

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM482158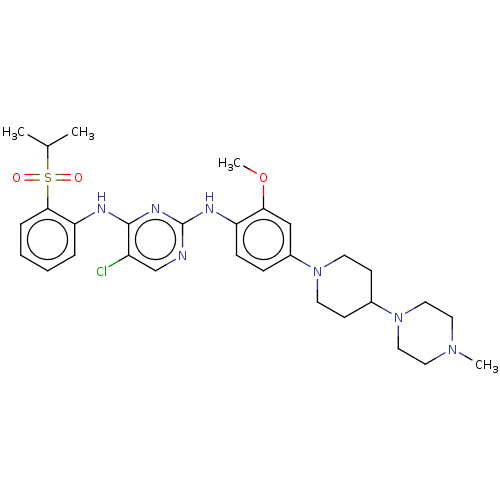 (BDBM50242742 | TAE684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM50062357 (AP26113 | CHEMBL3397300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192721 (KIRA analog, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50132003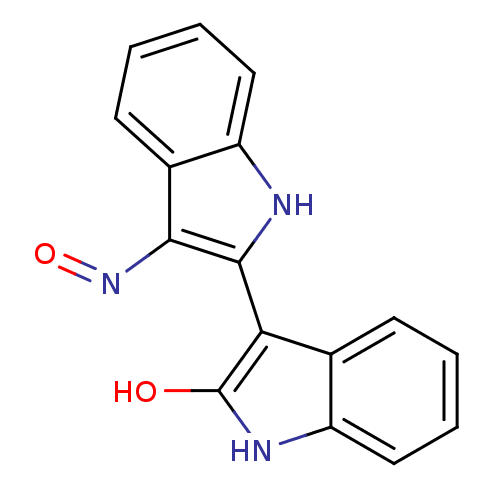 ((Z)-1H,1''H-[2,3'']Biindolylidene-3,2''-dione 3-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology Curated by ChEMBL | Assay Description Inhibition of cyclin-dependent kinase 5/p25 | J Med Chem 48: 671-9 (2005) Article DOI: 10.1021/jm049323m BindingDB Entry DOI: 10.7270/Q2MP52S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192751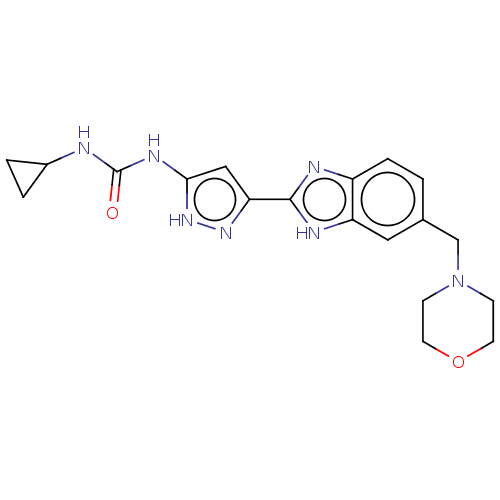 (AT9283) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192731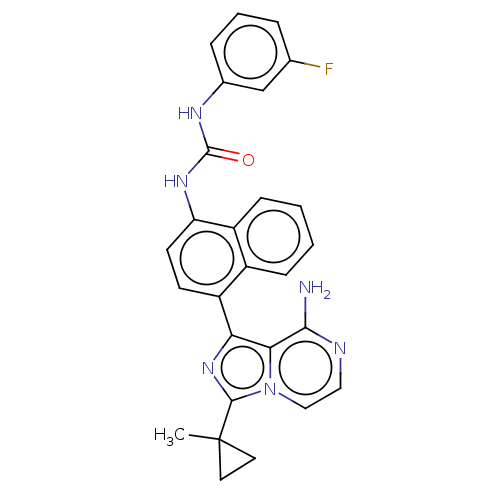 (KIRA analog, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192720 (KIRA analog, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192734 (KIRA analog, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192721 (KIRA analog, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192720 (KIRA analog, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192739 (KIRA analog, 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192722 (KIRA analog, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192730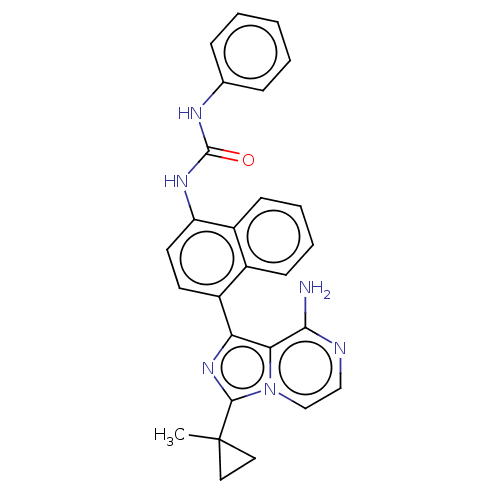 (KIRA analog, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192738 (KIRA analog, 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM7377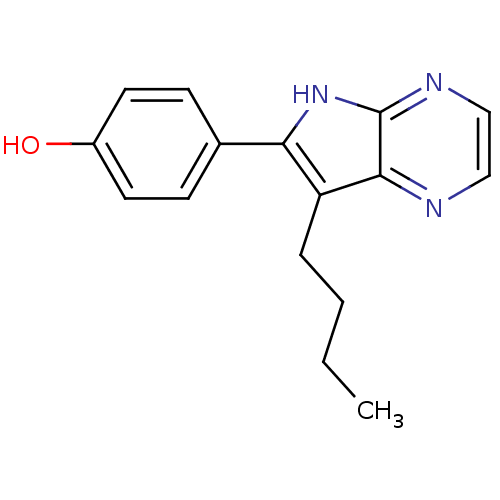 (4-{7-butyl-5H-pyrrolo[2,3-b]pyrazin-6-yl}phenol | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology Curated by ChEMBL | Assay Description Inhibition of cyclin-dependent kinase 5/p25; range 0.16-0.2 | J Med Chem 48: 671-9 (2005) Article DOI: 10.1021/jm049323m BindingDB Entry DOI: 10.7270/Q2MP52S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM7533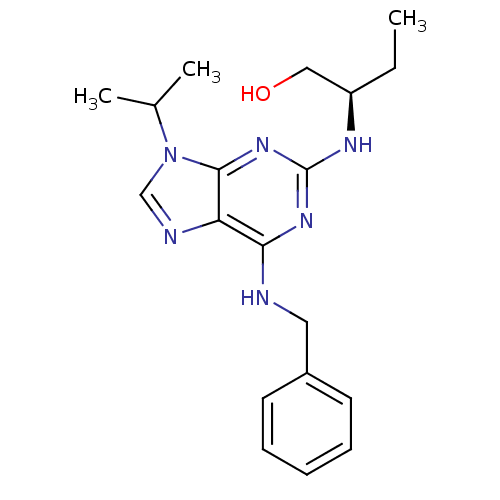 ((2R)-2-[[6-(benzylamino)-9-isopropyl-purin-2-yl]am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
European Institute of Oncology Curated by ChEMBL | Assay Description Inhibition of cyclin-dependent kinase 5/p25; range 0.1-0.2 | J Med Chem 48: 671-9 (2005) Article DOI: 10.1021/jm049323m BindingDB Entry DOI: 10.7270/Q2MP52S4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192731 (KIRA analog, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192732 (KIRA analog, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192723 (KIRA analog, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192732 (KIRA analog, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192735 (KIRA analog, 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192733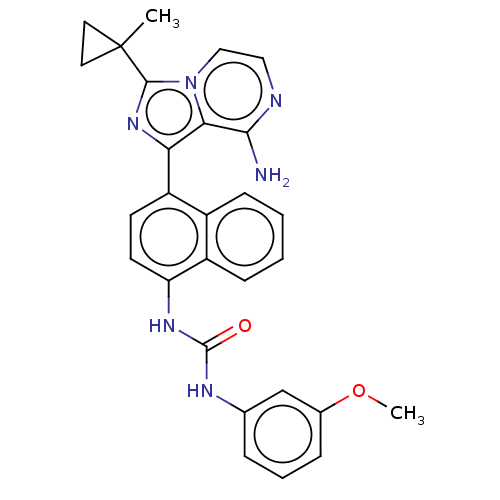 (KIRA analog, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192734 (KIRA analog, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192724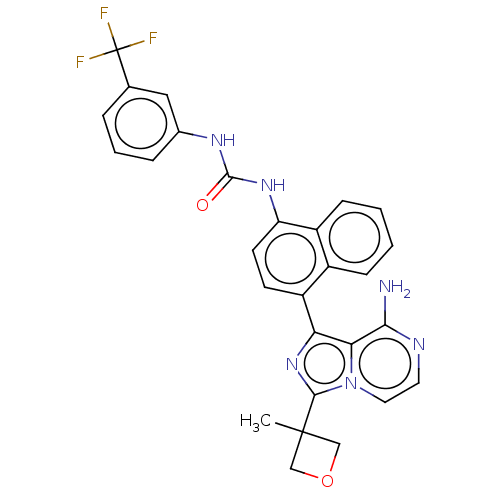 (KIRA analog, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192733 (KIRA analog, 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192739 (KIRA analog, 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192725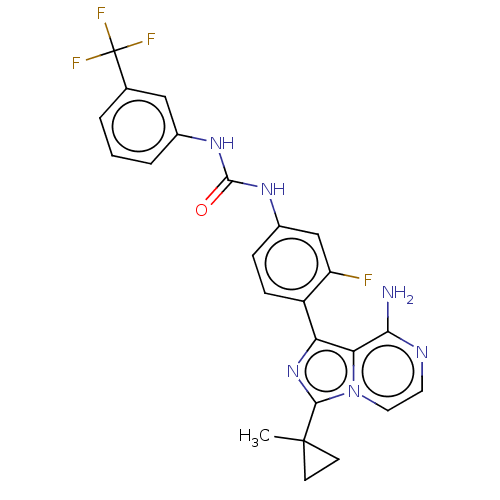 (KIRA analog, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192738 (KIRA analog, 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192730 (KIRA analog, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192723 (KIRA analog, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192722 (KIRA analog, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192736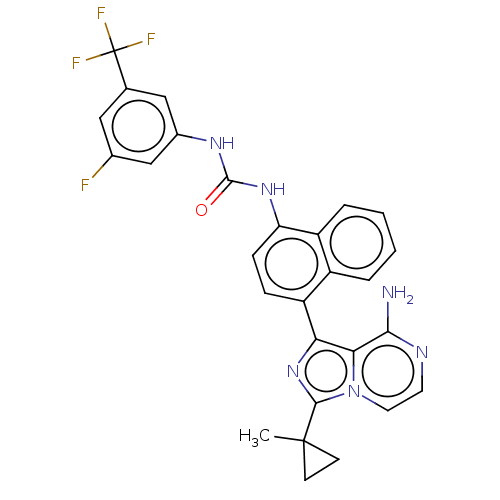 (KIRA analog, 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM50389803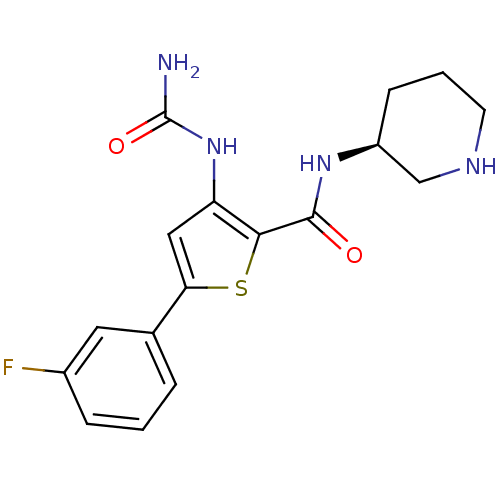 (AZD7762 | CHEMBL2041933) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192735 (KIRA analog, 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192724 (KIRA analog, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192752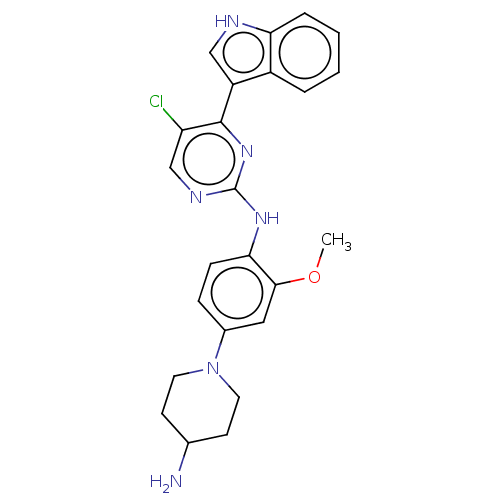 (AZD3463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM16018 (14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192753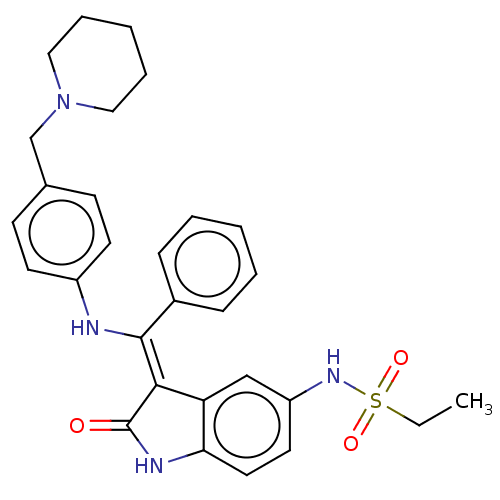 (Hesperadin | US10981896, Compound Hesperadin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM185149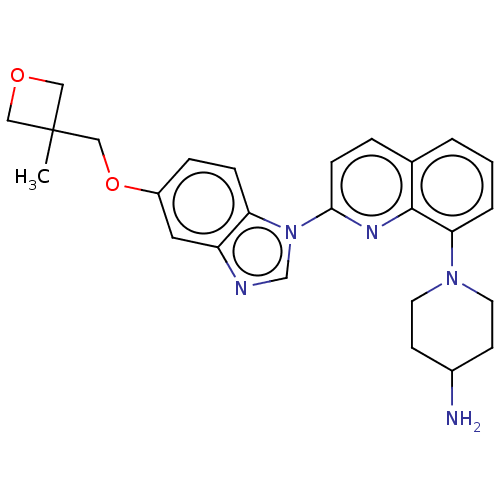 (1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192725 (KIRA analog, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM50334594 (2-(5-chloro-2-(2-methoxy-4-morpholinophenylamino)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192754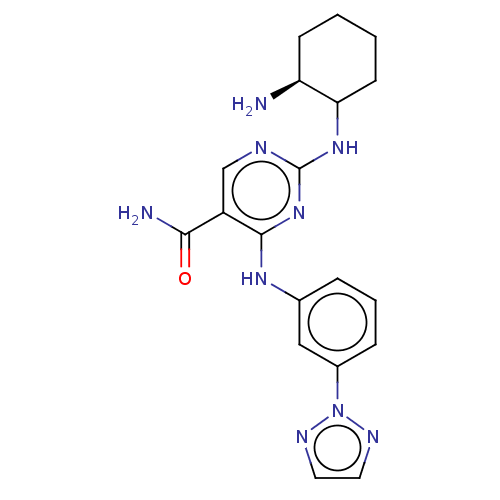 (PRT062607) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192736 (KIRA analog, 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192728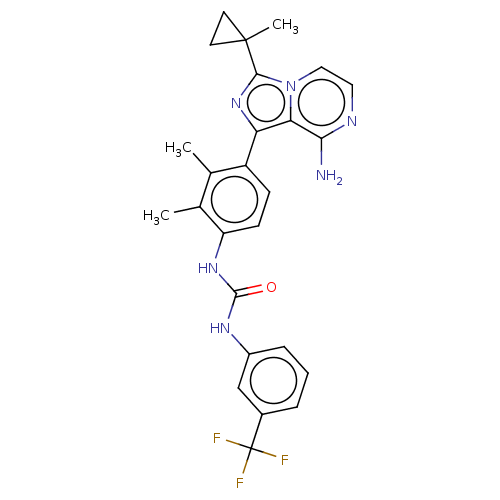 (KIRA analog, 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192719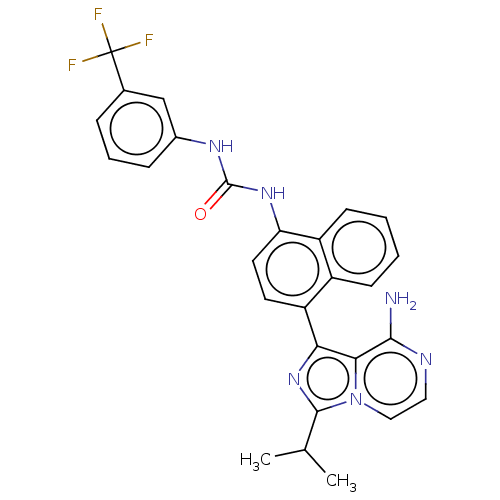 (KIRA analog, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM50130725 (3-(3-Chloro-4-hydroxy-phenylamino)-4-(2-nitro-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Washington | Assay Description Inhibitors (initial concentration 10 or 60 μM, three-fold serial dilutions) were incubated with IRE1α* in cleavage buffer (20 mM HEPES at p... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM60931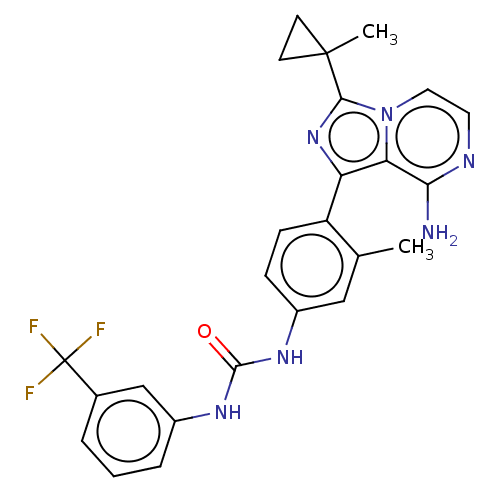 (KIRA analog, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192737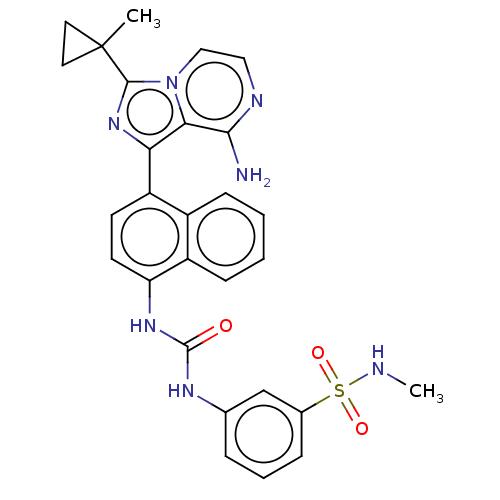 (KIRA analog, 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192719 (KIRA analog, 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 [547-977] (Homo sapiens (Human)) | BDBM192743 (KIRA analog, 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington | Assay Description 5′-Carboxyfluorescein (FAM)- and 3′-Black Hole Quencher (BHQ)-labeled XBP1 single stemloop mini-substrate (5′FAM-CUGAGUCCGCAGCACUCA... | ACS Chem Biol 11: 2195-205 (2016) Article DOI: 10.1021/acschembio.5b00940 BindingDB Entry DOI: 10.7270/Q2ZK5FGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 106 total ) | Next | Last >> |