

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM291172 (2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfuran-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM172369 (US9090596, 50) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The experiments were carried out on the FLIPR TETRA.RTM. platform from Molecular Devices. After the basal level had been read, the compounds were add... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM291181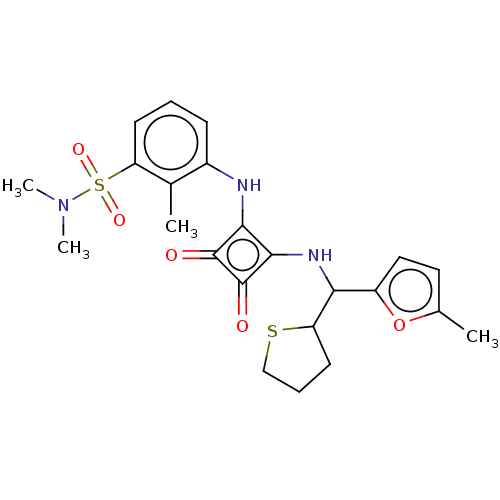 (6-chloro-2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM172372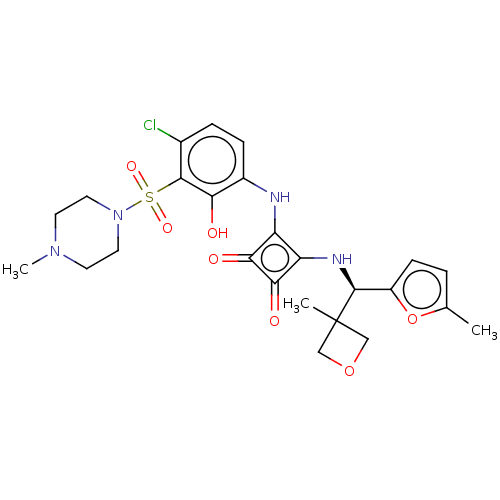 (US9090596, 53) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The experiments were carried out on the FLIPR TETRA.RTM. platform from Molecular Devices. After the basal level had been read, the compounds were add... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM291172 (2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfuran-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM236791 (US9388149, 22 | US9580412, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM291172 (2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfuran-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM172342 (US9090596, 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The experiments were carried out on the FLIPR TETRA.RTM. platform from Molecular Devices. After the basal level had been read, the compounds were add... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50181008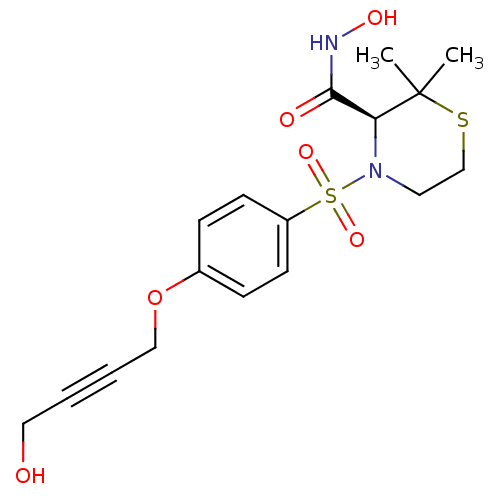 ((S)-N-hydroxy-4-(4-(4-hydroxybut-2-ynyloxy)phenyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GALDERMA RESEARCH & DEVLOPMENT US Patent | Assay Description The TACE enzyme is an internal production (carried out according to the publication protein Eng Des Sel 2006, 19, 155-161) and is added so as to have... | US Patent US9365529 (2016) BindingDB Entry DOI: 10.7270/Q29W0DC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM172328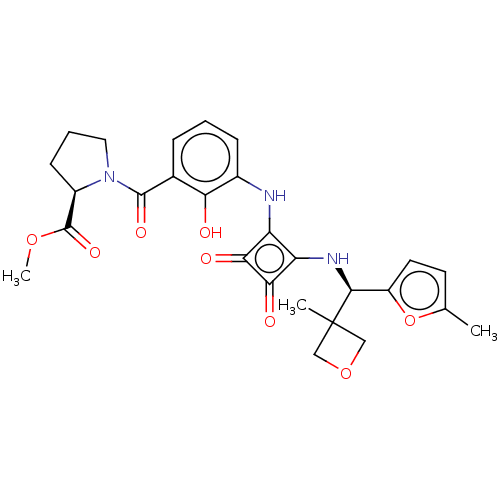 (US9090596, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The experiments were carried out on the FLIPR TETRA.RTM. platform from Molecular Devices. After the basal level had been read, the compounds were add... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM236795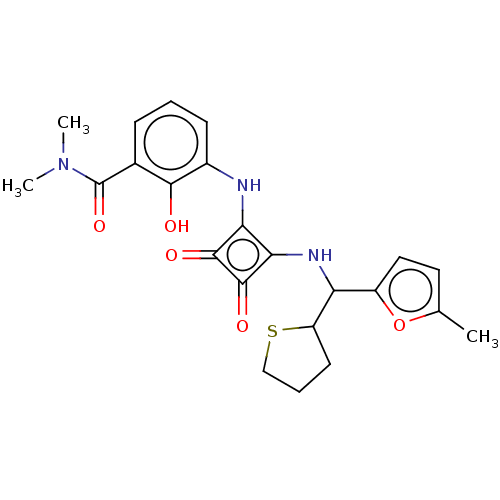 (US9388149, 2 (Diastereoisomer 1) | US9388149, 2 (D...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM236789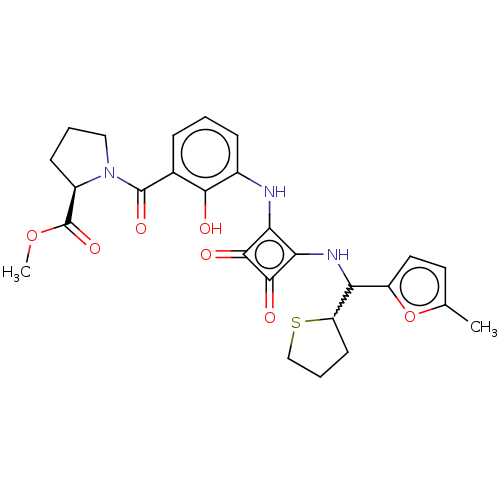 (US9388149, 20 | US9580412, Example 20) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM236795 (US9388149, 2 (Diastereoisomer 1) | US9388149, 2 (D...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM236788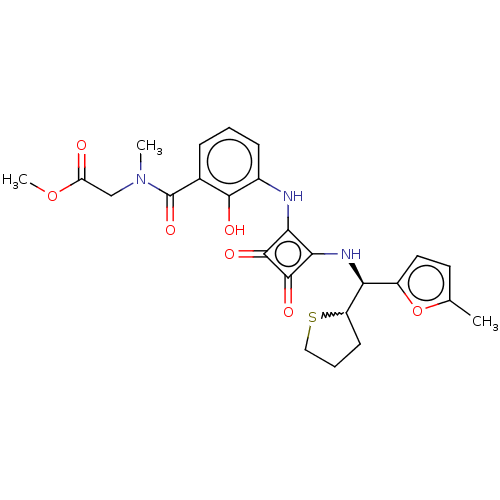 (US9388149, 19 | US9580412, Example 19) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM236789 (US9388149, 20 | US9580412, Example 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM236791 (US9388149, 22 | US9580412, Example 22) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM291177 (US9580412, Example 6 | US9580412, Example 7 | meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50181008 ((S)-N-hydroxy-4-(4-(4-hydroxybut-2-ynyloxy)phenyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVLOPMENT US Patent | Assay Description The molecules are dose-response tested on the following enzymes: MMP1, MMP3, MMP9, ADAM9 and ADAM10, according to the same protocol as that described... | US Patent US9365529 (2016) BindingDB Entry DOI: 10.7270/Q29W0DC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM236788 (US9388149, 19 | US9580412, Example 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236795 (US9388149, 2 (Diastereoisomer 1) | US9388149, 2 (D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236787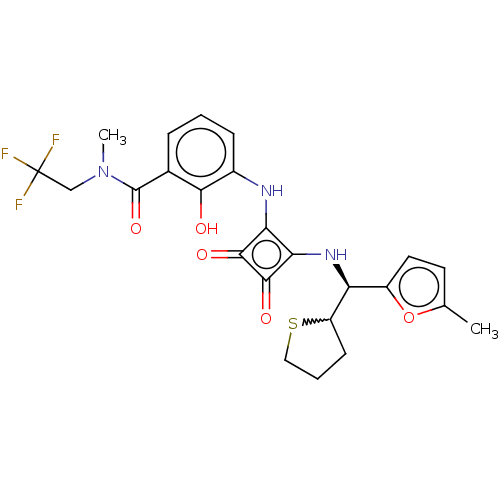 (US9388149, 18 | US9580412, Example 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236795 (US9388149, 2 (Diastereoisomer 1) | US9388149, 2 (D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236787 (US9388149, 18 | US9580412, Example 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM113592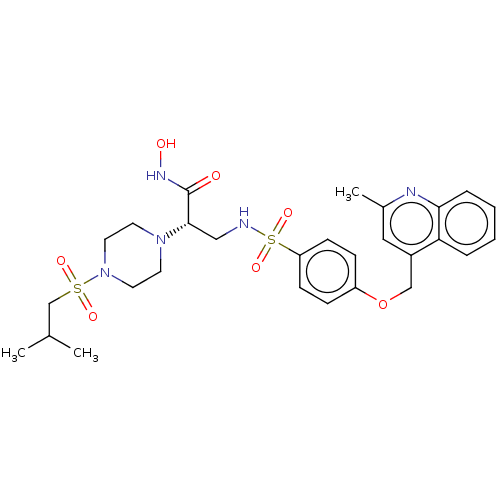 (US8633196, 24 | US9365529, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GALDERMA RESEARCH & DEVLOPMENT US Patent | Assay Description The TACE enzyme is an internal production (carried out according to the publication protein Eng Des Sel 2006, 19, 155-161) and is added so as to have... | US Patent US9365529 (2016) BindingDB Entry DOI: 10.7270/Q29W0DC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236813 (US9388149, 10 (Diastereoisomer 1) | US9388149, 10 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM291181 (6-chloro-2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM113593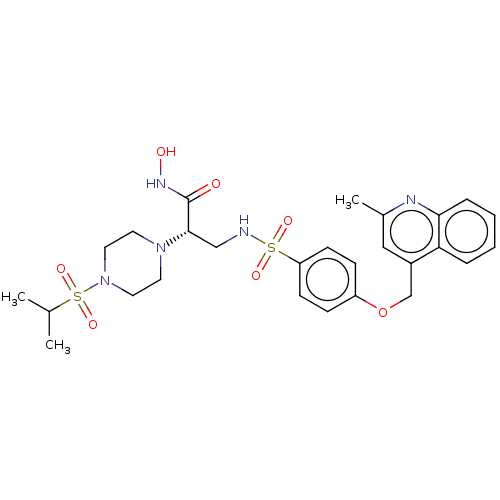 (US8633196, 26 | US9365529, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GALDERMA RESEARCH & DEVLOPMENT US Patent | Assay Description The TACE enzyme is an internal production (carried out according to the publication protein Eng Des Sel 2006, 19, 155-161) and is added so as to have... | US Patent US9365529 (2016) BindingDB Entry DOI: 10.7270/Q29W0DC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236791 (US9388149, 22 | US9580412, Example 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236791 (US9388149, 22 | US9580412, Example 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM291181 (6-chloro-2-hydroxy-N,N-dimethyl-3-(2-{[(5-methylfu...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236788 (US9388149, 19 | US9580412, Example 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM236813 (US9388149, 10 (Diastereoisomer 1) | US9388149, 10 ...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236788 (US9388149, 19 | US9580412, Example 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236789 (US9388149, 20 | US9580412, Example 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM113577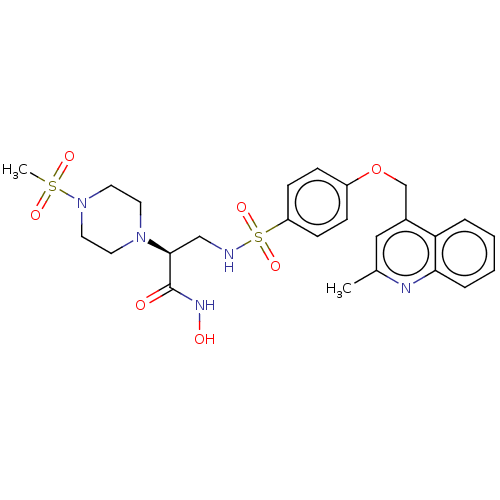 (US8633196, 14 | US8633196, 5 | US9365529, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GALDERMA RESEARCH & DEVLOPMENT US Patent | Assay Description The TACE enzyme is an internal production (carried out according to the publication protein Eng Des Sel 2006, 19, 155-161) and is added so as to have... | US Patent US9365529 (2016) BindingDB Entry DOI: 10.7270/Q29W0DC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236789 (US9388149, 20 | US9580412, Example 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM113577 (US8633196, 14 | US8633196, 5 | US9365529, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Galderma Research & Development US Patent | Assay Description The TACE enzyme is an internal production (carried out according to the publication protein Eng Des Sel 2006, 19, 155-161) and is added so as to have... | US Patent US8633196 (2014) BindingDB Entry DOI: 10.7270/Q2KK99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236785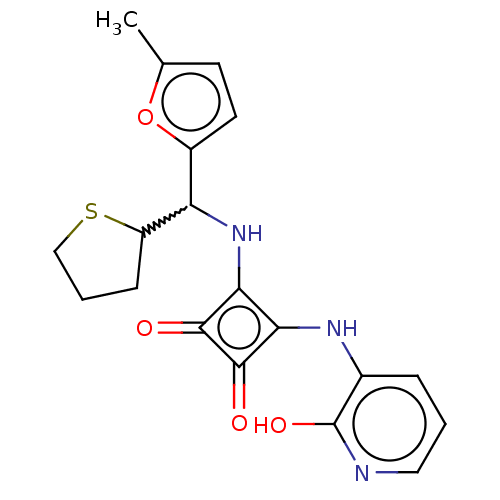 (US9388149, 16 | US9580412, Example 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236785 (US9388149, 16 | US9580412, Example 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM113583 (US8633196, 11 | US9365529, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.4 | 37 |
GALDERMA RESEARCH & DEVLOPMENT US Patent | Assay Description The TACE enzyme is an internal production (carried out according to the publication protein Eng Des Sel 2006, 19, 155-161) and is added so as to have... | US Patent US9365529 (2016) BindingDB Entry DOI: 10.7270/Q29W0DC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM113583 (US8633196, 11 | US9365529, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Galderma Research & Development US Patent | Assay Description The TACE enzyme is an internal production (carried out according to the publication protein Eng Des Sel 2006, 19, 155-161) and is added so as to have... | US Patent US8633196 (2014) BindingDB Entry DOI: 10.7270/Q2KK99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM172358 (US9090596, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the .beta.-a... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM172368 (US9090596, 48) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the .beta.-a... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM291177 (US9580412, Example 6 | US9580412, Example 7 | meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM236791 (US9388149, 22 | US9580412, Example 22) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the β-a... | US Patent US9580412 (2017) BindingDB Entry DOI: 10.7270/Q2222WT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM236806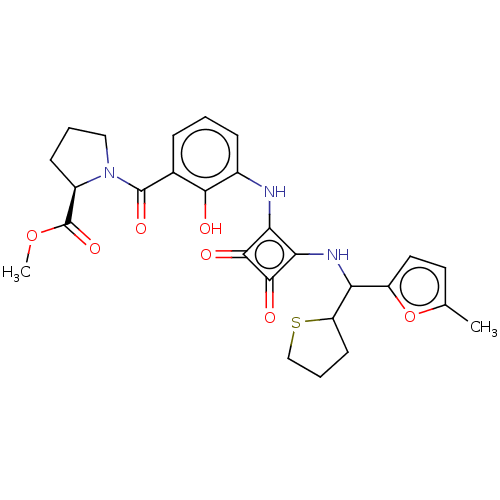 (US9388149, 6 | US9388149, 7 (Diastereoisomer 1)) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM236791 (US9388149, 22 | US9580412, Example 22) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 37 |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description ⿿PathHunter HEK293-CXCR2 or ⿿U2OS hCXCR1 β-arrestin cells (DiscoveRx Corporation) were seeded overnight at 10 000 cells/well (384-well... | US Patent US9388149 (2016) BindingDB Entry DOI: 10.7270/Q26W990K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM172372 (US9090596, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the .beta.-a... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM172342 (US9090596, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
GALDERMA RESEARCH & DEVELOPMENT US Patent | Assay Description The in vitro affinity of the compounds of the present invention for the CXCR1 and CXCR2 receptors was determined on a functional test of the .beta.-a... | US Patent US9090596 (2015) BindingDB Entry DOI: 10.7270/Q2M04462 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM113574 (US8633196, 2 | US9365529, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Galderma Research & Development US Patent | Assay Description The TACE enzyme is an internal production (carried out according to the publication protein Eng Des Sel 2006, 19, 155-161) and is added so as to have... | US Patent US8633196 (2014) BindingDB Entry DOI: 10.7270/Q2KK99FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 348 total ) | Next | Last >> |