 Found 26 hits with Last Name = 'simmons' and Initial = 'k'
Found 26 hits with Last Name = 'simmons' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337102
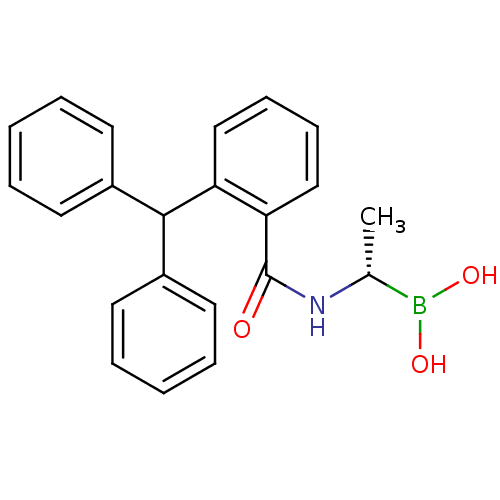
((S)-1-(2-(Diphenylmethyl)benzamido)ethaneboronate ...)Show SMILES C[C@@H](NC(=O)c1ccccc1C(c1ccccc1)c1ccccc1)B(O)O |r| Show InChI InChI=1S/C22H22BNO3/c1-16(23(26)27)24-22(25)20-15-9-8-14-19(20)21(17-10-4-2-5-11-17)18-12-6-3-7-13-18/h2-16,21,26-27H,1H3,(H,24,25)/t16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337101
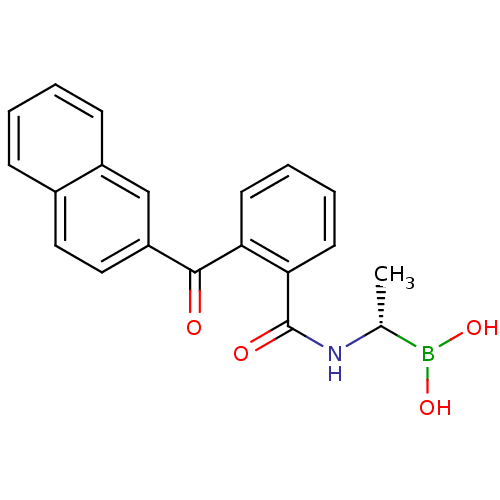
((S)-1-(2-(Naphthalen-2-ylcarbonyl)benzamido)ethane...)Show SMILES C[C@@H](NC(=O)c1ccccc1C(=O)c1ccc2ccccc2c1)B(O)O |r| Show InChI InChI=1S/C20H18BNO4/c1-13(21(25)26)22-20(24)18-9-5-4-8-17(18)19(23)16-11-10-14-6-2-3-7-15(14)12-16/h2-13,25-26H,1H3,(H,22,24)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
p2X7 purinoceptor
(Canis lupus familiaris) | BDBM50087267
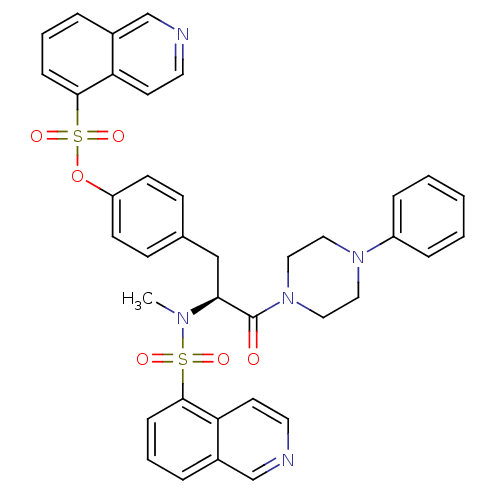
((1-(N,O-bis(1,5-isoquinolinesulfonyl)-N-methyl-L-t...)Show SMILES CN([C@@H](Cc1ccc(OS(=O)(=O)c2cccc3cnccc23)cc1)C(=O)N1CCN(CC1)c1ccccc1)S(=O)(=O)c1cccc2cnccc12 |r| Show InChI InChI=1S/C38H35N5O6S2/c1-41(50(45,46)36-11-5-7-29-26-39-19-17-33(29)36)35(38(44)43-23-21-42(22-24-43)31-9-3-2-4-10-31)25-28-13-15-32(16-14-28)49-51(47,48)37-12-6-8-30-27-40-20-18-34(30)37/h2-20,26-27,35H,21-25H2,1H3/t35-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of dog P2X7 receptor |
Bioorg Med Chem Lett 25: 3164-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.001
BindingDB Entry DOI: 10.7270/Q22N5427 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50404070
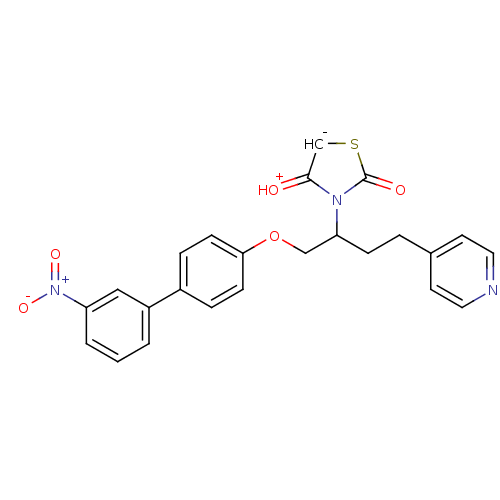
(CHEMBL338117)Show SMILES Oc1csc(=O)n1C(CCc1ccncc1)COc1ccc(cc1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C24H21N3O5S/c28-23-16-33-24(29)26(23)21(7-4-17-10-12-25-13-11-17)15-32-22-8-5-18(6-9-22)19-2-1-3-20(14-19)27(30)31/h1-3,5-6,8-14,16,21,28H,4,7,15H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of human P2X7 receptor |
Bioorg Med Chem Lett 25: 3164-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.001
BindingDB Entry DOI: 10.7270/Q22N5427 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887
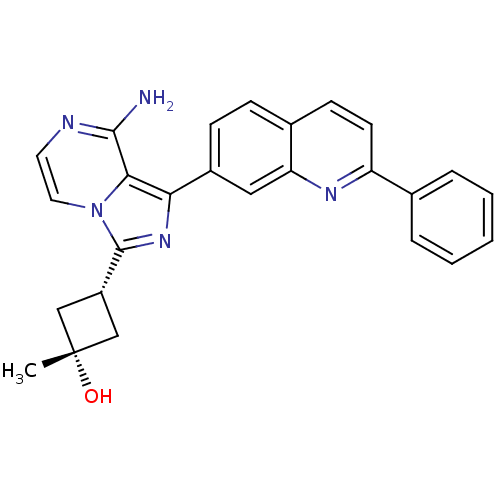
((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for the biological activity at the Beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337101

((S)-1-(2-(Naphthalen-2-ylcarbonyl)benzamido)ethane...)Show SMILES C[C@@H](NC(=O)c1ccccc1C(=O)c1ccc2ccccc2c1)B(O)O |r| Show InChI InChI=1S/C20H18BNO4/c1-13(21(25)26)22-20(24)18-9-5-4-8-17(18)19(23)16-11-10-14-6-2-3-7-15(14)12-16/h2-13,25-26H,1H3,(H,22,24)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157879
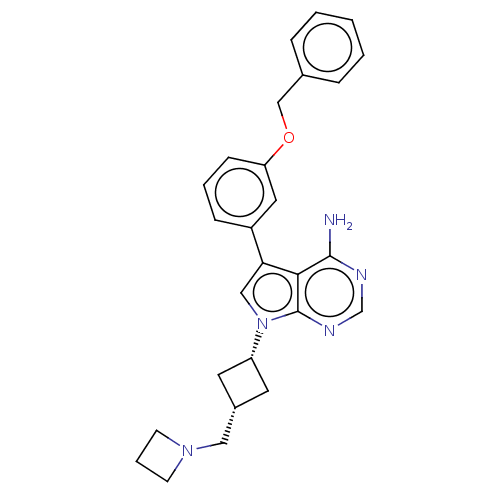
(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50087267

((1-(N,O-bis(1,5-isoquinolinesulfonyl)-N-methyl-L-t...)Show SMILES CN([C@@H](Cc1ccc(OS(=O)(=O)c2cccc3cnccc23)cc1)C(=O)N1CCN(CC1)c1ccccc1)S(=O)(=O)c1cccc2cnccc12 |r| Show InChI InChI=1S/C38H35N5O6S2/c1-41(50(45,46)36-11-5-7-29-26-39-19-17-33(29)36)35(38(44)43-23-21-42(22-24-43)31-9-3-2-4-10-31)25-28-13-15-32(16-14-28)49-51(47,48)37-12-6-8-30-27-40-20-18-34(30)37/h2-20,26-27,35H,21-25H2,1H3/t35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of human P2X7 receptor |
Bioorg Med Chem Lett 25: 3164-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.001
BindingDB Entry DOI: 10.7270/Q22N5427 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor
(Cavia porcellus) | BDBM50087267

((1-(N,O-bis(1,5-isoquinolinesulfonyl)-N-methyl-L-t...)Show SMILES CN([C@@H](Cc1ccc(OS(=O)(=O)c2cccc3cnccc23)cc1)C(=O)N1CCN(CC1)c1ccccc1)S(=O)(=O)c1cccc2cnccc12 |r| Show InChI InChI=1S/C38H35N5O6S2/c1-41(50(45,46)36-11-5-7-29-26-39-19-17-33(29)36)35(38(44)43-23-21-42(22-24-43)31-9-3-2-4-10-31)25-28-13-15-32(16-14-28)49-51(47,48)37-12-6-8-30-27-40-20-18-34(30)37/h2-20,26-27,35H,21-25H2,1H3/t35-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of guinea pig P2X7 receptor |
Bioorg Med Chem Lett 25: 3164-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.001
BindingDB Entry DOI: 10.7270/Q22N5427 |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337094
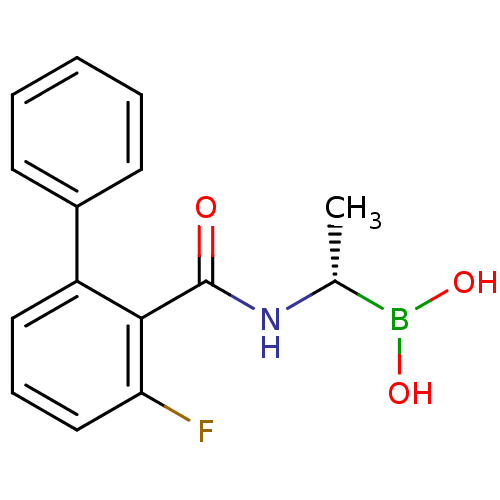
((S)-1-(2-Fluoro-6-phenylbenzamido)ethaneboronic ac...)Show SMILES C[C@@H](NC(=O)c1c(F)cccc1-c1ccccc1)B(O)O |r| Show InChI InChI=1S/C15H15BFNO3/c1-10(16(20)21)18-15(19)14-12(8-5-9-13(14)17)11-6-3-2-4-7-11/h2-10,20-21H,1H3,(H,18,19)/t10-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337100
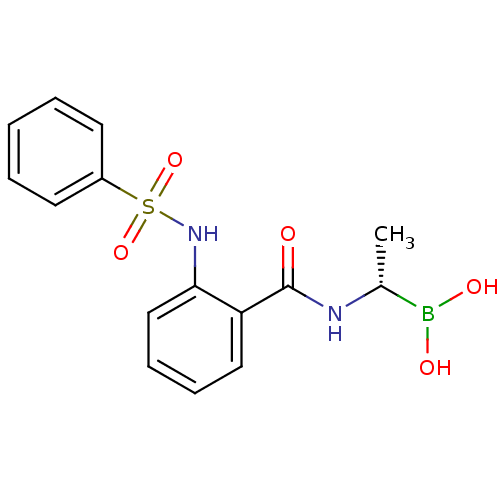
((S)-1-(2-[(Phenylsulfonyl)amino]benzamido)ethanebo...)Show SMILES C[C@@H](NC(=O)c1ccccc1NS(=O)(=O)c1ccccc1)B(O)O |r| Show InChI InChI=1S/C15H17BN2O5S/c1-11(16(20)21)17-15(19)13-9-5-6-10-14(13)18-24(22,23)12-7-3-2-4-8-12/h2-11,18,20-21H,1H3,(H,17,19)/t11-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337095

((S)-1-(2,6-Difluorobenzamido)ethaneboronic acid | ...)Show InChI InChI=1S/C9H10BF2NO3/c1-5(10(15)16)13-9(14)8-6(11)3-2-4-7(8)12/h2-5,15-16H,1H3,(H,13,14)/t5-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337093
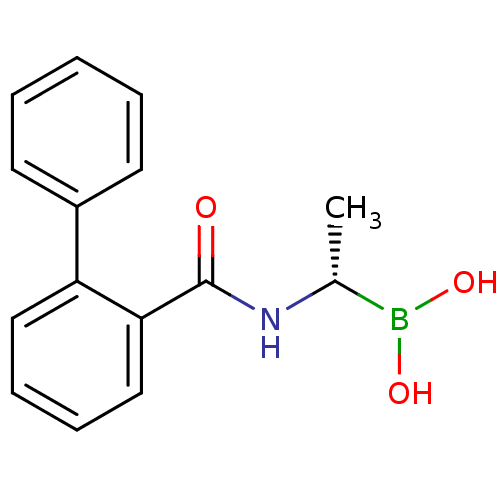
((S)-1-(2-Phenylbenzamido)ethaneboronic acid | CHEM...)Show InChI InChI=1S/C15H16BNO3/c1-11(16(19)20)17-15(18)14-10-6-5-9-13(14)12-7-3-2-4-8-12/h2-11,19-20H,1H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337092
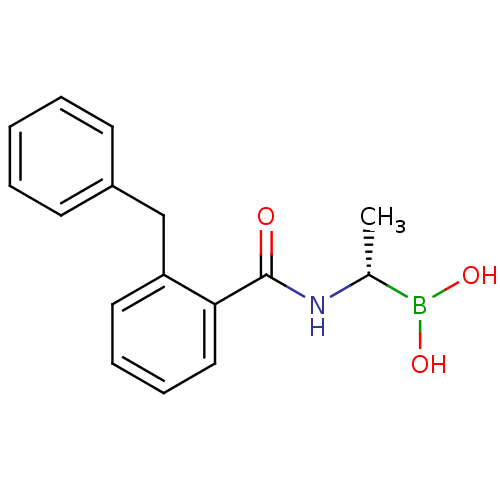
((S)-1-(2-Benzylbenzamido)ethaneboronic acid | CHEM...)Show InChI InChI=1S/C16H18BNO3/c1-12(17(20)21)18-16(19)15-10-6-5-9-14(15)11-13-7-3-2-4-8-13/h2-10,12,20-21H,11H2,1H3,(H,18,19)/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337091
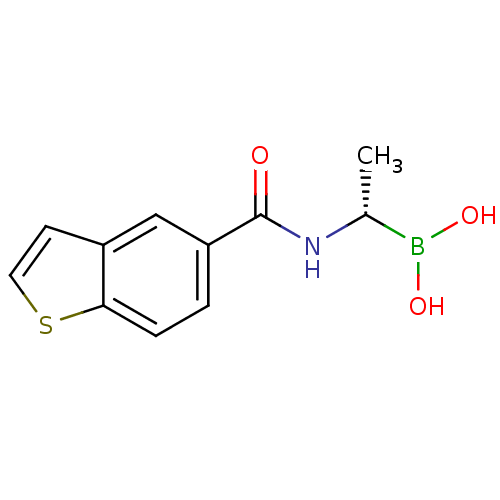
((S)-1-(1-Benzothiophene-5-carboxamido)ethaneboroni...)Show InChI InChI=1S/C11H12BNO3S/c1-7(12(15)16)13-11(14)9-2-3-10-8(6-9)4-5-17-10/h2-7,15-16H,1H3,(H,13,14)/t7-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50157879

(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337098
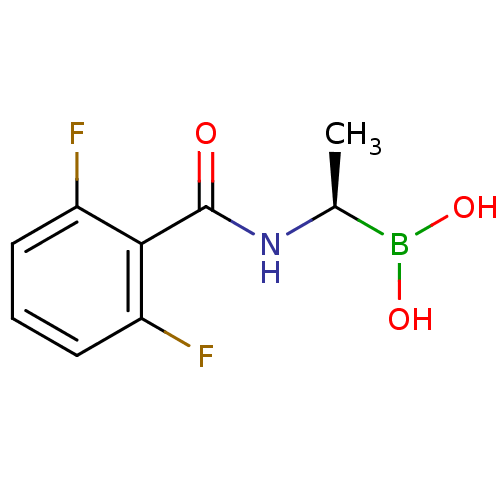
((R)-1-(2,6-Difluorobenzamido)ethaneboronic acid | ...)Show InChI InChI=1S/C9H10BF2NO3/c1-5(10(15)16)13-9(14)8-6(11)3-2-4-7(8)12/h2-5,15-16H,1H3,(H,13,14)/t5-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337096
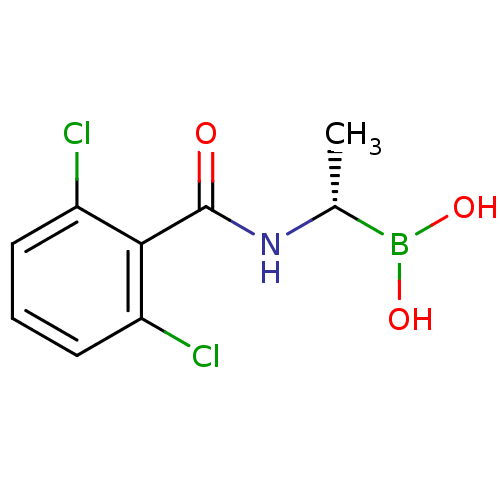
((S)-1-(2,6-Dichlorobenzamido)ethaneboronic acid | ...)Show InChI InChI=1S/C9H10BCl2NO3/c1-5(10(15)16)13-9(14)8-6(11)3-2-4-7(8)12/h2-5,15-16H,1H3,(H,13,14)/t5-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50045333
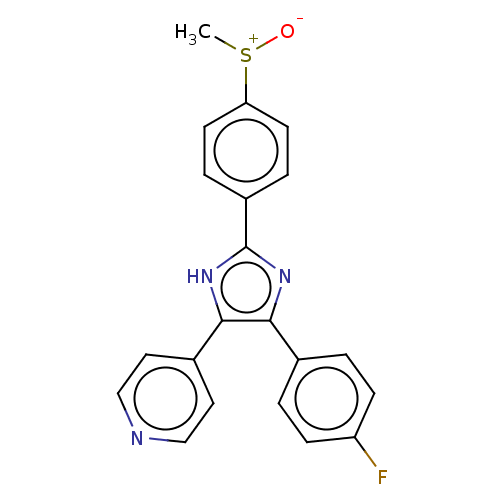
(CHEBI:90705 | SB-203580)Show SMILES C[S+]([O-])c1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of human P2X7 receptor |
Bioorg Med Chem Lett 25: 3164-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.001
BindingDB Entry DOI: 10.7270/Q22N5427 |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337099
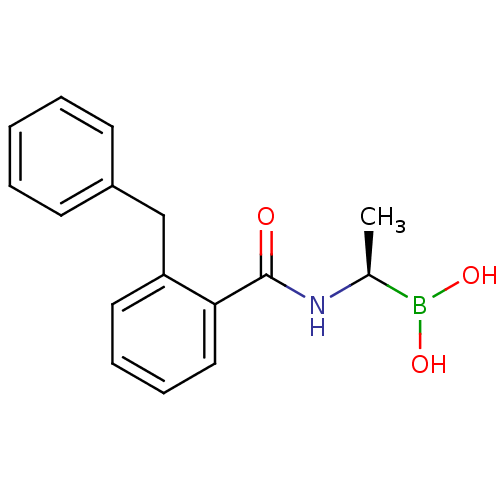
((R)-1-(2-Benzylbenzamido)ethaneboronic acid | CHEM...)Show InChI InChI=1S/C16H18BNO3/c1-12(17(20)21)18-16(19)15-10-6-5-9-14(15)11-13-7-3-2-4-8-13/h2-10,12,20-21H,11H2,1H3,(H,18,19)/t12-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337090
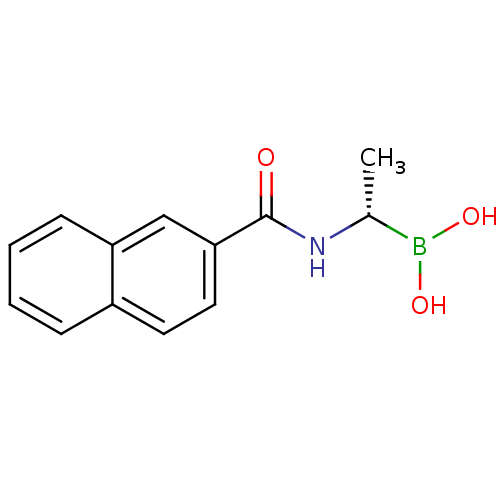
((S)-1-(Naphthalene-2-carboxamido)ethaneboronic aci...)Show InChI InChI=1S/C13H14BNO3/c1-9(14(17)18)15-13(16)12-7-6-10-4-2-3-5-11(10)8-12/h2-9,17-18H,1H3,(H,15,16)/t9-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Rattus norvegicus (Rat)) | BDBM50045333

(CHEBI:90705 | SB-203580)Show SMILES C[S+]([O-])c1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of rat P2X7 receptor |
Bioorg Med Chem Lett 25: 3164-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.001
BindingDB Entry DOI: 10.7270/Q22N5427 |
More data for this
Ligand-Target Pair | |
D-alanyl-D-alanine carboxypeptidase
(Actinomadura sp. (strain R39)) | BDBM50337097
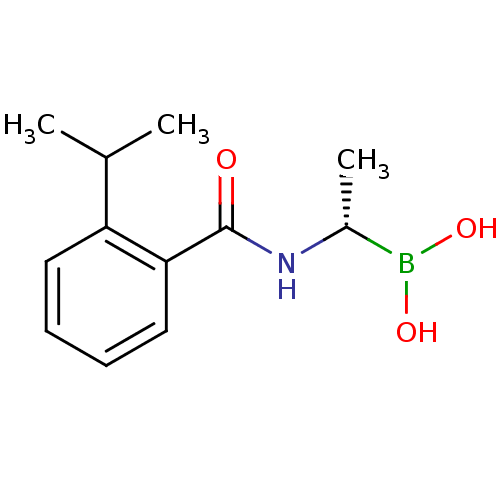
((S)-1-(2-(Propan-2-yl)benzamido)ethaneboronic acid...)Show InChI InChI=1S/C12H18BNO3/c1-8(2)10-6-4-5-7-11(10)12(15)14-9(3)13(16)17/h4-9,16-17H,1-3H3,(H,14,15)/t9-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Actinomadura R39 PBP after 60 mins |
ACS Med Chem Lett 2: 219-223 (2011)
Article DOI: 10.1021/ml100260x
BindingDB Entry DOI: 10.7270/Q25H7GJZ |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Rattus norvegicus (Rat)) | BDBM50087267

((1-(N,O-bis(1,5-isoquinolinesulfonyl)-N-methyl-L-t...)Show SMILES CN([C@@H](Cc1ccc(OS(=O)(=O)c2cccc3cnccc23)cc1)C(=O)N1CCN(CC1)c1ccccc1)S(=O)(=O)c1cccc2cnccc12 |r| Show InChI InChI=1S/C38H35N5O6S2/c1-41(50(45,46)36-11-5-7-29-26-39-19-17-33(29)36)35(38(44)43-23-21-42(22-24-43)31-9-3-2-4-10-31)25-28-13-15-32(16-14-28)49-51(47,48)37-12-6-8-30-27-40-20-18-34(30)37/h2-20,26-27,35H,21-25H2,1H3/t35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of rat P2X7 receptor |
Bioorg Med Chem Lett 25: 3164-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.001
BindingDB Entry DOI: 10.7270/Q22N5427 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Rattus norvegicus (Rat)) | BDBM50404070

(CHEMBL338117)Show SMILES Oc1csc(=O)n1C(CCc1ccncc1)COc1ccc(cc1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C24H21N3O5S/c28-23-16-33-24(29)26(23)21(7-4-17-10-12-25-13-11-17)15-32-22-8-5-18(6-9-22)19-2-1-3-20(14-19)27(30)31/h1-3,5-6,8-14,16,21,28H,4,7,15H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of rat P2X7 receptor |
Bioorg Med Chem Lett 25: 3164-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.001
BindingDB Entry DOI: 10.7270/Q22N5427 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data