

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged PPAR-alpha LBD expressed in Escherichia coli BL21 (DE3) PlysS after 30 mins in presence of fluorescein ligand FL... | ACS Med Chem Lett 7: 590-4 (2016) Article DOI: 10.1021/acsmedchemlett.6b00033 BindingDB Entry DOI: 10.7270/Q2CR5W99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | 4 | n/a | n/a | 8.0 | 22 |
Bristol-Myers Squibb Company | Assay Description For hPPAR alpha, percentage inhibition was calculated relative to unlabeled GW2331, which was used as the active site-specific competitive binder. Fl... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314811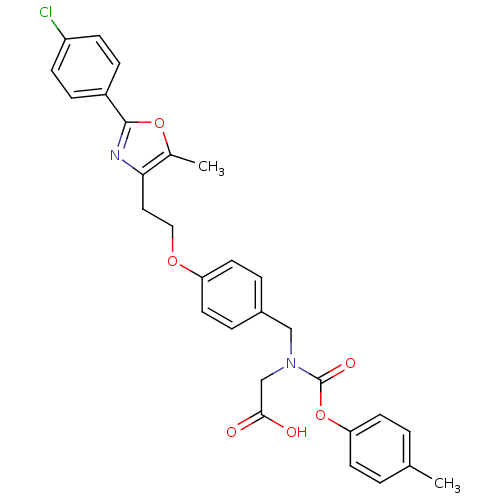 (2-((4-(2-(2-(4-chlorophenyl)-5-methyloxazol-4-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314813 (2-((3-(2-(2-(4-chlorophenyl)-5-methyloxazol-4-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314832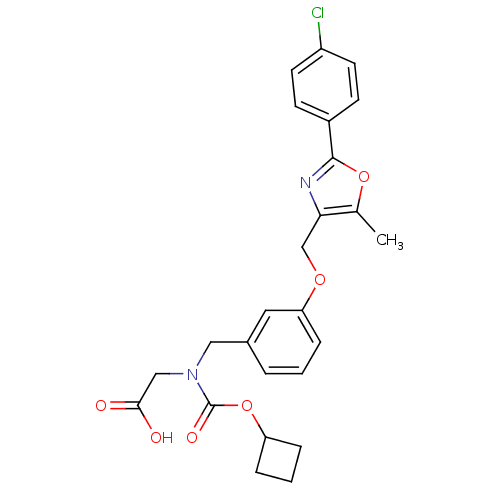 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314812 (2-((4-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28800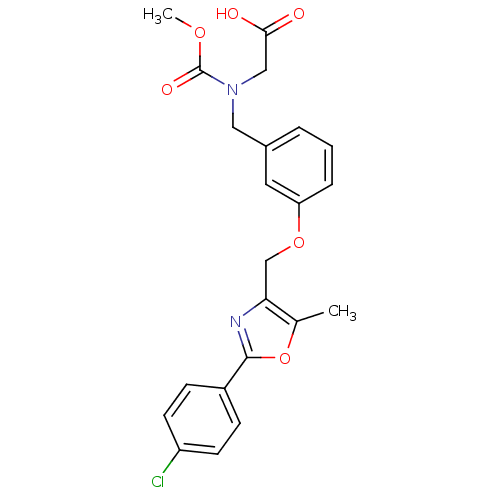 (2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28800 (2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 260 | n/a | 10 | n/a | n/a | 8.0 | 22 |
Bristol-Myers Squibb Company | Assay Description For hPPAR alpha, percentage inhibition was calculated relative to unlabeled GW2331, which was used as the active site-specific competitive binder. Fl... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314834 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314814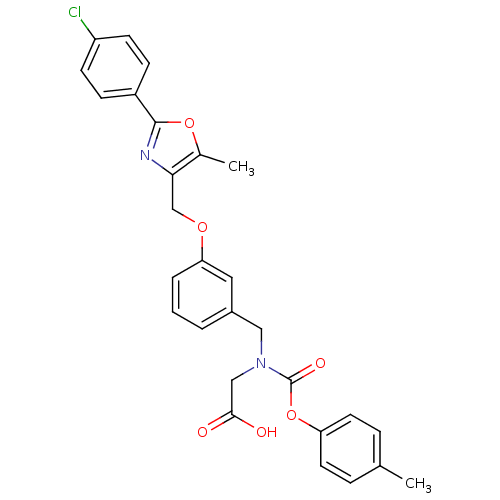 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314833 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 351 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314820 (2-((3-((2-(4-fluorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314823 (2-((3-((5-methyl-2-p-tolyloxazol-4-yl)methoxy)benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314822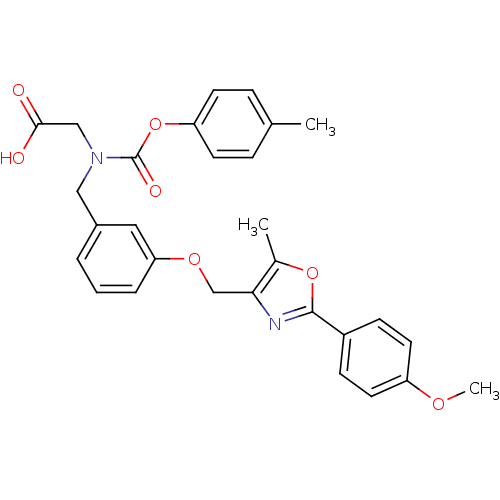 (2-((3-((2-(4-methoxyphenyl)-5-methyloxazol-4-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 375 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314819 (2-((3-((2-(3-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314831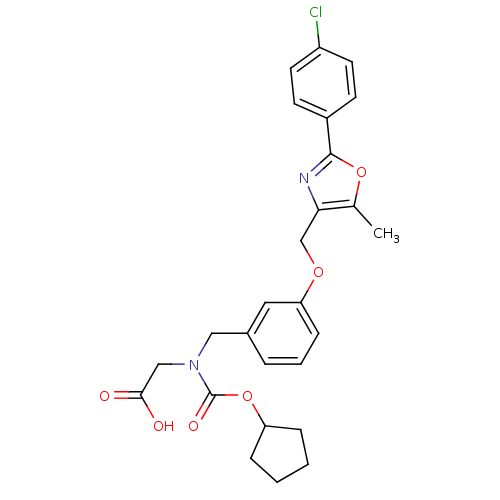 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 408 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314818 (2-((3-((2-(2-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314819 (2-((3-((2-(3-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314821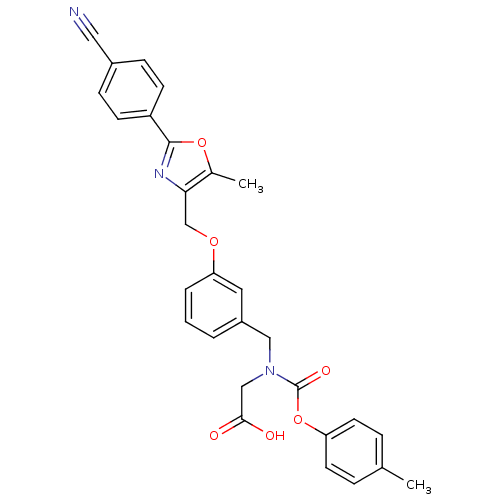 (2-((3-((2-(4-cyanophenyl)-5-methyloxazol-4-yl)meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 473 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314826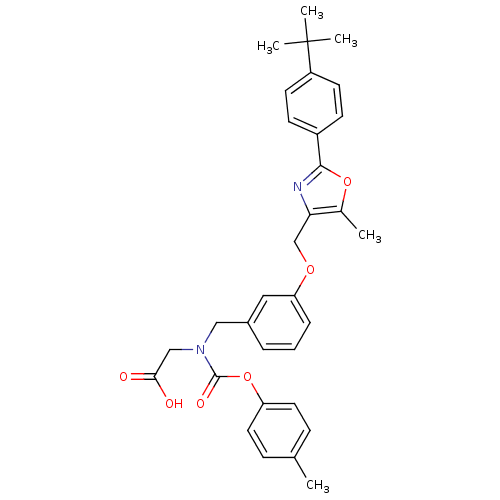 (2-((3-((2-(4-tert-butylphenyl)-5-methyloxazol-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 483 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314815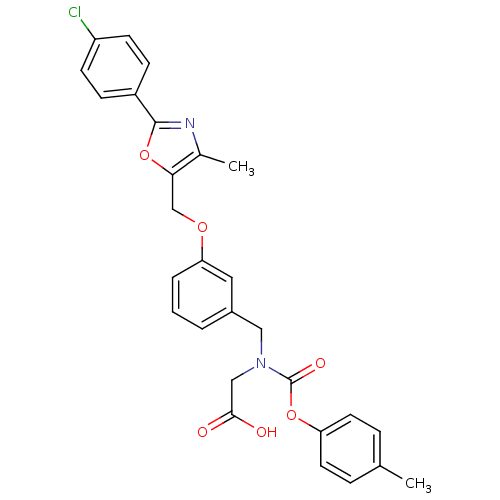 (2-((3-((2-(4-chlorophenyl)-4-methyloxazol-5-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 485 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314829 (2-((3-((2-(4-isopropylphenyl)-5-methyloxazol-4-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 497 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50314819 (2-((3-((2-(3-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314830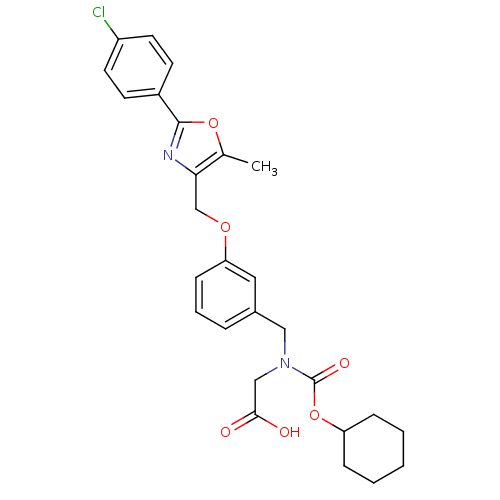 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314829 (2-((3-((2-(4-isopropylphenyl)-5-methyloxazol-4-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314824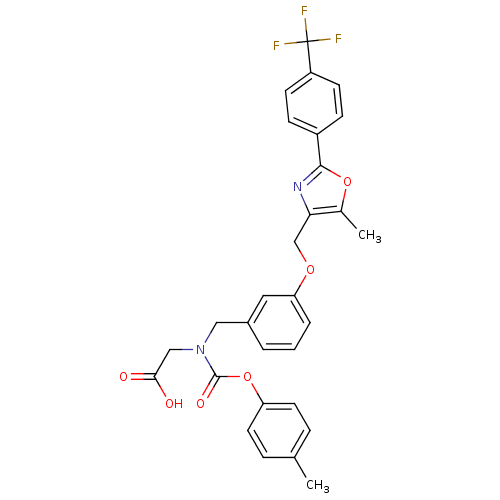 (2-((3-((5-methyl-2-(4-(trifluoromethyl)phenyl)oxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 569 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314817 (2-((3-((2-(4-chlorophenyl)-5-methylthiazol-4-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 582 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314813 (2-((3-(2-(2-(4-chlorophenyl)-5-methyloxazol-4-yl)e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50189566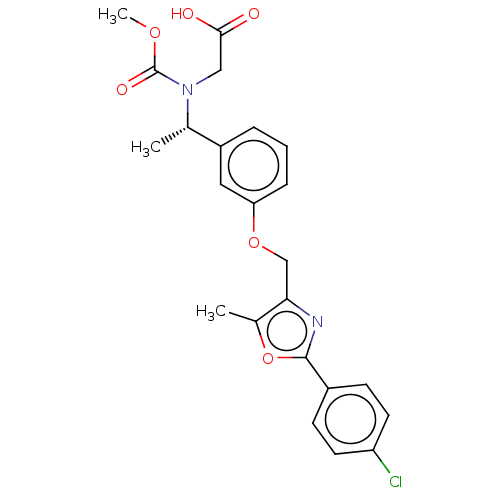 (CHEMBL3827820) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 671 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged PPAR-alpha LBD expressed in Escherichia coli BL21 (DE3) PlysS after 30 mins in presence of fluorescein ligand FL... | ACS Med Chem Lett 7: 590-4 (2016) Article DOI: 10.1021/acsmedchemlett.6b00033 BindingDB Entry DOI: 10.7270/Q2CR5W99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314816 (2-((3-((2-(4-chlorophenyl)-4-methylthiazol-5-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314812 (2-((4-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 811 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314822 (2-((3-((2-(4-methoxyphenyl)-5-methyloxazol-4-yl)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 873 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314811 (2-((4-(2-(2-(4-chlorophenyl)-5-methyloxazol-4-yl)e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 942 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314818 (2-((3-((2-(2-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314828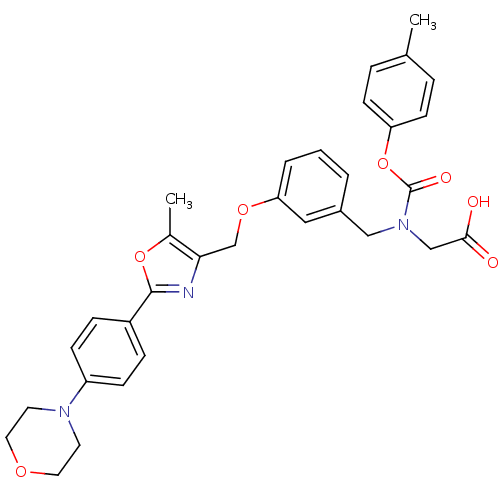 (2-((3-((5-methyl-2-(4-morpholinophenyl)oxazol-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50314814 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314826 (2-((3-((2-(4-tert-butylphenyl)-5-methyloxazol-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50189575 (CHEMBL3827335) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged PPAR-alpha LBD expressed in Escherichia coli BL21 (DE3) PlysS after 30 mins in presence of fluorescein ligand FL... | ACS Med Chem Lett 7: 590-4 (2016) Article DOI: 10.1021/acsmedchemlett.6b00033 BindingDB Entry DOI: 10.7270/Q2CR5W99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314823 (2-((3-((5-methyl-2-p-tolyloxazol-4-yl)methoxy)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50189567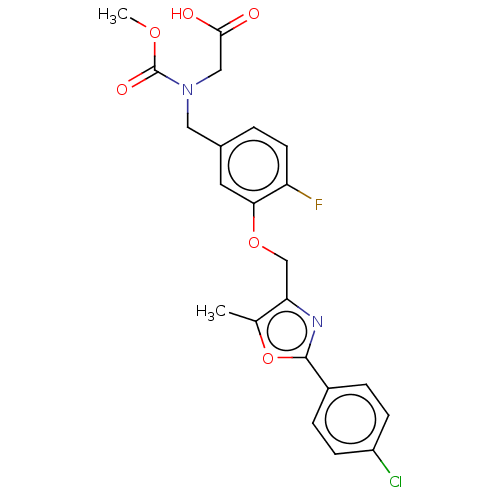 (CHEMBL3828232) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged PPAR-alpha LBD expressed in Escherichia coli BL21 (DE3) PlysS after 30 mins in presence of fluorescein ligand FL... | ACS Med Chem Lett 7: 590-4 (2016) Article DOI: 10.1021/acsmedchemlett.6b00033 BindingDB Entry DOI: 10.7270/Q2CR5W99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314828 (2-((3-((5-methyl-2-(4-morpholinophenyl)oxazol-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314827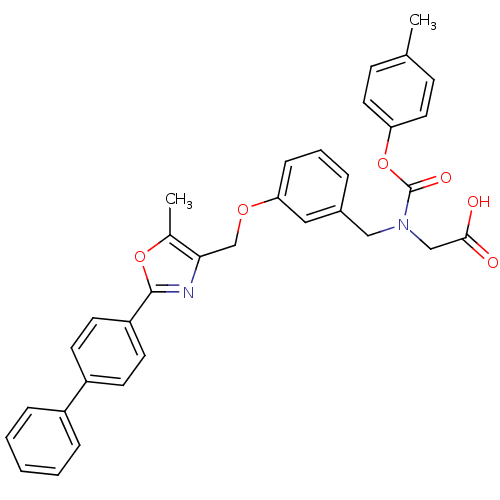 (2-((3-((2-(biphenyl-4-yl)-5-methyloxazol-4-yl)meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314820 (2-((3-((2-(4-fluorophenyl)-5-methyloxazol-4-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314824 (2-((3-((5-methyl-2-(4-(trifluoromethyl)phenyl)oxaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314814 (2-((3-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50314822 (2-((3-((2-(4-methoxyphenyl)-5-methyloxazol-4-yl)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50314827 (2-((3-((2-(biphenyl-4-yl)-5-methyloxazol-4-yl)meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARalpha LBD (E196-Y468) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314817 (2-((3-((2-(4-chlorophenyl)-5-methylthiazol-4-yl)me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human His-tagged PPARgamma LBD (Q203-Y477) expressed in Escherichia coli BL21 (DE3) by fluorescence polarization assay | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50314815 (2-((3-((2-(4-chlorophenyl)-4-methyloxazol-5-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50314823 (2-((3-((5-methyl-2-p-tolyloxazol-4-yl)methoxy)benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 53: 2854-64 (2010) Article DOI: 10.1021/jm9016812 BindingDB Entry DOI: 10.7270/Q2B56JW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 214 total ) | Next | Last >> |