

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin D (Homo sapiens (Human)) | BDBM50030745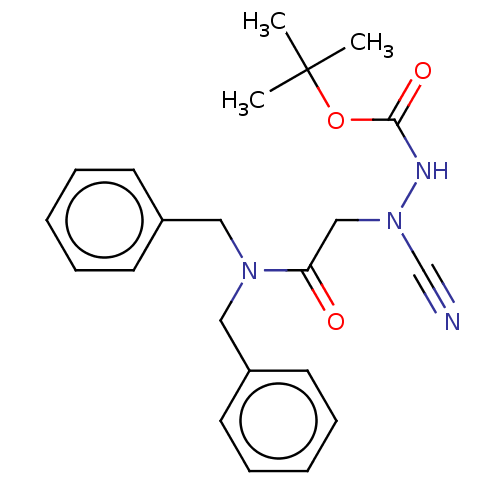 (CHEMBL3342185 | acs.jmedchem.1c00409_ST.412) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50030746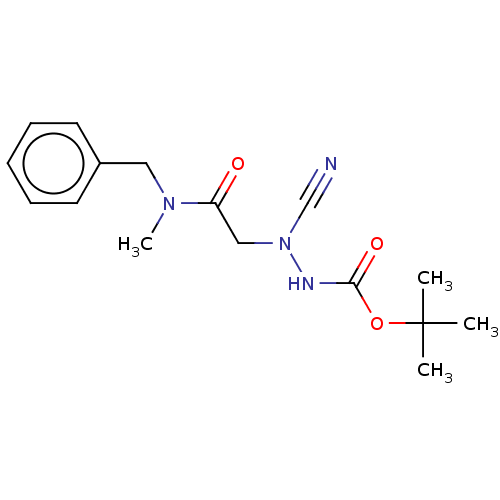 (CHEMBL3342184 | acs.jmedchem.1c00409_ST.413) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50030747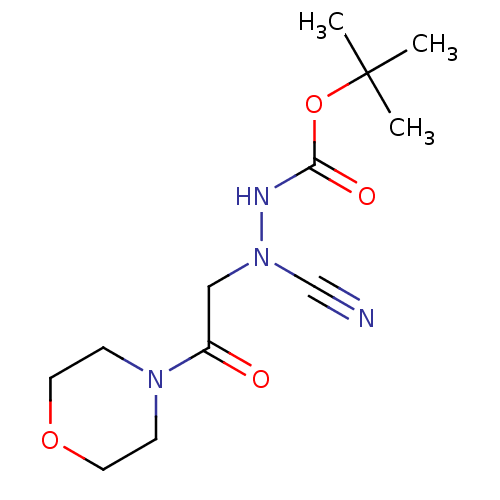 (CHEMBL3342183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50030745 (CHEMBL3342185 | acs.jmedchem.1c00409_ST.412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S using Z-Phe-Arg-AMC fluorogenic substrate fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50030746 (CHEMBL3342184 | acs.jmedchem.1c00409_ST.413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S using Z-Phe-Arg-AMC fluorogenic substrate fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q/T423H mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50030745 (CHEMBL3342185 | acs.jmedchem.1c00409_ST.412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.406 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Z-Arg-Arg-pNA chromogenic substrate fluorogenic substrate incubated for 30 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M2 receptor expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 W422A mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50030746 (CHEMBL3342184 | acs.jmedchem.1c00409_ST.413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.459 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Z-Arg-Arg-pNA chromogenic substrate fluorogenic substrate incubated for 30 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50392215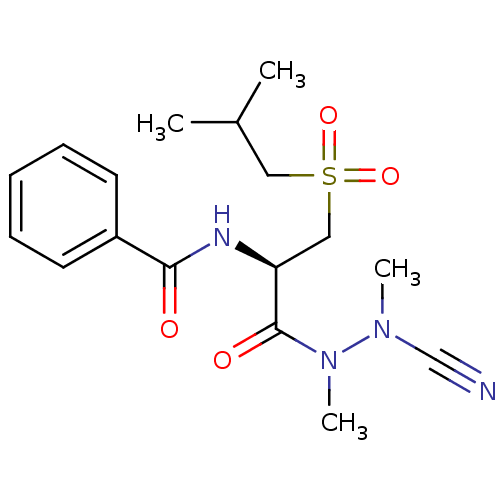 (CHEMBL2153161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S using Z-Phe-Val-Arg-pNA as substrate after 80 mins by spectrophotometric analysis | J Med Chem 55: 5982-6 (2012) Article DOI: 10.1021/jm300734k BindingDB Entry DOI: 10.7270/Q2833T40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50392215 (CHEMBL2153161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin K using Z-Leu-Arg-AMC as substrate after 80 mins by fluorimetric analysis | J Med Chem 55: 5982-6 (2012) Article DOI: 10.1021/jm300734k BindingDB Entry DOI: 10.7270/Q2833T40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50510762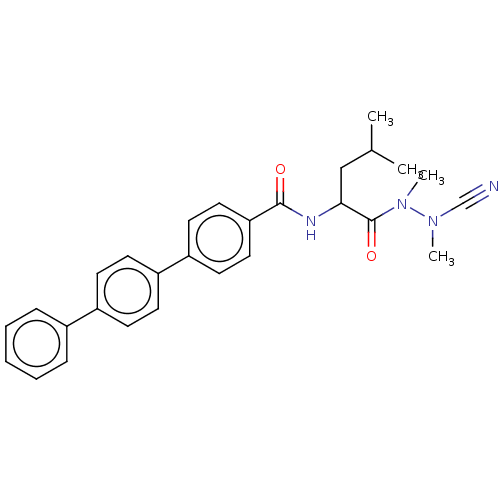 (CHEMBL4442025) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin B using Z-Phe-Arg-AMC as substrate incubated for 30 mins by fluorescence based assay | Bioorg Med Chem 27: 1-15 (2019) Article DOI: 10.1016/j.bmc.2018.10.017 BindingDB Entry DOI: 10.7270/Q21G0QK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50030747 (CHEMBL3342183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.777 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin S using Z-Phe-Arg-AMC fluorogenic substrate fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M5 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50168941 (CHEMBL3804928) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin L using Cbz-Phe-Arg-pNA as substrate incubated for 30 mins measured for 20 mins by photometrical analysis | ACS Med Chem Lett 7: 211-6 (2016) Article DOI: 10.1021/acsmedchemlett.5b00474 BindingDB Entry DOI: 10.7270/Q2TH8PMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM19855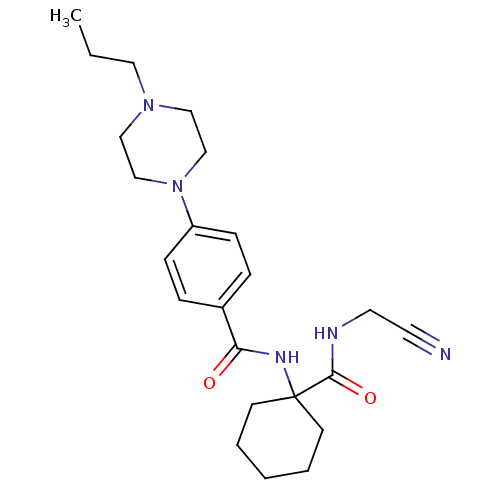 (Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin K using Z-Leu-Arg-AMC fluorogenic substrate incubated for 60 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M5 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497843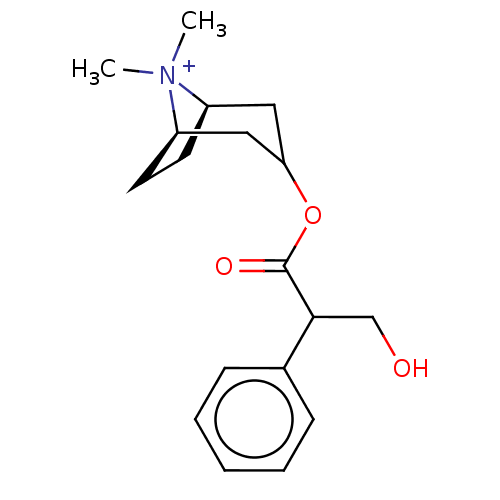 (CHEMBL1197177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M2 receptor expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50510761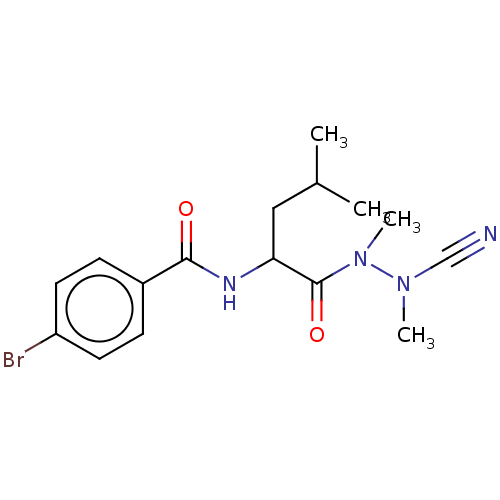 (CHEMBL4440655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin B using Z-Phe-Arg-AMC as substrate incubated for 30 mins by fluorescence based assay | Bioorg Med Chem 27: 1-15 (2019) Article DOI: 10.1016/j.bmc.2018.10.017 BindingDB Entry DOI: 10.7270/Q21G0QK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50448377 (CHEMBL3121473) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q/T423H mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50448377 (CHEMBL3121473) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M2 receptor expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497842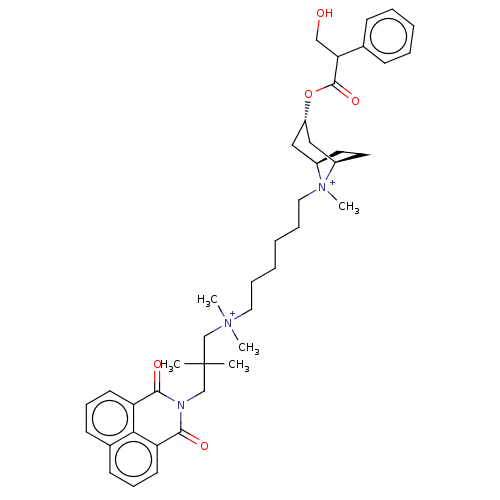 (CHEMBL3323281) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q/T423H mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497835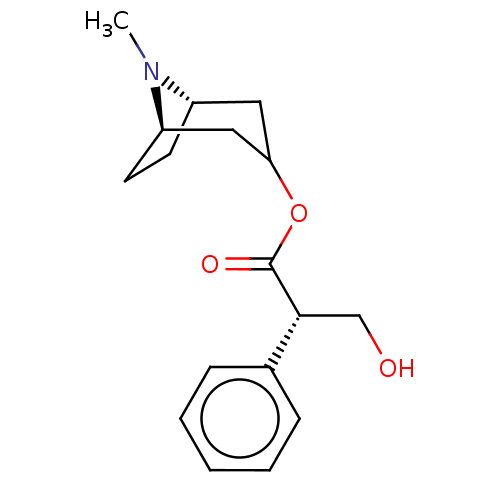 (ENDO-ATROPINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 W422A mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497842 (CHEMBL3323281) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M2 receptor expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M2 receptor expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50497835 (ENDO-ATROPINE) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M5 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 W422A mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497834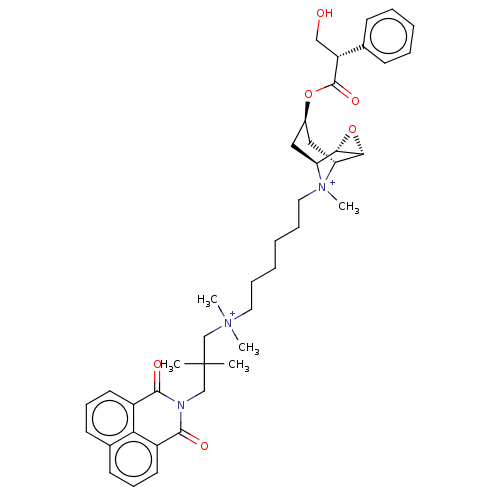 (CHEMBL3323284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q/T423H mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50452855 (Isoptpo Hyoscine | Scopolamine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q/T423H mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50030747 (CHEMBL3342183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Z-Arg-Arg-pNA chromogenic substrate fluorogenic substrate incubated for 30 mins | ACS Med Chem Lett 5: 1076-81 (2014) Article DOI: 10.1021/ml500238q BindingDB Entry DOI: 10.7270/Q20P11NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497835 (ENDO-ATROPINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497835 (ENDO-ATROPINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q/T423H mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50510759 (CHEMBL4459206) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin B using Z-Phe-Arg-AMC as substrate incubated for 30 mins by fluorescence based assay | Bioorg Med Chem 27: 1-15 (2019) Article DOI: 10.1016/j.bmc.2018.10.017 BindingDB Entry DOI: 10.7270/Q21G0QK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497835 (ENDO-ATROPINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M2 receptor expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497841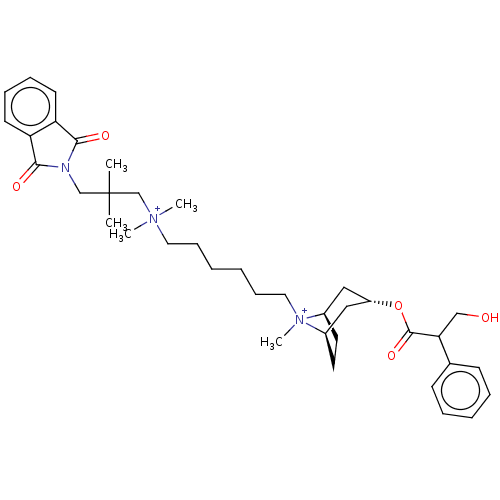 (CHEMBL3323280) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M2 receptor expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497841 (CHEMBL3323280) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q/T423H mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50497834 (CHEMBL3323284) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M5 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50392215 (CHEMBL2153161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human cathepsin B using Z-Arg-Arg-pNA as substrate after 80 mins by spectrophotometric analysis | J Med Chem 55: 5982-6 (2012) Article DOI: 10.1021/jm300734k BindingDB Entry DOI: 10.7270/Q2833T40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497834 (CHEMBL3323284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M2 receptor expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497834 (CHEMBL3323284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 W422A mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497837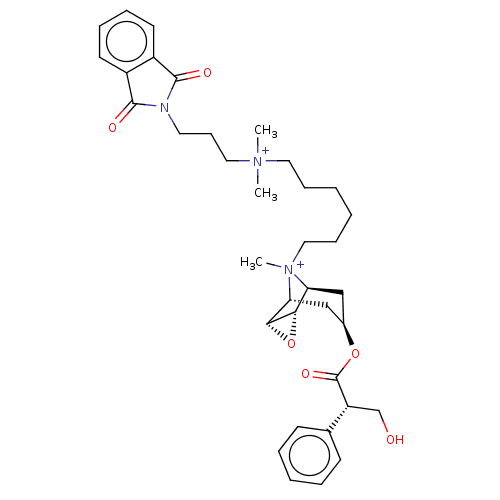 (CHEMBL3323283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 W422A mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497837 (CHEMBL3323283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q/T423H mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497837 (CHEMBL3323283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497837 (CHEMBL3323283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from wild-type human muscarinic M2 receptor expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497842 (CHEMBL3323281) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50448377 (CHEMBL3121473) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50497834 (CHEMBL3323284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 Y177Q mutant expressed in Flp-In-CHO cells by liquid scintillation counting | J Med Chem 57: 6739-50 (2014) Article DOI: 10.1021/jm500790x BindingDB Entry DOI: 10.7270/Q2RJ4NGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 303 total ) | Next | Last >> |