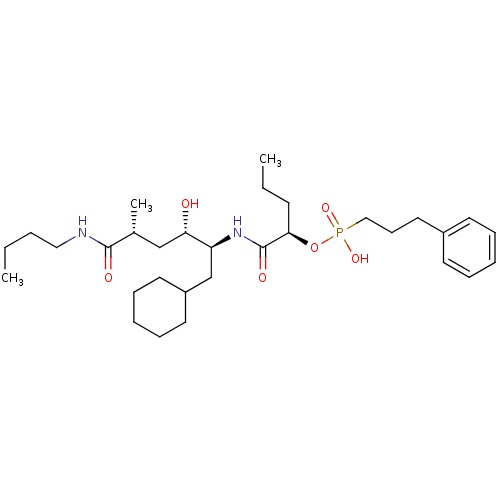 ((3-Phenyl-propyl)-phosphonic acid mono-[(R)-1-((1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibition of human renin. | J Med Chem 37: 486-97 (1994) BindingDB Entry DOI: 10.7270/Q2MW2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010417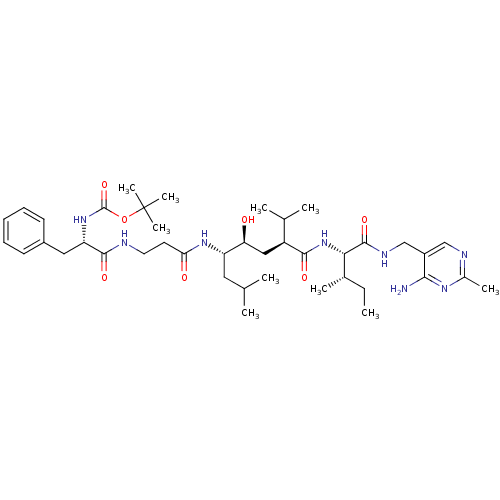 (BOC-Phe-beta-Ala-Leu-psi[CHOHCH2]Val-Ile N-[(4-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50040383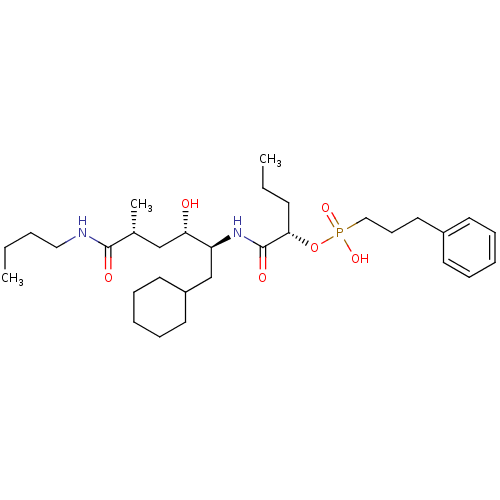 ((3-Phenyl-propyl)-phosphonic acid mono-[(S)-1-((1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibition of human renin. | J Med Chem 37: 486-97 (1994) BindingDB Entry DOI: 10.7270/Q2MW2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010413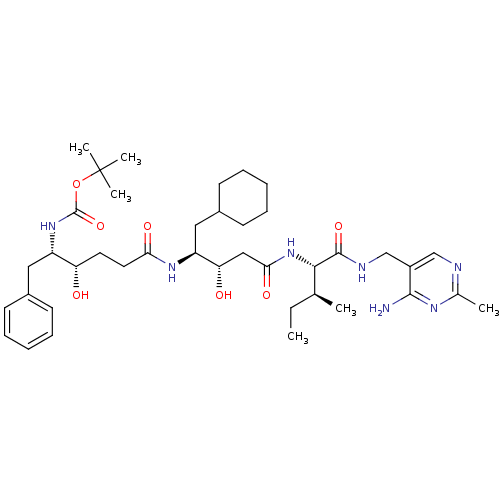 (CHEMBL324624 | [4-(3-{1-[(4-Amino-2-methyl-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50368157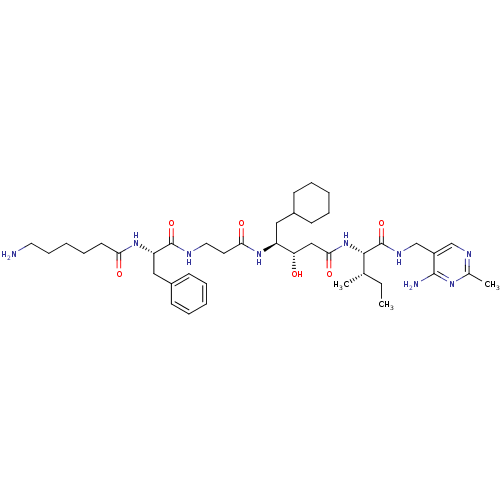 (CHEMBL1203095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070892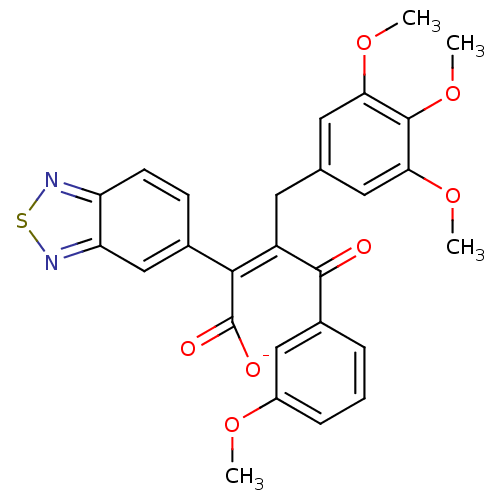 (CHEMBL299572 | Sodium; (Z)-2-benzo[1,2,5]thiadiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50368155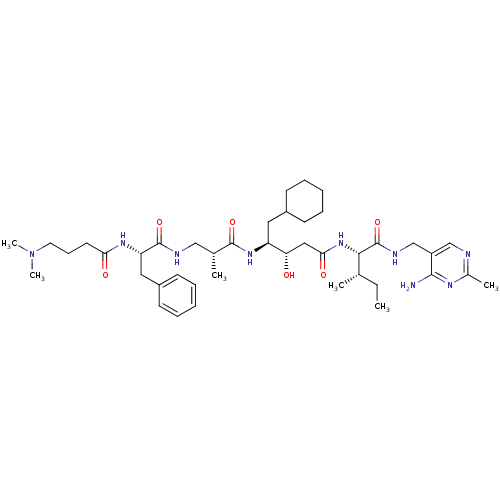 (CHEMBL1203094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50040382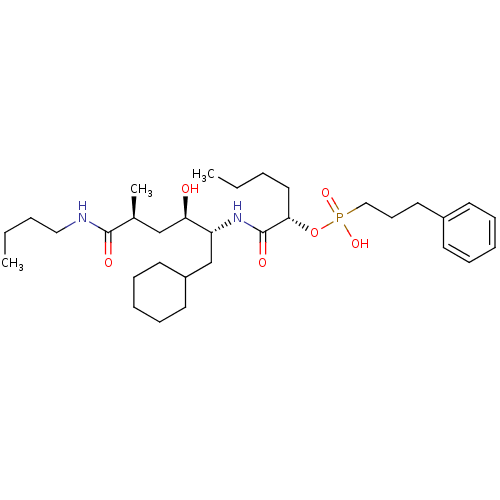 ((3-Phenyl-propyl)-phosphonic acid mono-[(S)-1-((1R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibition of human renin. | J Med Chem 37: 486-97 (1994) BindingDB Entry DOI: 10.7270/Q2MW2HTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50002985 (4-Amino-piperidine-1-carboxylic acid 1-{1-[1-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 5.5 | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro inhibitory activity against human plasma renin at pH 5.5 for suppression of angiotensin I formation. | J Med Chem 35: 3525-36 (1992) BindingDB Entry DOI: 10.7270/Q2DB80S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010428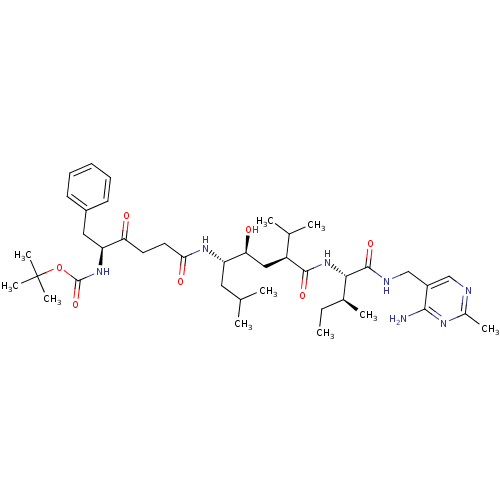 (BOC-Phe-psi[COCH2]Gly-Leu-psi-[CHOHCH2]Val-Ile N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010412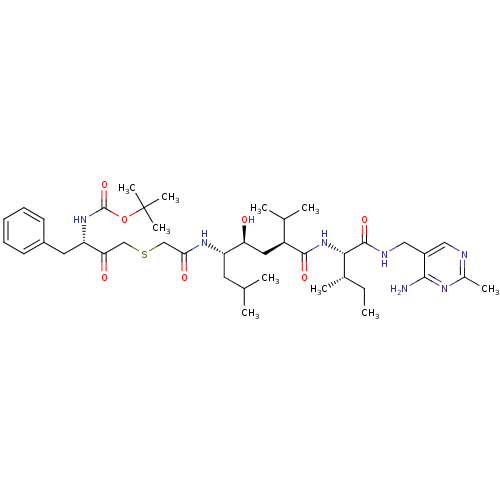 (BOC-Phe-psi[COCH2S]Gly-Leu-psi-[CHOHCH2]Val-Ile N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010425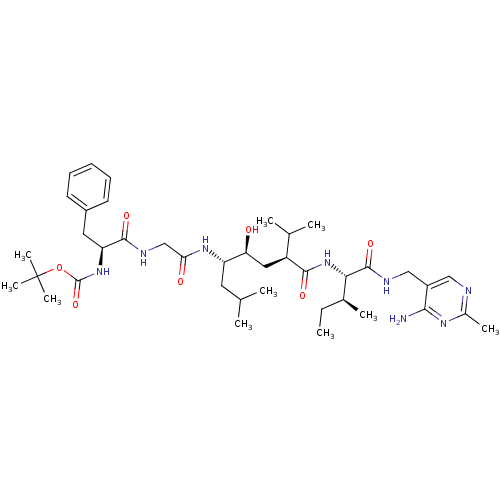 (BOC-Phe-Gly-Leu-psi[CHOHCH2]Gly-Ile N-[(4-amino-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010409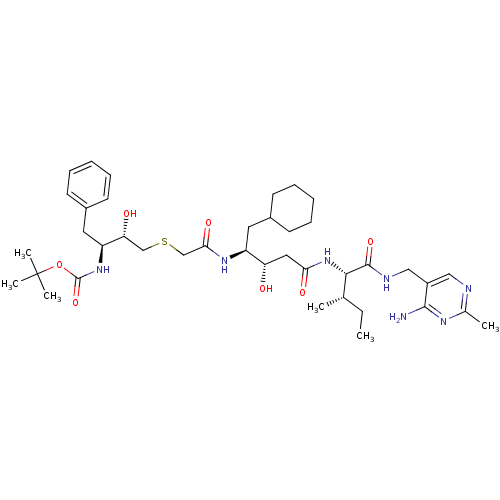 (CHEMBL112091 | {3-[(3-{1-[(4-Amino-2-methyl-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010405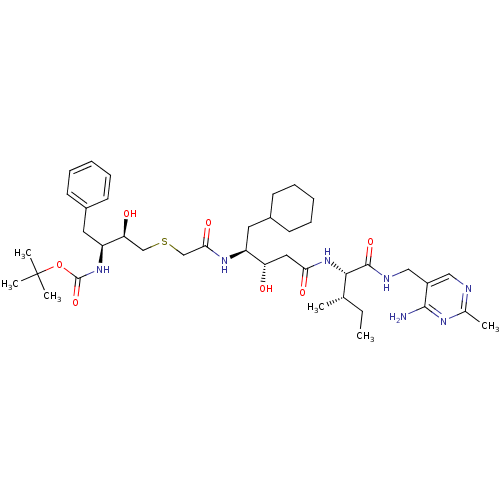 (CHEMBL113593 | {3-[(3-{1-[(4-Amino-2-methyl-pyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50070876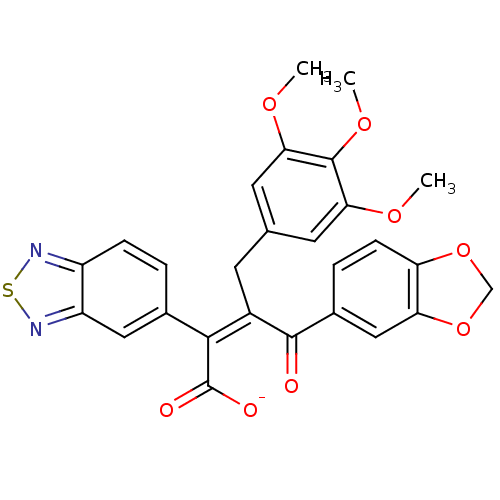 (CHEMBL299328 | Sodium; (Z)-4-benzo[1,3]dioxol-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50057155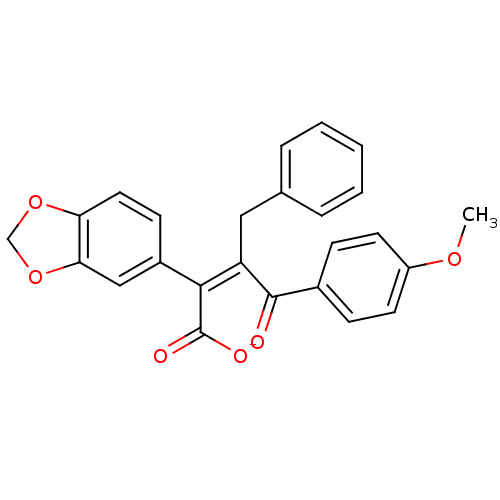 ((Z)-2-Benzo[1,3]dioxol-5-yl-3-benzyl-4-(4-methoxy-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description In vitro ability to inhibit specific [125I]ET1 binding to rat aorta membranes Endothelin A receptor | Bioorg Med Chem Lett 8: 1771-6 (1999) BindingDB Entry DOI: 10.7270/Q2TM7BM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50010406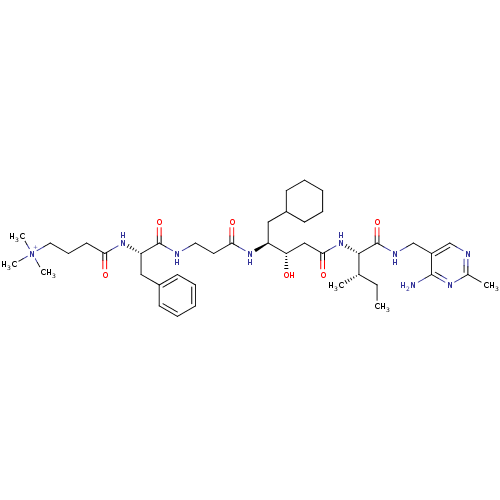 (CHEMBL325329 | [4-(trimethylammonio)butyryl]-Phe-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
E. Merck Darmstadt Curated by ChEMBL | Assay Description In vitro for inhibitory activity against human renin | J Med Chem 34: 3267-80 (1991) BindingDB Entry DOI: 10.7270/Q2G161FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 438 total ) | Next | Last >> |