 Found 388 hits with Last Name = 'srinivas' and Initial = 'k'
Found 388 hits with Last Name = 'srinivas' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542790
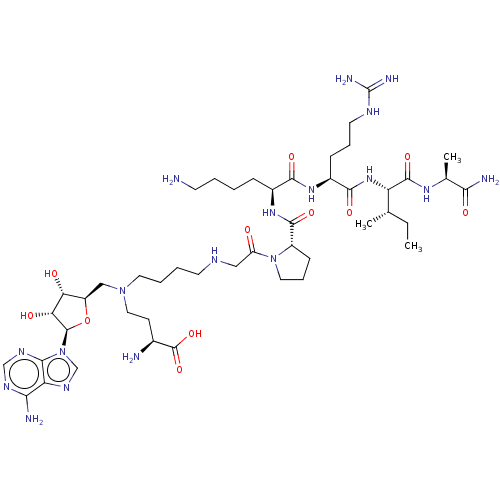
(CHEMBL4647584)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C46H80N18O11/c1-4-25(2)33(43(72)58-26(3)38(50)68)61-41(70)29(12-9-17-54-46(51)52)59-40(69)28(11-5-6-15-47)60-42(71)30-13-10-19-63(30)32(65)21-53-16-7-8-18-62(20-14-27(48)45(73)74)22-31-35(66)36(67)44(75-31)64-24-57-34-37(49)55-23-56-39(34)64/h23-31,33,35-36,44,53,66-67H,4-22,47-48H2,1-3H3,(H2,50,68)(H,58,72)(H,59,69)(H,60,71)(H,61,70)(H,73,74)(H2,49,55,56)(H4,51,52,54)/t25-,26-,27-,28-,29-,30-,31+,33-,35+,36+,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 120 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence ... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542790

(CHEMBL4647584)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C46H80N18O11/c1-4-25(2)33(43(72)58-26(3)38(50)68)61-41(70)29(12-9-17-54-46(51)52)59-40(69)28(11-5-6-15-47)60-42(71)30-13-10-19-63(30)32(65)21-53-16-7-8-18-62(20-14-27(48)45(73)74)22-31-35(66)36(67)44(75-31)64-24-57-34-37(49)55-23-56-39(34)64/h23-31,33,35-36,44,53,66-67H,4-22,47-48H2,1-3H3,(H2,50,68)(H,58,72)(H,59,69)(H,60,71)(H,61,70)(H,73,74)(H2,49,55,56)(H4,51,52,54)/t25-,26-,27-,28-,29-,30-,31+,33-,35+,36+,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 30 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542790

(CHEMBL4647584)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C46H80N18O11/c1-4-25(2)33(43(72)58-26(3)38(50)68)61-41(70)29(12-9-17-54-46(51)52)59-40(69)28(11-5-6-15-47)60-42(71)30-13-10-19-63(30)32(65)21-53-16-7-8-18-62(20-14-27(48)45(73)74)22-31-35(66)36(67)44(75-31)64-24-57-34-37(49)55-23-56-39(34)64/h23-31,33,35-36,44,53,66-67H,4-22,47-48H2,1-3H3,(H2,50,68)(H,58,72)(H,59,69)(H,60,71)(H,61,70)(H,73,74)(H2,49,55,56)(H4,51,52,54)/t25-,26-,27-,28-,29-,30-,31+,33-,35+,36+,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 90 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542790

(CHEMBL4647584)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C46H80N18O11/c1-4-25(2)33(43(72)58-26(3)38(50)68)61-41(70)29(12-9-17-54-46(51)52)59-40(69)28(11-5-6-15-47)60-42(71)30-13-10-19-63(30)32(65)21-53-16-7-8-18-62(20-14-27(48)45(73)74)22-31-35(66)36(67)44(75-31)64-24-57-34-37(49)55-23-56-39(34)64/h23-31,33,35-36,44,53,66-67H,4-22,47-48H2,1-3H3,(H2,50,68)(H,58,72)(H,59,69)(H,60,71)(H,61,70)(H,73,74)(H2,49,55,56)(H4,51,52,54)/t25-,26-,27-,28-,29-,30-,31+,33-,35+,36+,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 60 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542789
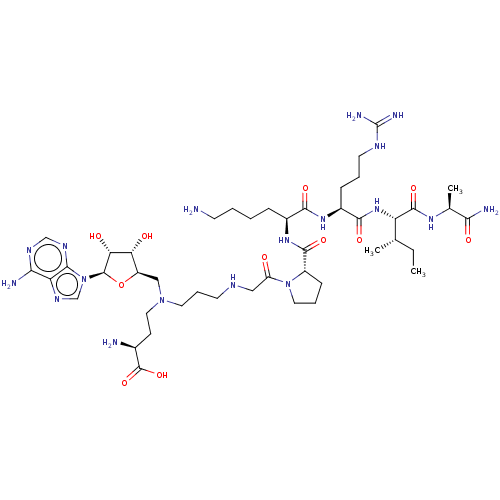
(CHEMBL4647726)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C45H78N18O11/c1-4-24(2)32(42(71)57-25(3)37(49)67)60-40(69)28(11-7-16-53-45(50)51)58-39(68)27(10-5-6-14-46)59-41(70)29-12-8-18-62(29)31(64)20-52-15-9-17-61(19-13-26(47)44(72)73)21-30-34(65)35(66)43(74-30)63-23-56-33-36(48)54-22-55-38(33)63/h22-30,32,34-35,43,52,65-66H,4-21,46-47H2,1-3H3,(H2,49,67)(H,57,71)(H,58,68)(H,59,70)(H,60,69)(H,72,73)(H2,48,54,55)(H4,50,51,53)/t24-,25-,26-,27-,28-,29-,30+,32-,34+,35+,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 60 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542789

(CHEMBL4647726)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C45H78N18O11/c1-4-24(2)32(42(71)57-25(3)37(49)67)60-40(69)28(11-7-16-53-45(50)51)58-39(68)27(10-5-6-14-46)59-41(70)29-12-8-18-62(29)31(64)20-52-15-9-17-61(19-13-26(47)44(72)73)21-30-34(65)35(66)43(74-30)63-23-56-33-36(48)54-22-55-38(33)63/h22-30,32,34-35,43,52,65-66H,4-21,46-47H2,1-3H3,(H2,49,67)(H,57,71)(H,58,68)(H,59,70)(H,60,69)(H,72,73)(H2,48,54,55)(H4,50,51,53)/t24-,25-,26-,27-,28-,29-,30+,32-,34+,35+,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 120 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence ... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542789

(CHEMBL4647726)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C45H78N18O11/c1-4-24(2)32(42(71)57-25(3)37(49)67)60-40(69)28(11-7-16-53-45(50)51)58-39(68)27(10-5-6-14-46)59-41(70)29-12-8-18-62(29)31(64)20-52-15-9-17-61(19-13-26(47)44(72)73)21-30-34(65)35(66)43(74-30)63-23-56-33-36(48)54-22-55-38(33)63/h22-30,32,34-35,43,52,65-66H,4-21,46-47H2,1-3H3,(H2,49,67)(H,57,71)(H,58,68)(H,59,70)(H,60,69)(H,72,73)(H2,48,54,55)(H4,50,51,53)/t24-,25-,26-,27-,28-,29-,30+,32-,34+,35+,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 30 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542789

(CHEMBL4647726)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C45H78N18O11/c1-4-24(2)32(42(71)57-25(3)37(49)67)60-40(69)28(11-7-16-53-45(50)51)58-39(68)27(10-5-6-14-46)59-41(70)29-12-8-18-62(29)31(64)20-52-15-9-17-61(19-13-26(47)44(72)73)21-30-34(65)35(66)43(74-30)63-23-56-33-36(48)54-22-55-38(33)63/h22-30,32,34-35,43,52,65-66H,4-21,46-47H2,1-3H3,(H2,49,67)(H,57,71)(H,58,68)(H,59,70)(H,60,69)(H,72,73)(H2,48,54,55)(H4,50,51,53)/t24-,25-,26-,27-,28-,29-,30+,32-,34+,35+,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 90 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542788
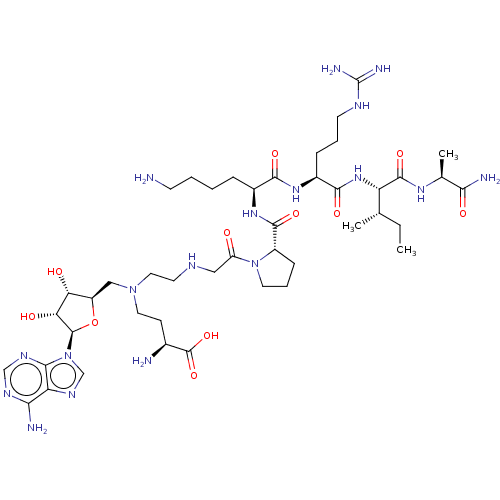
(CHEMBL4645959)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C44H76N18O11/c1-4-23(2)31(41(70)56-24(3)36(48)66)59-39(68)27(10-7-14-52-44(49)50)57-38(67)26(9-5-6-13-45)58-40(69)28-11-8-16-61(28)30(63)19-51-15-18-60(17-12-25(46)43(71)72)20-29-33(64)34(65)42(73-29)62-22-55-32-35(47)53-21-54-37(32)62/h21-29,31,33-34,42,51,64-65H,4-20,45-46H2,1-3H3,(H2,48,66)(H,56,70)(H,57,67)(H,58,69)(H,59,68)(H,71,72)(H2,47,53,54)(H4,49,50,52)/t23-,24-,25-,26-,27-,28-,29+,31-,33+,34+,42+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 90 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542788

(CHEMBL4645959)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C44H76N18O11/c1-4-23(2)31(41(70)56-24(3)36(48)66)59-39(68)27(10-7-14-52-44(49)50)57-38(67)26(9-5-6-13-45)58-40(69)28-11-8-16-61(28)30(63)19-51-15-18-60(17-12-25(46)43(71)72)20-29-33(64)34(65)42(73-29)62-22-55-32-35(47)53-21-54-37(32)62/h21-29,31,33-34,42,51,64-65H,4-20,45-46H2,1-3H3,(H2,48,66)(H,56,70)(H,57,67)(H,58,69)(H,59,68)(H,71,72)(H2,47,53,54)(H4,49,50,52)/t23-,24-,25-,26-,27-,28-,29+,31-,33+,34+,42+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 120 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence ... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542788

(CHEMBL4645959)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C44H76N18O11/c1-4-23(2)31(41(70)56-24(3)36(48)66)59-39(68)27(10-7-14-52-44(49)50)57-38(67)26(9-5-6-13-45)58-40(69)28-11-8-16-61(28)30(63)19-51-15-18-60(17-12-25(46)43(71)72)20-29-33(64)34(65)42(73-29)62-22-55-32-35(47)53-21-54-37(32)62/h21-29,31,33-34,42,51,64-65H,4-20,45-46H2,1-3H3,(H2,48,66)(H,56,70)(H,57,67)(H,58,69)(H,59,68)(H,71,72)(H2,47,53,54)(H4,49,50,52)/t23-,24-,25-,26-,27-,28-,29+,31-,33+,34+,42+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 30 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542788

(CHEMBL4645959)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C44H76N18O11/c1-4-23(2)31(41(70)56-24(3)36(48)66)59-39(68)27(10-7-14-52-44(49)50)57-38(67)26(9-5-6-13-45)58-40(69)28-11-8-16-61(28)30(63)19-51-15-18-60(17-12-25(46)43(71)72)20-29-33(64)34(65)42(73-29)62-22-55-32-35(47)53-21-54-37(32)62/h21-29,31,33-34,42,51,64-65H,4-20,45-46H2,1-3H3,(H2,48,66)(H,56,70)(H,57,67)(H,58,69)(H,59,68)(H,71,72)(H2,47,53,54)(H4,49,50,52)/t23-,24-,25-,26-,27-,28-,29+,31-,33+,34+,42+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 60 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542790

(CHEMBL4647584)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C46H80N18O11/c1-4-25(2)33(43(72)58-26(3)38(50)68)61-41(70)29(12-9-17-54-46(51)52)59-40(69)28(11-5-6-15-47)60-42(71)30-13-10-19-63(30)32(65)21-53-16-7-8-18-62(20-14-27(48)45(73)74)22-31-35(66)36(67)44(75-31)64-24-57-34-37(49)55-23-56-39(34)64/h23-31,33,35-36,44,53,66-67H,4-22,47-48H2,1-3H3,(H2,50,68)(H,58,72)(H,59,69)(H,60,71)(H,61,70)(H,73,74)(H2,49,55,56)(H4,51,52,54)/t25-,26-,27-,28-,29-,30-,31+,33-,35+,36+,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 10 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542789

(CHEMBL4647726)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C45H78N18O11/c1-4-24(2)32(42(71)57-25(3)37(49)67)60-40(69)28(11-7-16-53-45(50)51)58-39(68)27(10-5-6-14-46)59-41(70)29-12-8-18-62(29)31(64)20-52-15-9-17-61(19-13-26(47)44(72)73)21-30-34(65)35(66)43(74-30)63-23-56-33-36(48)54-22-55-38(33)63/h22-30,32,34-35,43,52,65-66H,4-21,46-47H2,1-3H3,(H2,49,67)(H,57,71)(H,58,68)(H,59,70)(H,60,69)(H,72,73)(H2,48,54,55)(H4,50,51,53)/t24-,25-,26-,27-,28-,29-,30+,32-,34+,35+,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 10 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542788

(CHEMBL4645959)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C44H76N18O11/c1-4-23(2)31(41(70)56-24(3)36(48)66)59-39(68)27(10-7-14-52-44(49)50)57-38(67)26(9-5-6-13-45)58-40(69)28-11-8-16-61(28)30(63)19-51-15-18-60(17-12-25(46)43(71)72)20-29-33(64)34(65)42(73-29)62-22-55-32-35(47)53-21-54-37(32)62/h21-29,31,33-34,42,51,64-65H,4-20,45-46H2,1-3H3,(H2,48,66)(H,56,70)(H,57,67)(H,58,69)(H,59,68)(H,71,72)(H2,47,53,54)(H4,49,50,52)/t23-,24-,25-,26-,27-,28-,29+,31-,33+,34+,42+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of NTMT1 (unknown origin) pre-incubated for 10 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence a... |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242265
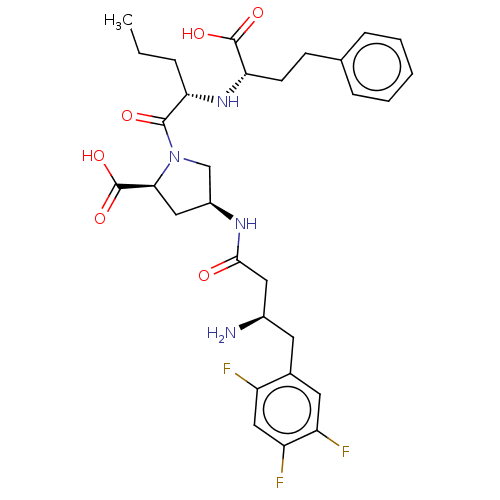
(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50242266
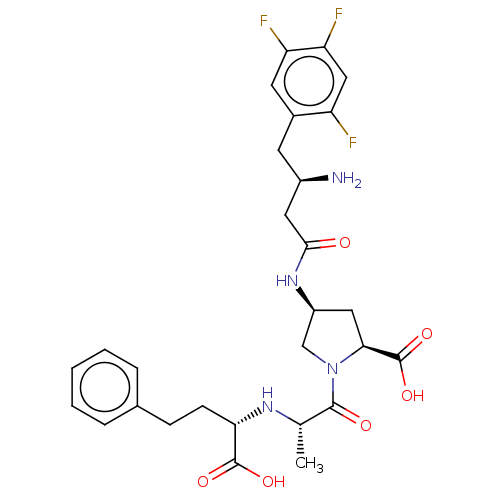
(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542790

(CHEMBL4647584)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C46H80N18O11/c1-4-25(2)33(43(72)58-26(3)38(50)68)61-41(70)29(12-9-17-54-46(51)52)59-40(69)28(11-5-6-15-47)60-42(71)30-13-10-19-63(30)32(65)21-53-16-7-8-18-62(20-14-27(48)45(73)74)22-31-35(66)36(67)44(75-31)64-24-57-34-37(49)55-23-56-39(34)64/h23-31,33,35-36,44,53,66-67H,4-22,47-48H2,1-3H3,(H2,50,68)(H,58,72)(H,59,69)(H,60,71)(H,61,70)(H,73,74)(H2,49,55,56)(H4,51,52,54)/t25-,26-,27-,28-,29-,30-,31+,33-,35+,36+,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of NTMT1 (unknown origin) pre-incubated for 10 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence assay |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542793

(CHEMBL4635903)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)CNCCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C37H64N16O9/c38-11-2-1-7-23(33(58)49-22(31(41)57)8-5-13-45-37(42)43)50-34(59)24-9-6-15-52(24)26(54)17-44-12-3-4-14-51(16-10-21(39)36(60)61)18-25-28(55)29(56)35(62-25)53-20-48-27-30(40)46-19-47-32(27)53/h19-25,28-29,35,44,55-56H,1-18,38-39H2,(H2,41,57)(H,49,58)(H,50,59)(H,60,61)(H2,40,46,47)(H4,42,43,45)/t21-,22-,23-,24-,25+,28+,29+,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of NTMT1 (unknown origin) pre-incubated for 10 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence assay |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Mus musculus) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50049730
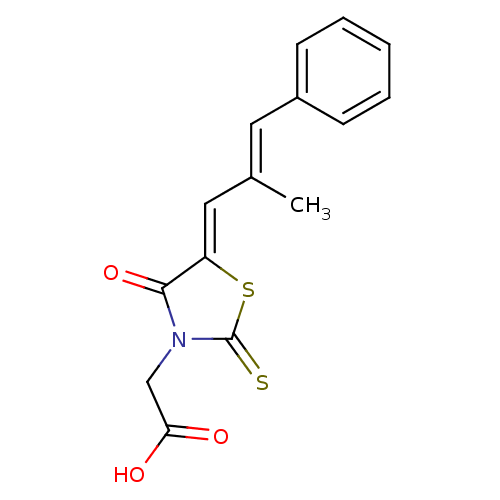
(2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxot...)Show InChI InChI=1S/C15H13NO3S2/c1-10(7-11-5-3-2-4-6-11)8-12-14(19)16(9-13(17)18)15(20)21-12/h2-8H,9H2,1H3,(H,17,18)/b10-7+,12-8- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... |
Eur J Med Chem 71: 53-66 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.043
BindingDB Entry DOI: 10.7270/Q2H996P7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242274
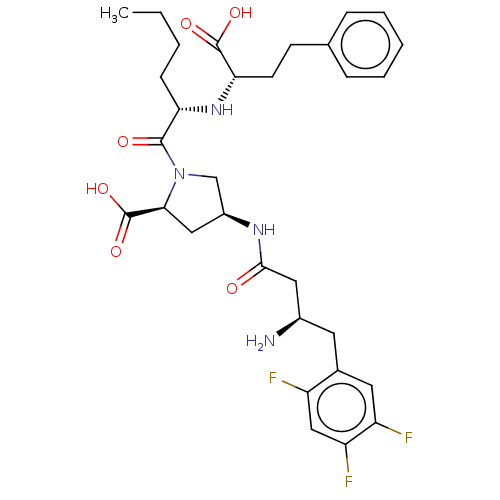
(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542789

(CHEMBL4647726)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C45H78N18O11/c1-4-24(2)32(42(71)57-25(3)37(49)67)60-40(69)28(11-7-16-53-45(50)51)58-39(68)27(10-5-6-14-46)59-41(70)29-12-8-18-62(29)31(64)20-52-15-9-17-61(19-13-26(47)44(72)73)21-30-34(65)35(66)43(74-30)63-23-56-33-36(48)54-22-55-38(33)63/h22-30,32,34-35,43,52,65-66H,4-21,46-47H2,1-3H3,(H2,49,67)(H,57,71)(H,58,68)(H,59,70)(H,60,69)(H,72,73)(H2,48,54,55)(H4,50,51,53)/t24-,25-,26-,27-,28-,29-,30+,32-,34+,35+,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of NTMT1 (unknown origin) pre-incubated for 10 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence assay |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542792
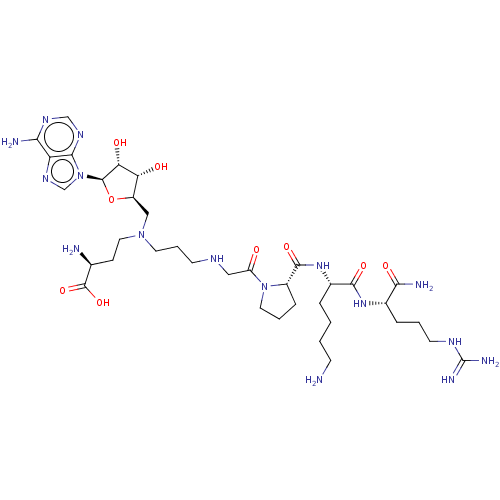
(CHEMBL4636288)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)CNCCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C36H62N16O9/c37-10-2-1-6-22(32(57)48-21(30(40)56)7-3-12-44-36(41)42)49-33(58)23-8-4-14-51(23)25(53)16-43-11-5-13-50(15-9-20(38)35(59)60)17-24-27(54)28(55)34(61-24)52-19-47-26-29(39)45-18-46-31(26)52/h18-24,27-28,34,43,54-55H,1-17,37-38H2,(H2,40,56)(H,48,57)(H,49,58)(H,59,60)(H2,39,45,46)(H4,41,42,44)/t20-,21-,22-,23-,24+,27+,28+,34+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of NTMT1 (unknown origin) pre-incubated for 10 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence assay |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50444638
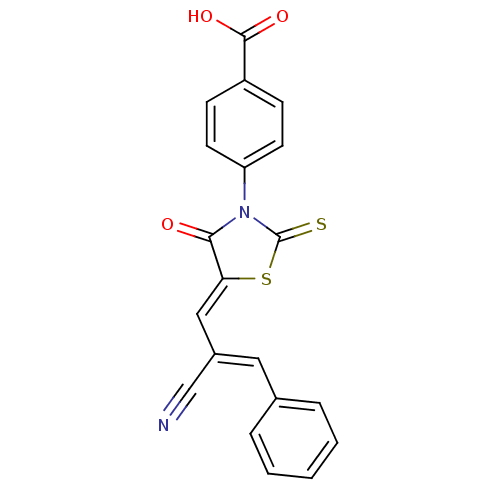
(CHEMBL3098361)Show SMILES OC(=O)c1ccc(cc1)N1C(=S)S\C(=C/C(=C/c2ccccc2)/C#N)C1=O Show InChI InChI=1S/C20H12N2O3S2/c21-12-14(10-13-4-2-1-3-5-13)11-17-18(23)22(20(26)27-17)16-8-6-15(7-9-16)19(24)25/h1-11H,(H,24,25)/b14-10-,17-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... |
Eur J Med Chem 71: 53-66 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.043
BindingDB Entry DOI: 10.7270/Q2H996P7 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542788

(CHEMBL4645959)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)CNCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C44H76N18O11/c1-4-23(2)31(41(70)56-24(3)36(48)66)59-39(68)27(10-7-14-52-44(49)50)57-38(67)26(9-5-6-13-45)58-40(69)28-11-8-16-61(28)30(63)19-51-15-18-60(17-12-25(46)43(71)72)20-29-33(64)34(65)42(73-29)62-22-55-32-35(47)53-21-54-37(32)62/h21-29,31,33-34,42,51,64-65H,4-20,45-46H2,1-3H3,(H2,48,66)(H,56,70)(H,57,67)(H,58,69)(H,59,68)(H,71,72)(H2,47,53,54)(H4,49,50,52)/t23-,24-,25-,26-,27-,28-,29+,31-,33+,34+,42+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of NTMT1 (unknown origin) pre-incubated for 10 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence assay |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50242274

(CHEMBL4090635)Show SMILES CCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C31H39F3N4O6/c1-2-3-9-25(37-26(30(41)42)11-10-18-7-5-4-6-8-18)29(40)38-17-21(15-27(38)31(43)44)36-28(39)14-20(35)12-19-13-23(33)24(34)16-22(19)32/h4-8,13,16,20-21,25-27,37H,2-3,9-12,14-15,17,35H2,1H3,(H,36,39)(H,41,42)(H,43,44)/t20-,21+,25+,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
N-terminal Xaa-Pro-Lys N-methyltransferase 1
(Homo sapiens) | BDBM50542791

(CHEMBL4633108)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)CNCCN(CC[C@H](N)C(O)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C35H60N16O9/c36-9-2-1-5-21(31(56)47-20(29(39)55)6-3-10-43-35(40)41)48-32(57)22-7-4-12-50(22)24(52)15-42-11-14-49(13-8-19(37)34(58)59)16-23-26(53)27(54)33(60-23)51-18-46-25-28(38)44-17-45-30(25)51/h17-23,26-27,33,42,53-54H,1-16,36-37H2,(H2,39,55)(H,47,56)(H,48,57)(H,58,59)(H2,38,44,45)(H4,40,41,43)/t19-,20-,21-,22-,23+,26+,27+,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of NTMT1 (unknown origin) pre-incubated for 10 mins before GPKRIA peptide substrate addition by SAHH coupled fluorescence assay |
J Med Chem 63: 8419-8431 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00770
BindingDB Entry DOI: 10.7270/Q2B85CQB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50242266

(CHEMBL4067185)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O6/c1-15(33-23(27(38)39)8-7-16-5-3-2-4-6-16)26(37)35-14-19(12-24(35)28(40)41)34-25(36)11-18(32)9-17-10-21(30)22(31)13-20(17)29/h2-6,10,13,15,18-19,23-24,33H,7-9,11-12,14,32H2,1H3,(H,34,36)(H,38,39)(H,40,41)/t15-,18+,19-,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Mus musculus) | BDBM50242264

(CHEMBL4086264)Show SMILES CC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C29H35F3N4O6/c1-2-23(35-24(28(39)40)9-8-16-6-4-3-5-7-16)27(38)36-15-19(13-25(36)29(41)42)34-26(37)12-18(33)10-17-11-21(31)22(32)14-20(17)30/h3-7,11,14,18-19,23-25,35H,2,8-10,12-13,15,33H2,1H3,(H,34,37)(H,39,40)(H,41,42)/t18-,19+,23+,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 488 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of ob/ob mouse plasma DPP4 |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50444641

(CHEMBL3098447)Show SMILES OC(=O)CN1C(=S)S\C(=C/C(=C/c2ccco2)/C#N)C1=O Show InChI InChI=1S/C13H8N2O4S2/c14-6-8(4-9-2-1-3-19-9)5-10-12(18)15(7-11(16)17)13(20)21-10/h1-5H,7H2,(H,16,17)/b8-4-,10-5- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... |
Eur J Med Chem 71: 53-66 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.043
BindingDB Entry DOI: 10.7270/Q2H996P7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50444640
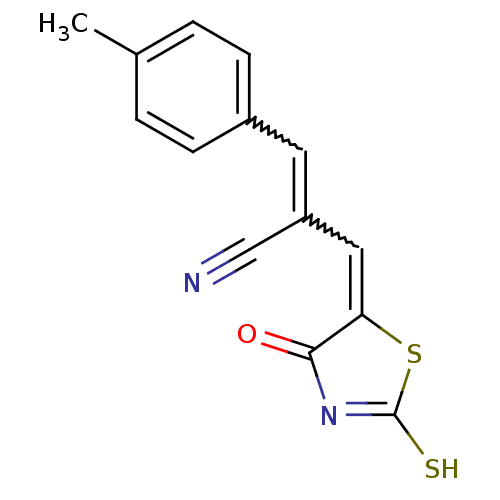
(CHEMBL3098450)Show SMILES Cc1ccc(C=C(C=C2SC(S)=NC2=O)C#N)cc1 |w:5.4,7.6,c:11| Show InChI InChI=1S/C14H10N2OS2/c1-9-2-4-10(5-3-9)6-11(8-15)7-12-13(17)16-14(18)19-12/h2-7H,1H3,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... |
Eur J Med Chem 71: 53-66 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.043
BindingDB Entry DOI: 10.7270/Q2H996P7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50444642

(CHEMBL3098459)Show SMILES OC(=O)CN1C(=S)S\C(=C/C(=C/c2ccccc2)/C#N)C1=O Show InChI InChI=1S/C15H10N2O3S2/c16-8-11(6-10-4-2-1-3-5-10)7-12-14(20)17(9-13(18)19)15(21)22-12/h1-7H,9H2,(H,18,19)/b11-6-,12-7- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... |
Eur J Med Chem 71: 53-66 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.043
BindingDB Entry DOI: 10.7270/Q2H996P7 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1
(Rattus norvegicus) | BDBM50444639
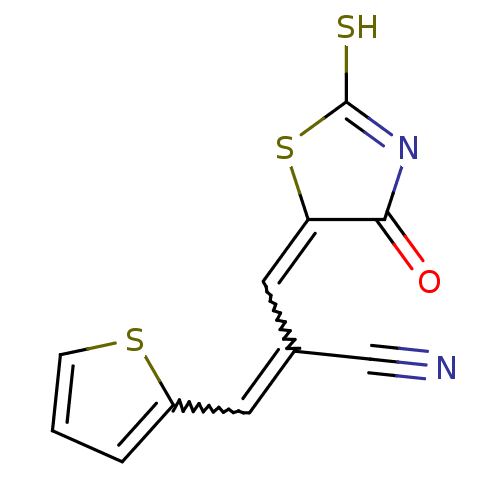
(CHEMBL3098360)Show SMILES SC1=NC(=O)C(S1)=CC(=Cc1cccs1)C#N |w:9.10,7.8,t:1| Show InChI InChI=1S/C11H6N2OS3/c12-6-7(4-8-2-1-3-16-8)5-9-10(14)13-11(15)17-9/h1-5H,(H,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of rat kidney NADPH-dependent aldose reductase assessed as DL-glyceraldehyde conversion to glycerol preincubated for 20 mins followed by N... |
Eur J Med Chem 71: 53-66 (2014)
Article DOI: 10.1016/j.ejmech.2013.10.043
BindingDB Entry DOI: 10.7270/Q2H996P7 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50242265

(CHEMBL4078112)Show SMILES CCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1C[C@H](C[C@H]1C(O)=O)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C30H37F3N4O6/c1-2-6-24(36-25(29(40)41)10-9-17-7-4-3-5-8-17)28(39)37-16-20(14-26(37)30(42)43)35-27(38)13-19(34)11-18-12-22(32)23(33)15-21(18)31/h3-5,7-8,12,15,19-20,24-26,36H,2,6,9-11,13-14,16,34H2,1H3,(H,35,38)(H,40,41)(H,42,43)/t19-,20+,24+,25+,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 893 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat plasma ACE |
Bioorg Med Chem Lett 27: 2313-2318 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.036
BindingDB Entry DOI: 10.7270/Q2ZC858S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data