 Found 132 hits with Last Name = 'sinz' and Initial = 'mw'
Found 132 hits with Last Name = 'sinz' and Initial = 'mw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614709

(CHEMBL5273615) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614704
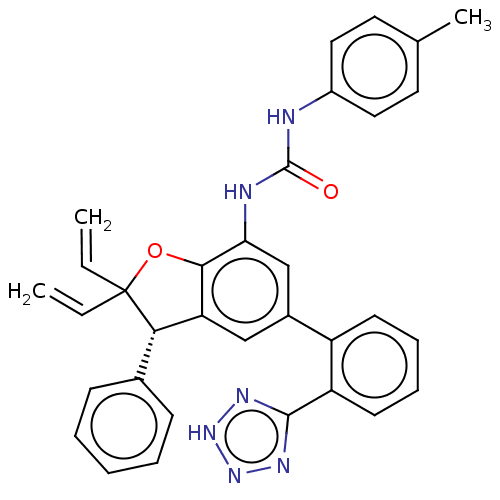
(CHEMBL5279540) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614710
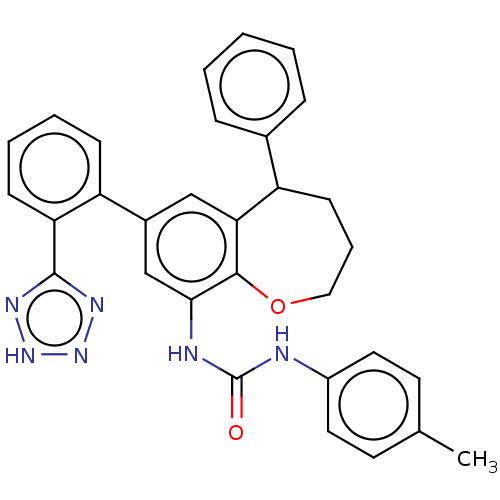
(CHEMBL5267407) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614709

(CHEMBL5273615) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614703
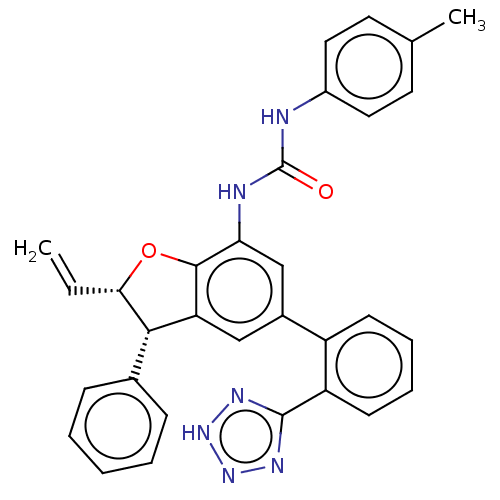
(CHEMBL5269992) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614705
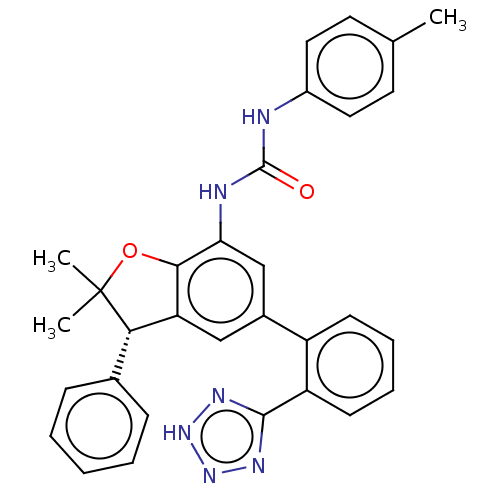
(CHEMBL5287356) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50454785
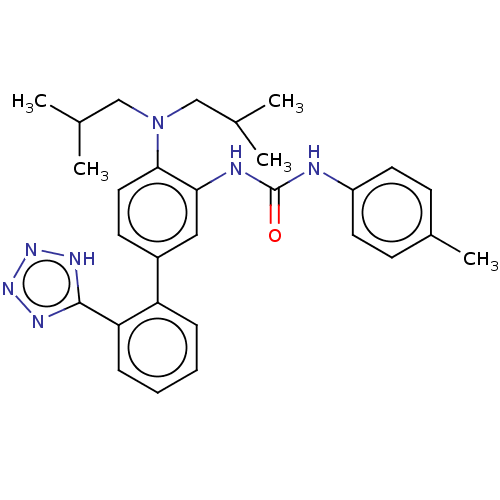
(CHEMBL4207581)Show SMILES CC(C)CN(CC(C)C)c1ccc(cc1NC(=O)Nc1ccc(C)cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H35N7O/c1-19(2)17-36(18-20(3)4)27-15-12-22(24-8-6-7-9-25(24)28-32-34-35-33-28)16-26(27)31-29(37)30-23-13-10-21(5)11-14-23/h6-16,19-20H,17-18H2,1-5H3,(H2,30,31,37)(H,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614708
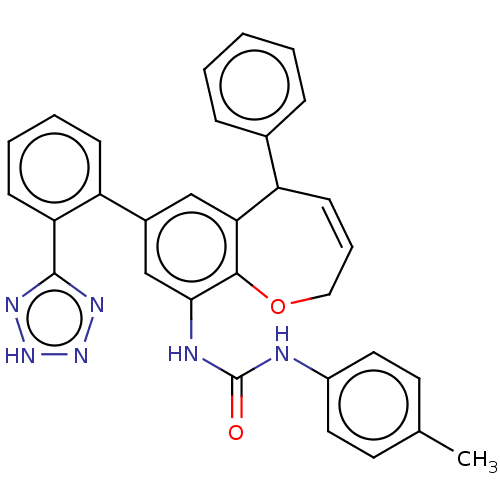
(CHEMBL5272014) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614702

(CHEMBL5285519) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614706
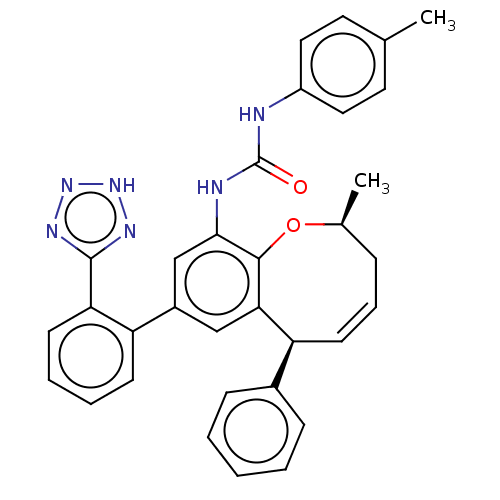
(CHEMBL5280327) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614693
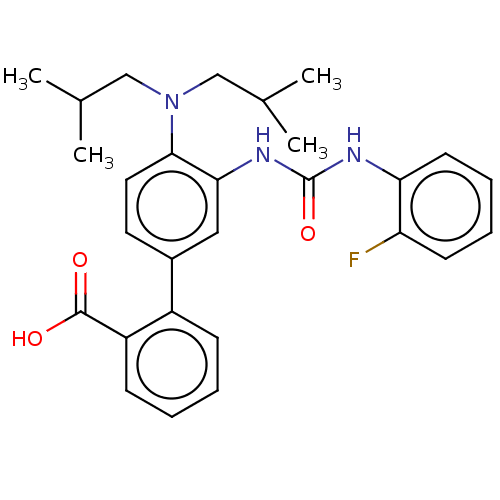
(CHEMBL5272231) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614710

(CHEMBL5267407) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27887

(4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-...)Show SMILES COCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NCCn2cc(Cl)cn2)cc[nH]c1=O Show InChI InChI=1S/C26H32ClN7O2/c1-17-13-19(18-4-8-33(9-5-18)11-12-36-2)14-22-24(17)32-25(31-22)23-21(3-6-29-26(23)35)28-7-10-34-16-20(27)15-30-34/h3,6,13-16,18H,4-5,7-12H2,1-2H3,(H,31,32)(H2,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company
| Assay Description
The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27881
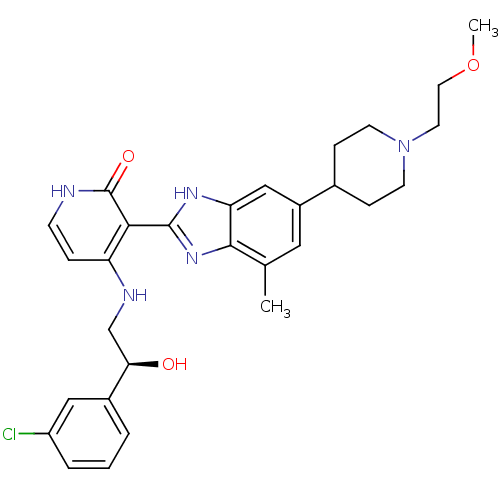
(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES COCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C29H34ClN5O3/c1-18-14-21(19-7-10-35(11-8-19)12-13-38-2)16-24-27(18)34-28(33-24)26-23(6-9-31-29(26)37)32-17-25(36)20-4-3-5-22(30)15-20/h3-6,9,14-16,19,25,36H,7-8,10-13,17H2,1-2H3,(H,33,34)(H2,31,32,37)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company
| Assay Description
The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27884
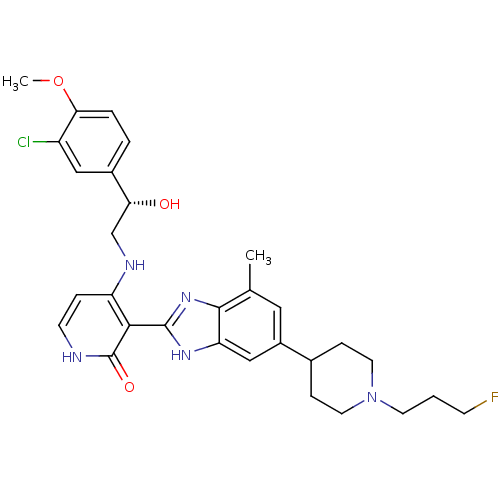
(4-{[(2S)-2-(3-chloro-4-methoxyphenyl)-2-hydroxyeth...)Show SMILES COc1ccc(cc1Cl)[C@H](O)CNc1cc[nH]c(=O)c1-c1nc2c(C)cc(cc2[nH]1)C1CCN(CCCF)CC1 |r| Show InChI InChI=1S/C30H35ClFN5O3/c1-18-14-21(19-7-12-37(13-8-19)11-3-9-32)16-24-28(18)36-29(35-24)27-23(6-10-33-30(27)39)34-17-25(38)20-4-5-26(40-2)22(31)15-20/h4-6,10,14-16,19,25,38H,3,7-9,11-13,17H2,1-2H3,(H,35,36)(H2,33,34,39)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company
| Assay Description
The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27880

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES CN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H30ClN5O2/c1-16-12-19(17-7-10-33(2)11-8-17)14-22-25(16)32-26(31-22)24-21(6-9-29-27(24)35)30-15-23(34)18-4-3-5-20(28)13-18/h3-6,9,12-14,17,23,34H,7-8,10-11,15H2,1-2H3,(H,31,32)(H2,29,30,35)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company
| Assay Description
The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27883
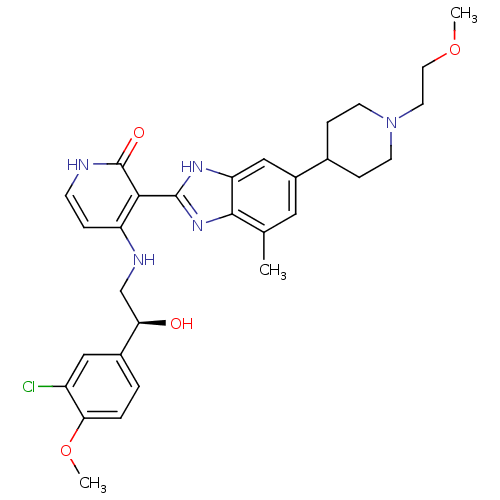
(4-{[(2S)-2-(3-chloro-4-methoxyphenyl)-2-hydroxyeth...)Show SMILES COCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2ccc(OC)c(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C30H36ClN5O4/c1-18-14-21(19-7-10-36(11-8-19)12-13-39-2)16-24-28(18)35-29(34-24)27-23(6-9-32-30(27)38)33-17-25(37)20-4-5-26(40-3)22(31)15-20/h4-6,9,14-16,19,25,37H,7-8,10-13,17H2,1-3H3,(H,34,35)(H2,32,33,38)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company
| Assay Description
The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614707
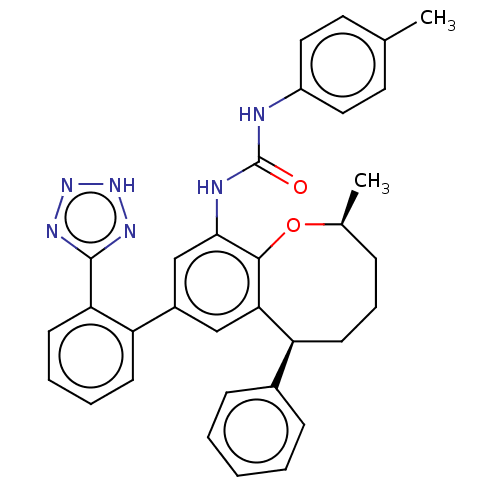
(CHEMBL5287058) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27886

(4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NCCn2cc(Cl)cn2)cc[nH]c1=O)C1CCN(CCO)CC1 Show InChI InChI=1S/C25H30ClN7O2/c1-16-12-18(17-3-7-32(8-4-17)10-11-34)13-21-23(16)31-24(30-21)22-20(2-5-28-25(22)35)27-6-9-33-15-19(26)14-29-33/h2,5,12-15,17,34H,3-4,6-11H2,1H3,(H,30,31)(H2,27,28,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company
| Assay Description
The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614699
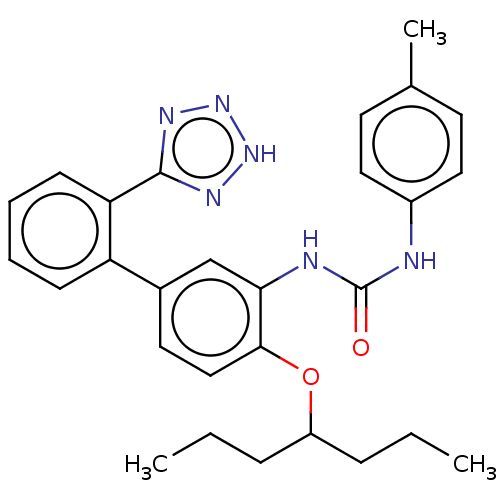
(CHEMBL5267732) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27885

(4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-[4-...)Show SMILES CN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NCCn2cc(Cl)cn2)cc[nH]c1=O Show InChI InChI=1S/C24H28ClN7O/c1-15-11-17(16-4-8-31(2)9-5-16)12-20-22(15)30-23(29-20)21-19(3-6-27-24(21)33)26-7-10-32-14-18(25)13-28-32/h3,6,11-14,16H,4-5,7-10H2,1-2H3,(H,29,30)(H2,26,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company
| Assay Description
The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614698
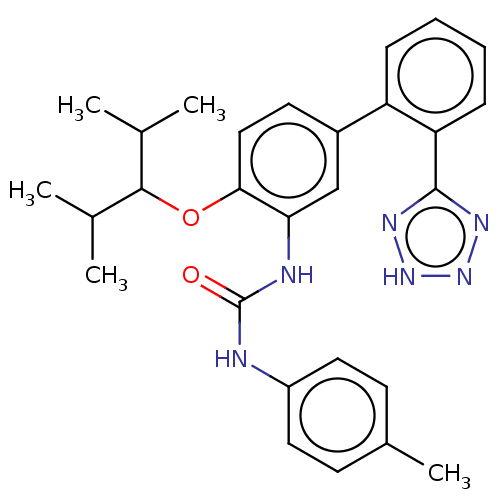
(CHEMBL5286959) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27882

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)C1CCN(CCCF)CC1 |r| Show InChI InChI=1S/C29H33ClFN5O2/c1-18-14-21(19-7-12-36(13-8-19)11-3-9-31)16-24-27(18)35-28(34-24)26-23(6-10-32-29(26)38)33-17-25(37)20-4-2-5-22(30)15-20/h2,4-6,10,14-16,19,25,37H,3,7-9,11-13,17H2,1H3,(H,34,35)(H2,32,33,38)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company
| Assay Description
The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614701
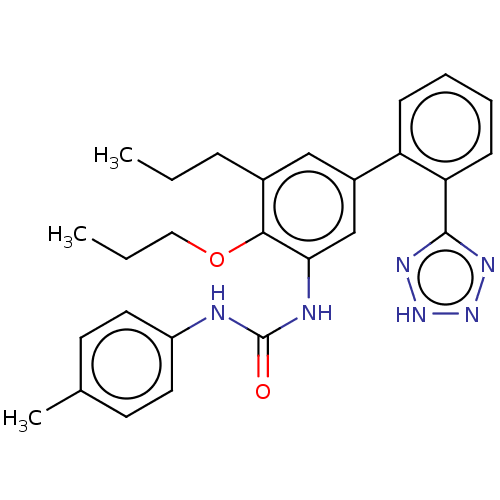
(CHEMBL5270232) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27888

(4-{[2-(4-chloro-1H-pyrazol-1-yl)ethyl]amino}-3-{6-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NCCn2cc(Cl)cn2)cc[nH]c1=O)C1CCN(CCCF)CC1 Show InChI InChI=1S/C26H31ClFN7O/c1-17-13-19(18-4-10-34(11-5-18)9-2-6-28)14-22-24(17)33-25(32-22)23-21(3-7-30-26(23)36)29-8-12-35-16-20(27)15-31-35/h3,7,13-16,18H,2,4-6,8-12H2,1H3,(H,32,33)(H2,29,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company
| Assay Description
The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM27879

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company
| Assay Description
The inhibition of recombinant human CYP3A4 was measured as the ability to perform a dealkylation of 7-benzyloxy-4-trifluoromethylcoumarin (BFC). Befo... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM27879

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Bristol-Myers Squibb Company
| Assay Description
The IGF-1 receptor tyrosine kinase was assayed using the synthetic polymer poly(Glu/Tyr) as a phosphoacceptor substrate. Incorporated radioactivity w... |
J Med Chem 51: 5897-900 (2008)
Article DOI: 10.1021/jm800832q
BindingDB Entry DOI: 10.7270/Q2513WJK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM340794
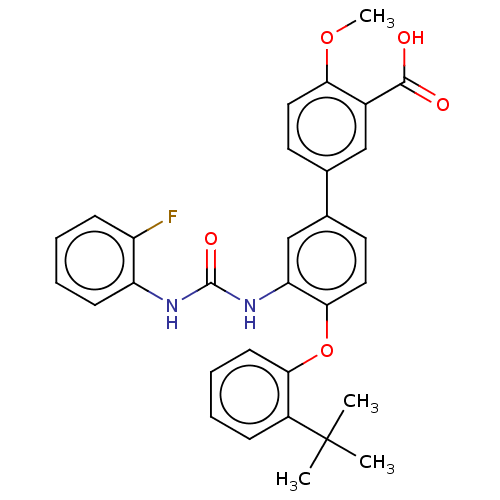
(4'-(2-(tert-butyl) phenoxy)-3'-(3-(2- fluorophenyl...)Show SMILES COc1ccc(cc1C(O)=O)-c1ccc(Oc2ccccc2C(C)(C)C)c(NC(=O)Nc2ccccc2F)c1 Show InChI InChI=1S/C31H29FN2O5/c1-31(2,3)22-9-5-8-12-27(22)39-28-16-14-20(19-13-15-26(38-4)21(17-19)29(35)36)18-25(28)34-30(37)33-24-11-7-6-10-23(24)32/h5-18H,1-4H3,(H,35,36)(H2,33,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614700
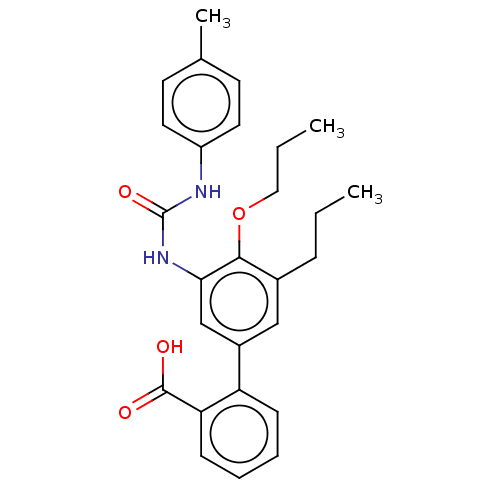
(CHEMBL5288516) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614695
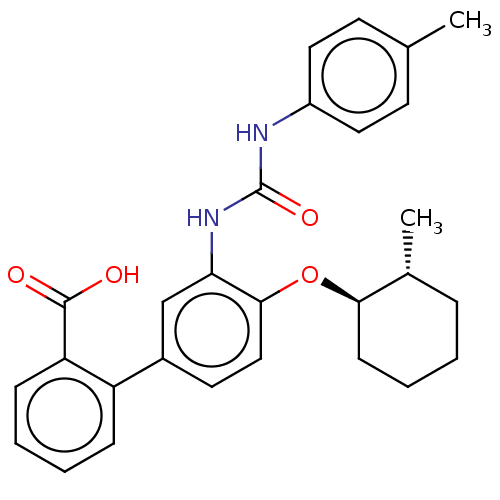
(CHEMBL5286917) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50614704

(CHEMBL5279540) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50614705

(CHEMBL5287356) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614694

(CHEMBL5283336) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50614709

(CHEMBL5273615) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50614703

(CHEMBL5269992) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614697
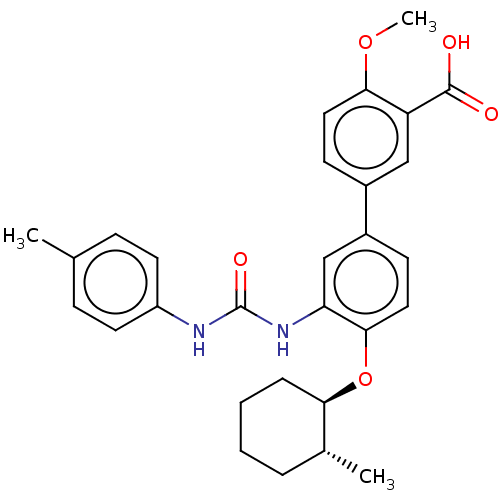
(CHEMBL5286519) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50614702

(CHEMBL5285519) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50614709

(CHEMBL5273615) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50614698

(CHEMBL5286959) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50614696

(CHEMBL5266607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50614699

(CHEMBL5267732) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50614706

(CHEMBL5280327) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50461550
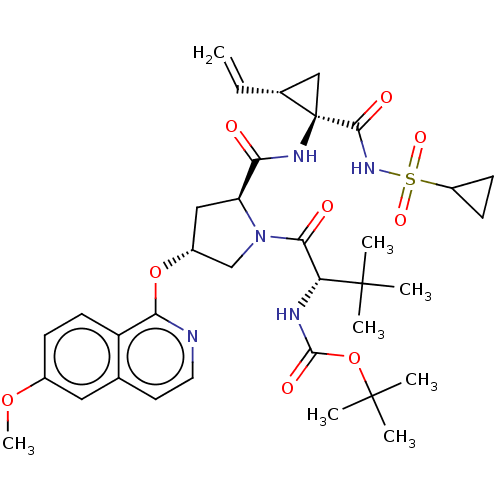
(CHEMBL2403888)Show SMILES COc1ccc2c(O[C@@H]3C[C@H](N(C3)C(=O)[C@@H](NC(=O)OC(C)(C)C)C(C)(C)C)C(=O)N[C@@]3(C[C@H]3C=C)C(=O)NS(=O)(=O)C3CC3)nccc2c1 |r| Show InChI InChI=1S/C35H47N5O9S/c1-9-21-18-35(21,31(43)39-50(45,46)24-11-12-24)38-28(41)26-17-23(48-29-25-13-10-22(47-8)16-20(25)14-15-36-29)19-40(26)30(42)27(33(2,3)4)37-32(44)49-34(5,6)7/h9-10,13-16,21,23-24,26-27H,1,11-12,17-19H2,2-8H3,(H,37,44)(H,38,41)(H,39,43)/t21-,23-,26+,27-,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 1708-29 (2014)
Article DOI: 10.1021/jm401840s
BindingDB Entry DOI: 10.7270/Q2WW7MPQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50454785

(CHEMBL4207581)Show SMILES CC(C)CN(CC(C)C)c1ccc(cc1NC(=O)Nc1ccc(C)cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H35N7O/c1-19(2)17-36(18-20(3)4)27-15-12-22(24-8-6-7-9-25(24)28-32-34-35-33-28)16-26(27)31-29(37)30-23-13-10-21(5)11-14-23/h6-16,19-20H,17-18H2,1-5H3,(H2,30,31,37)(H,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50454785

(CHEMBL4207581)Show SMILES CC(C)CN(CC(C)C)c1ccc(cc1NC(=O)Nc1ccc(C)cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C29H35N7O/c1-19(2)17-36(18-20(3)4)27-15-12-22(24-8-6-7-9-25(24)28-32-34-35-33-28)16-26(27)31-29(37)30-23-13-10-21(5)11-14-23/h6-16,19-20H,17-18H2,1-5H3,(H2,30,31,37)(H,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50614708

(CHEMBL5272014) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50614707

(CHEMBL5287058) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50614710

(CHEMBL5267407) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50614710

(CHEMBL5267407) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM340794

(4'-(2-(tert-butyl) phenoxy)-3'-(3-(2- fluorophenyl...)Show SMILES COc1ccc(cc1C(O)=O)-c1ccc(Oc2ccccc2C(C)(C)C)c(NC(=O)Nc2ccccc2F)c1 Show InChI InChI=1S/C31H29FN2O5/c1-31(2,3)22-9-5-8-12-27(22)39-28-16-14-20(19-13-15-26(38-4)21(17-19)29(35)36)18-25(28)34-30(37)33-24-11-7-6-10-23(24)32/h5-18H,1-4H3,(H,35,36)(H2,33,34,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data