

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.00360 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antgonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM315131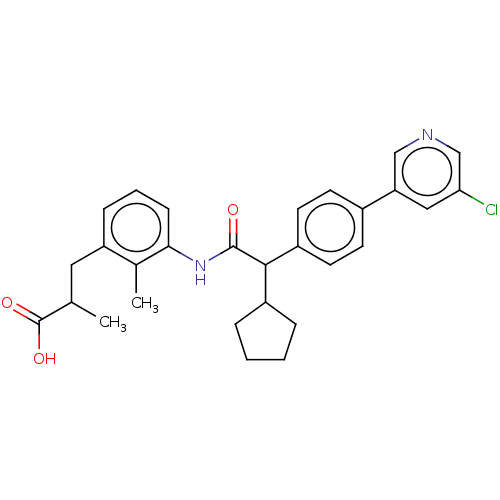 ((2R/S)-3-[3-({(2R/S)-2-[4-(5-Chloropyridin-3-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.188 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (MOUSE) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in NIH3T3 cells expressing glucocorticoid receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM314872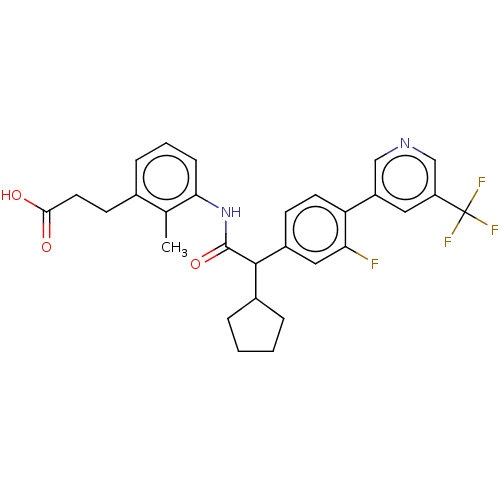 ((−) 3-{3-[(Cyclopentyl{3-fluoro-4-[5-(triflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389389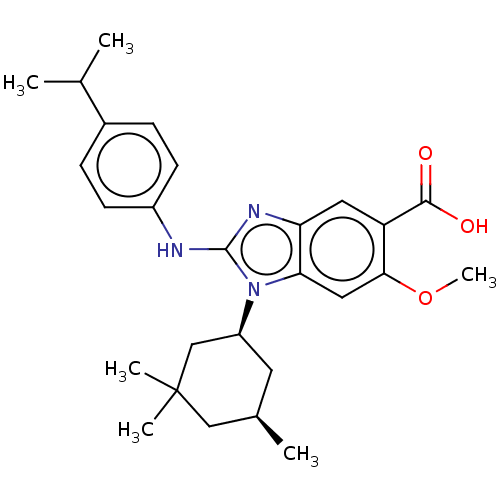 (US9951027, 2-155-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM315173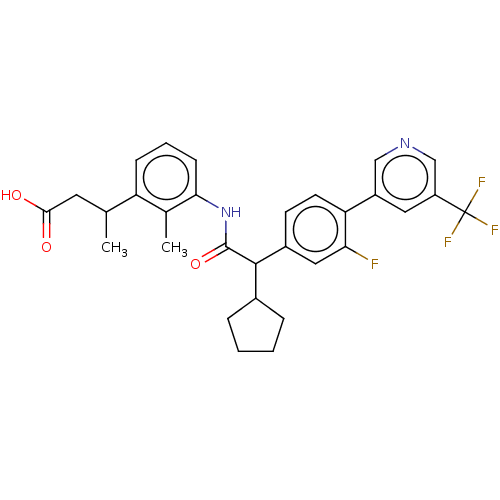 ((3R/S)-3-(3-{[(2R/S)-2-Cyclopentyl-2-{3-fluoro-4-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.49 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389290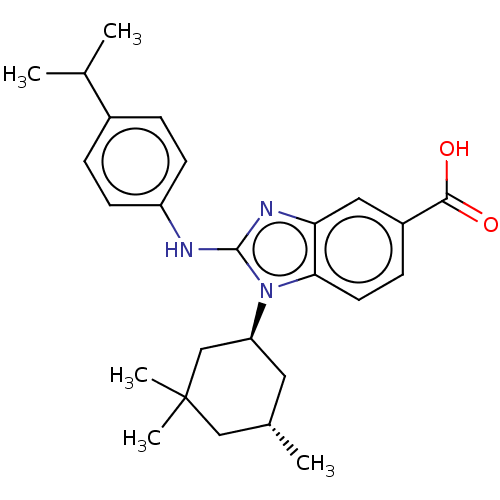 (US9951027, 2-76-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389381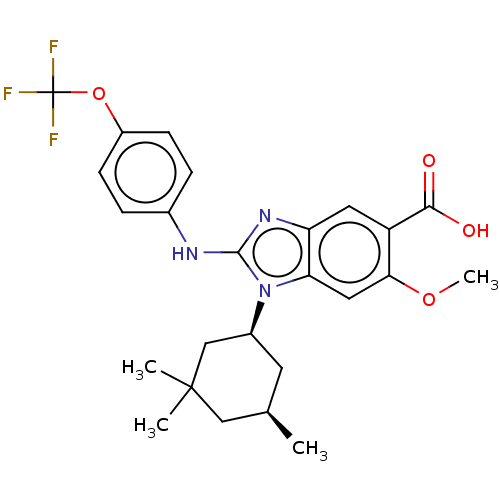 ((+-) 6-methoxy-2-{[4-(trifluoromethoxy)phenyl]amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389364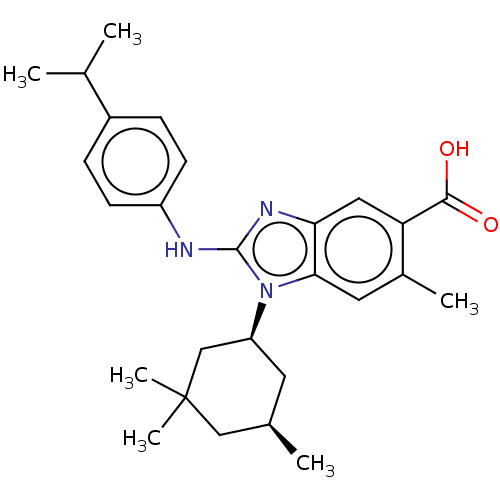 (US9951027, 2-144-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389324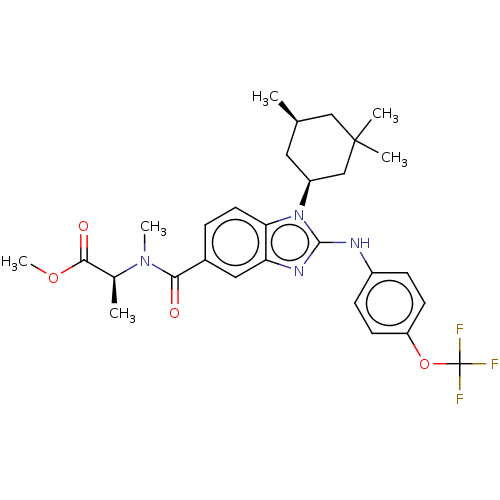 (US9951027, 2-95-2 | US9951027, 2-96-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390499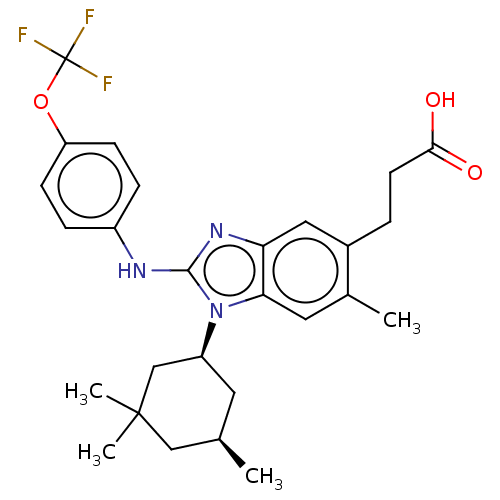 (US10442772, Example 2-162-2 | US9957235, 2-162-1 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390498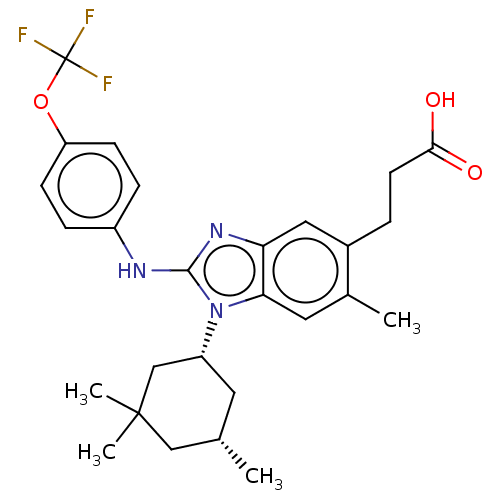 ((�) 3-(6-methyl-2-{[4-(trifluoromethoxy)phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM315178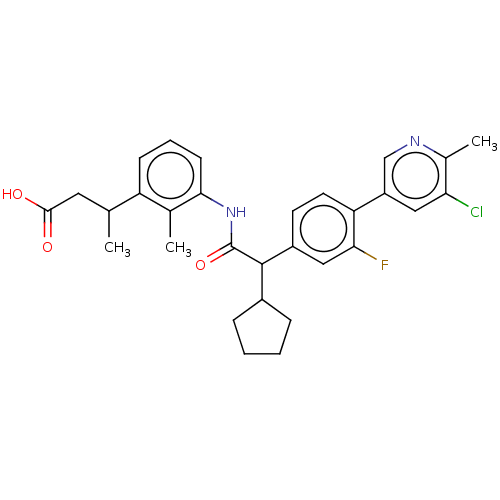 ((3R/S)-3-[3-({(2R/S)-2-[4-(5-Chloro-6-methylpyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.06 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM315183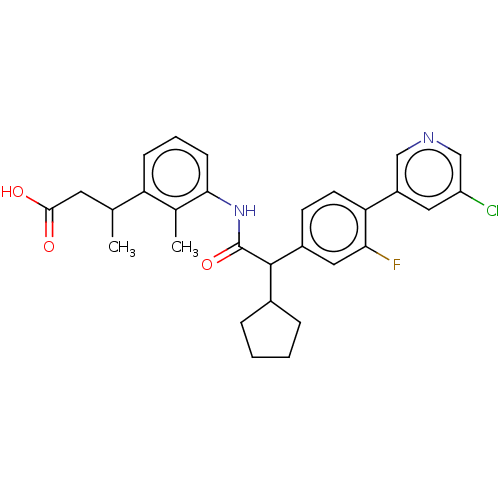 ((3R/S)-3-[3-({(2R/S)-2-[4-(5-Chloropyridin-3-yl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389223 (US9951027, 2-42-2 | US9951027, 2-75-1 | US9951027,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389336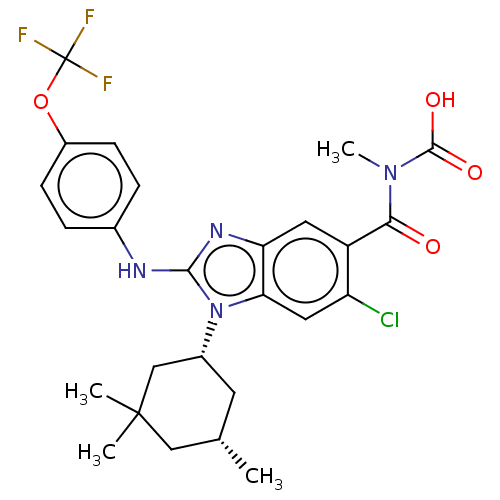 (US9951027, 2-102-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389172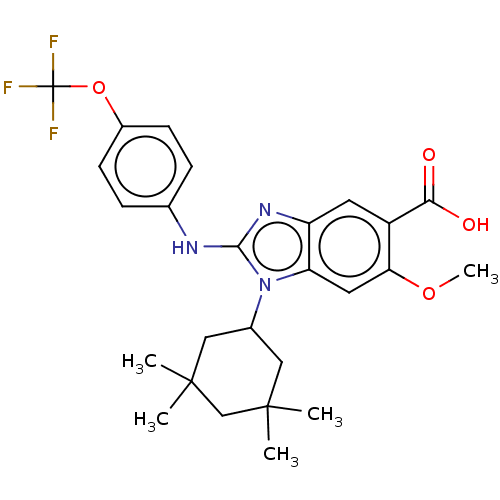 (6-methoxy-1-(3,3,5,5-tetramethylcyclohexyl)-2-{[4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM315251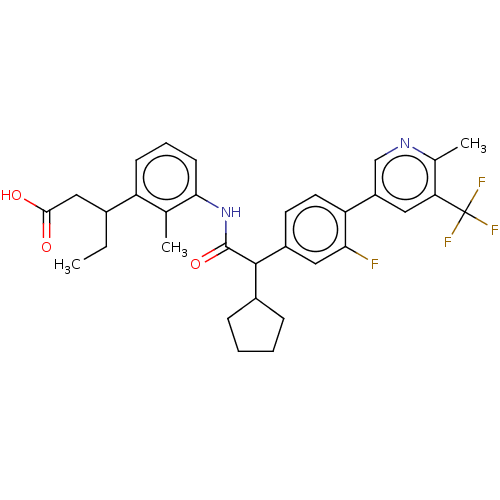 (3-(3-{[(2R)-2-cyclopentyl-2-{4-[6-methyl-5-(triflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.57 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM315244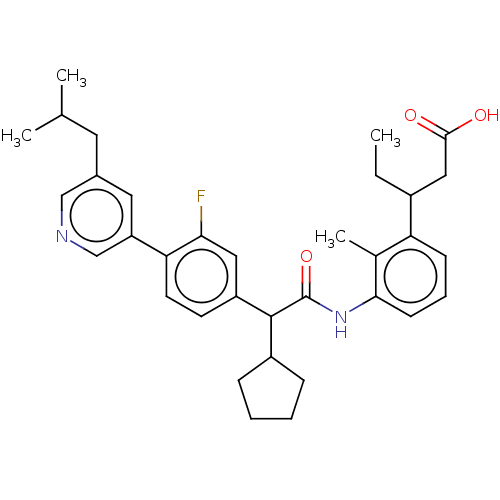 (3-[3-({cyclopentyl[3-fluoro-4-(5-isobutylpyridin-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.82 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389208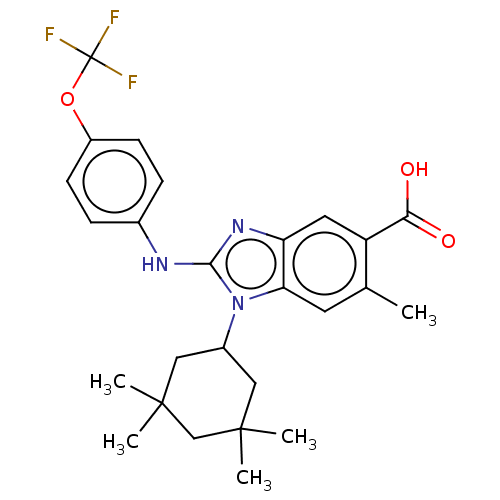 (6-methyl-1-(3,3,5,5-tetramethylcyclohexyl)-2-{[4-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM315188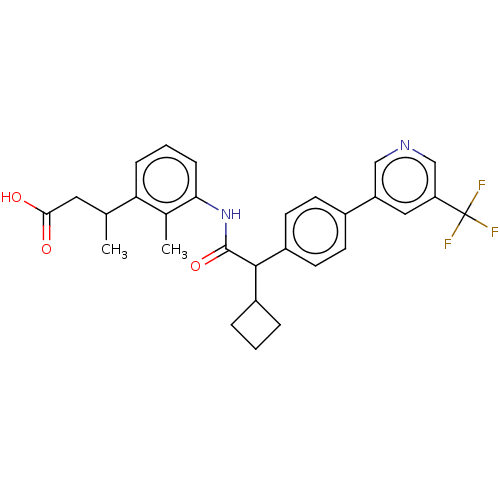 ((3R/S)-3-(3-{[(2R/S)-2-Cyclobutyl-2-{4-[5-(trifluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.59 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390662 (US10442772, Example 2-247-2 | US9957235, 2-247-1 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM415800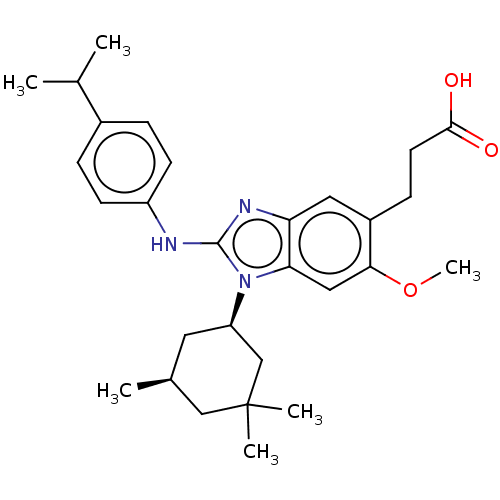 (US10442772, Example 2-170 | US10442772, Example 2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM315143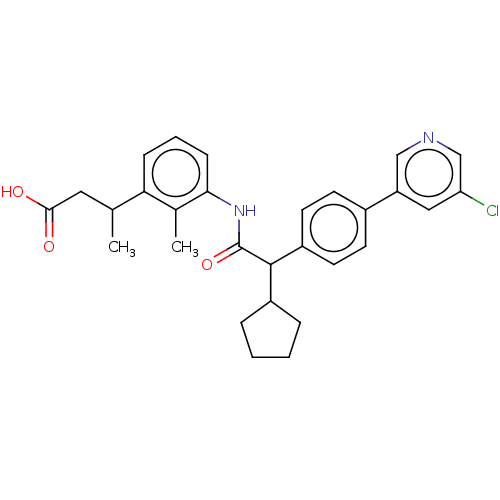 ((3R/S)-3-[3-({(2R/S)-2-[4-(5-Chloropyridin-3-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390662 (US10442772, Example 2-247-2 | US9957235, 2-247-1 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390522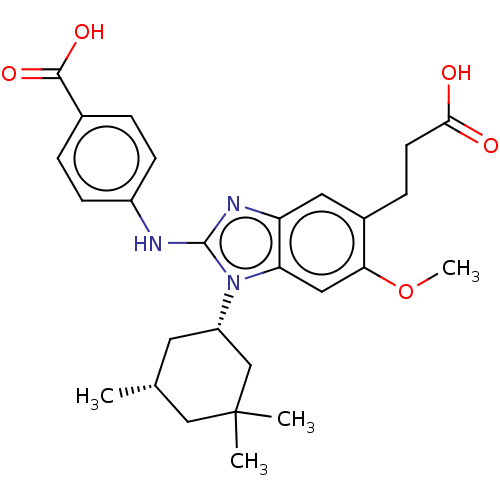 (US9957235, 2-170 | US9957235, 2-170-1 | US9957235,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389358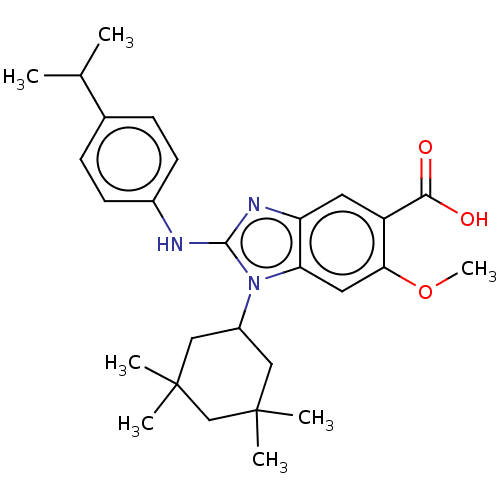 (6-methoxy-2-{[4-(propan-2-yl)phenyl]amino}-1-(3,3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389381 ((+-) 6-methoxy-2-{[4-(trifluoromethoxy)phenyl]amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389387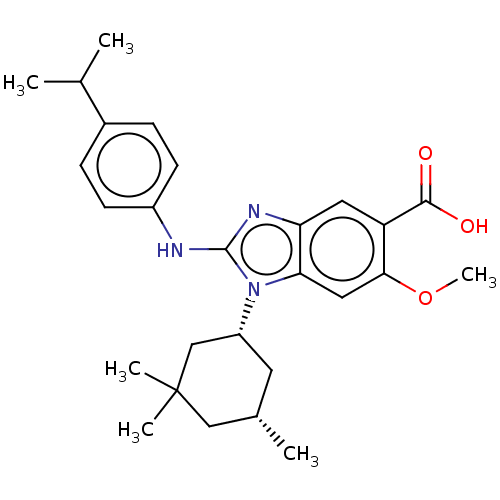 (US9951027, 2-155) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390528 (US10442772, Example 2-175-2 | US9957235, 2-172 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390543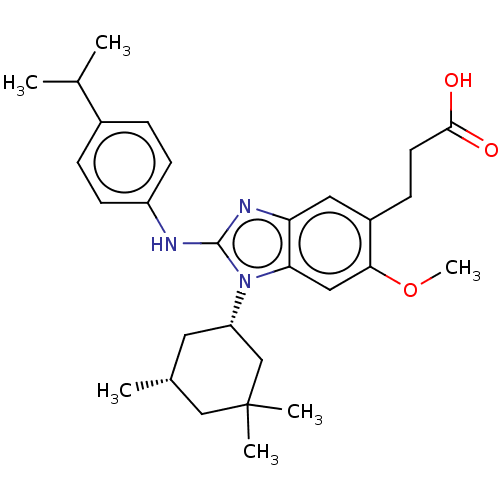 (US10442772, Example 2-170-2 | US9957235, 2-177 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390525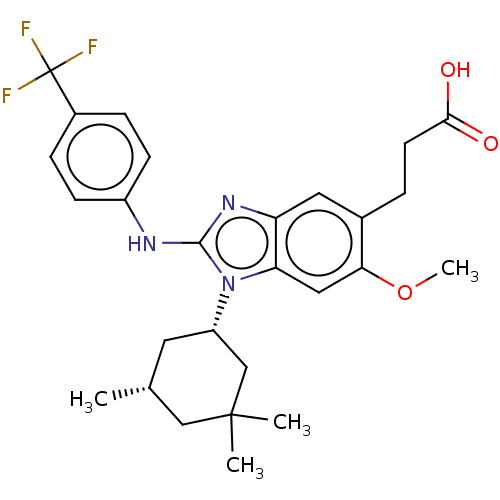 (US10442772, Example 2-171-2 | US9957235, 2-171 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390528 (US10442772, Example 2-175-2 | US9957235, 2-172 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM415929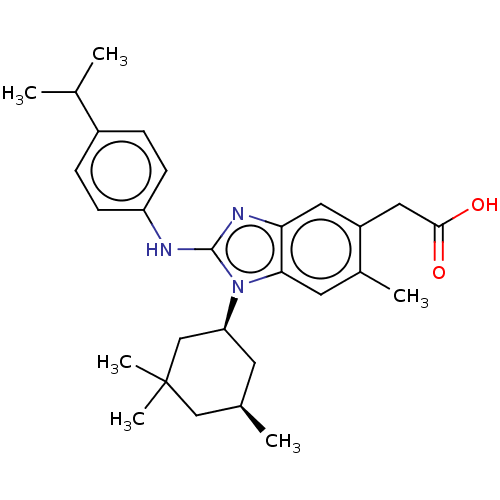 (US10442772, Example 2-237-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390499 (US10442772, Example 2-162-2 | US9957235, 2-162-1 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... | US Patent US10442772 (2019) BindingDB Entry DOI: 10.7270/Q21R6SVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389229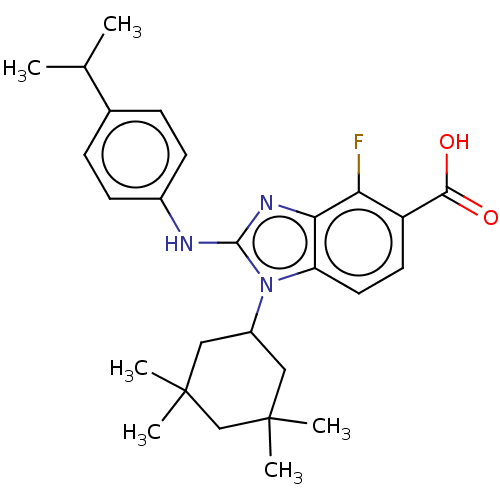 (US9951027, 2-48) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389362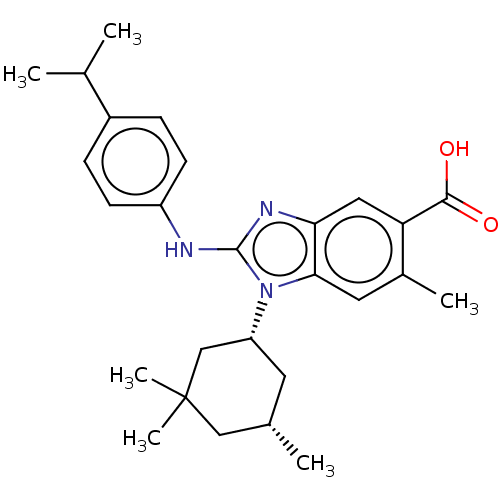 (US9951027, 2-144 | US9951027, 2-144-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389392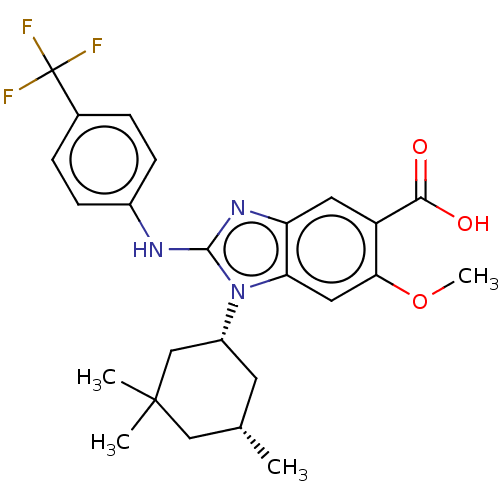 (US9951027, 2-156-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390647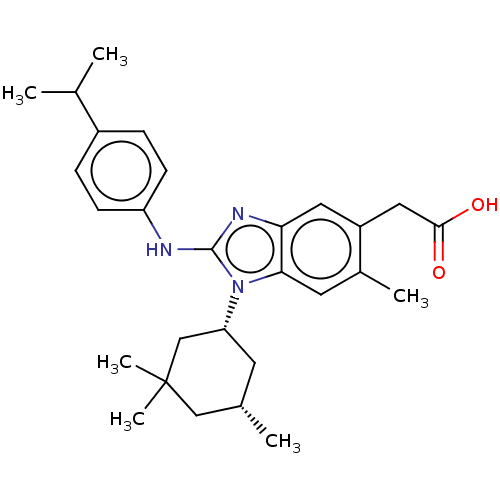 (US10442772, Example 2-237-1 | US9957235, 2-237 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390499 (US10442772, Example 2-162-2 | US9957235, 2-162-1 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390522 (US9957235, 2-170 | US9957235, 2-170-1 | US9957235,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM390525 (US10442772, Example 2-171-2 | US9957235, 2-171 | U...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | Bioorg Med Chem 14: 1378-90 (2006) BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM315143 ((3R/S)-3-[3-({(2R/S)-2-[4-(5-Chloropyridin-3-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.67 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM314852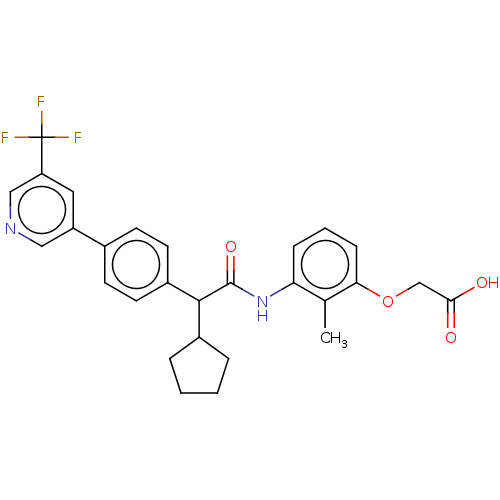 ((−) {3-[(Cyclopentyl{4-[5-(trifluoromethyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.85 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description Recombinant proteins (human isoforms) of PTGES containing a FLAG tag, expressed in baculovirus infected insect cells (Hi-5) and purified by affinity ... | US Patent US10172814 (2019) BindingDB Entry DOI: 10.7270/Q20K2BNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389288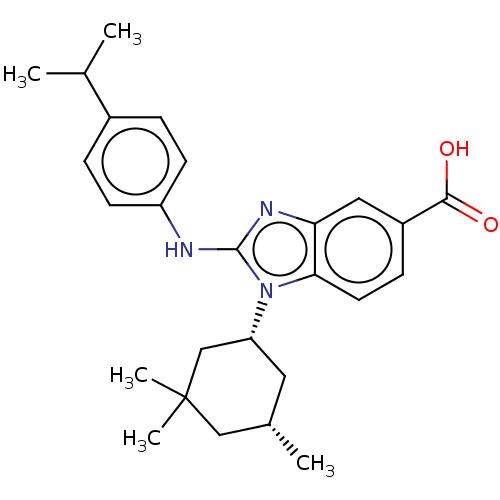 (US9951027, 2-76) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in CV-1 cells expressing androgen receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM389390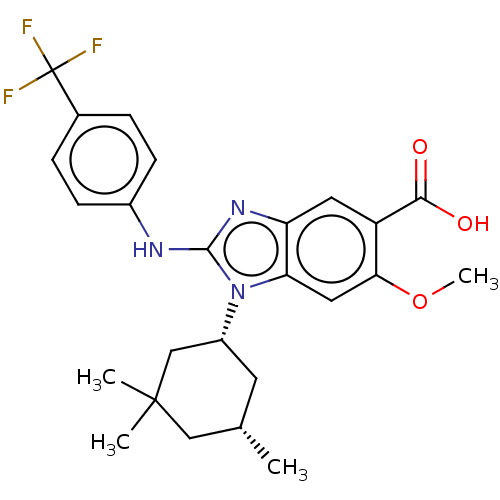 (US9951027, 2-156) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... | J Med Chem 52: 514-23 (2009) BindingDB Entry DOI: 10.7270/Q2S75JN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1119 total ) | Next | Last >> |